Effects of Intermittent Feeding and Exercise Training on the Carbohydrate Utilization of Oreochromis niloticus
Received: 29-Mar-2018 / Accepted Date: 05-May-2018 / Published Date: 10-May-2018 DOI: 10.4172/2332-2608.1000266
Abstract
The effects of exercise training and intermittent feeding on the capacity of carbohydrate utilization in Nile tilapia (Oreochromis niloticus), an omnivorous fish, were examined under laboratory conditions, with the aim to increase the content of carbohydrate in the feed. The experimental fish were fed with a diet of 45% carbohydrate level for 48 days. Two feeding modes, namely continuous feeding group A and intermittent feeding group B (1 day starvation, 5 day feeding) were set up. Two exercise intensity groups (0 BL/s and 1.5 BL/s) were set up for each feeding mode. After feeding for 48 days, the results showed that exercise training could effectively reduce the negative effects on growth, body nutrition and liver health caused by high sugar diet. Cyclical deprivation and re-feeding regimes stimulated overcompensatory growth effects of O. niloticus fed by carbohydrate-rich diets. The combination of intermittent feeding and exercise training can further improve the utilization rate of carbohydrates in O. niloticus, reduce the accumulation of excessive glycogen and fat in fish, and alleviate the lipidty liver symptoms caused by high carbohydrate diets.
Keywords: Exercise training; Intermittent feeding; Oreochromis niloticus; Lipidty liver
Introduction
Carbohydrate is a kind of cheap diet material. Adding carbohydrate to fish feed can save protein or fat [1,2]. But fish have a poor ability to use carbohydrate [3]. Excess digestible carbohydrate in feed can cause excess accumulation of liver fat or glycogen and damage liver tissue, inhibit fish growth, cause fish death, etc. [4,5].
When fish return to normal feeding after starvation, they exhibit a faster growth rate than normal, known as compensatory growth or catch-up growth of fish [6-8]. Intermittent feeding can not only avoid the loss of compensatory growth ability due to excessive starvation, but also avoid the destruction of fish physiological function [9,10]. And intermittent feeding help to stimulate the compensatory growth potential of fish and increase the compensatory growth time of fish [11]. In addition, cyclical deprivation and re-feeding regimes can also be used to change various substances and energy metabolism in fish, thereby changing the content of energy nutrients such as protein, fat and glycogen [12-14]. However, there are few reports of intermittent feeding in the process of exercise training.
Tilapia belongs to Perciformes, Cichlidae. It is one of the excellent breeding varieties recommended by FAO to the world [15]. Therefore, this experiment took Oreochromis niloticus as the experimental object. The effects of exercise training and intermittent feeding on the carbohydrate utilization of O. niloticus were studied in this experiment, with two main purposes: 1) in order to enhance the glucose tolerance of fish and slow down the occurrence of metabolic diseases, such as fatty liver and 2) in order to provide a theoretical basis for improving the efficiency of aquaculture.
Materials and Methods
Animal handling and experimental feed
Nile tilapia (O. niloticus) was obtained from Fisheries Seedling Breeding Center (Guangzhou, China). The care and use of the fish in our laboratory complied with legal regulations in Guangdong, China, and with animal experimental principles and guidelines [16]. The fish were kept under natural photoperiod conditions in well-oxygenated water at 27 ± 2°C for 2 weeks (before and throughout the experiments). The light intensity is 1200 LX ± 100 LX. The fish was fed with commercial feed twice a day at 9: 00 and 18: 00. The main composition of their diet was 35% protein, 6% lipid, 28% carbohydrate, 6% ash, and 1.5% vitamin and mineral premix. At the beginning of the experiment, the mean body mass of the fish was 41.78 ± 5.38 g, and their mean length was 10.61 ± 4.90 mm.
The feed used in this experiment is a feed with a Carbohydrate content of 45%.The formula and nutritional level of experimental feed are shown in Table 1.
| Ingredients | Scale (%) |
|---|---|
| White fish meal (CP 60.2%) | 5 |
| Soybean meal (CP 47.9%) | 25 |
| Casein (CP 84.4%) | 18 |
| Flour | 5 |
| Corn starch | 38 |
| Cellulose | 3 |
| Fish oil | 2 |
| Bean oil | 2 |
| Mineral premix | 1 |
| Vitamins premix | 1 |
| Ca(H2PO4)2 | 2 |
| Vitamin C | 0.5 |
| Choline chloride | 0.5 |
| Total | 100 |
| proximate composition( dry weight) | |
| Ash | 5.61 |
| Crude protein | 30.35 |
| Crude lipid | 4.58 |
| Carbohydrate | 45.26 |
Table 1: Composition and nutrient levels of experimental diets (Dry matter basis).
Experimental design
The method of this experiment is to induce the counter current movement of fish by flowing water [17]. The unit of flow velocity is expressed as a multiple of the body length of the experimental fish (BL/s). In the course of the experiment, the experimental fish were placed in a self-designed swimming device [18].
The fish were hungry for 48 h before the beginning of the experiment. Two feeding methods were set up in this experiment: continuous feeding group A (daily feeding) and intermittent feeding group B (starvation 1 day, feeding 5 days). Two water current velocities, 0 BL/s and 1.5 BL/s, were set up for each feeding mode. The four experimental group are 0A, 0B, 1.5A, 1.5B. There are 20 fish in each group. Each combination has three parallel experiments. The whole experiment went on for 48 days. The exercise group was trained for 12 hours a day from 20: 00 to 8: 00. The feeding time and environmental conditions during the experiment were the same as those in the temporary culture.
Sample collection
After the experiment, five fish were randomly selected from each group. And then these fish were starved for 24 hours and anaesthetized with MS-222 (100 mg/L; Sigma, St. Louis, MO, USA). The body mass and body length, the weight gain rate, the specific growth rate, condition factor and other indicators of these fish were calculated. The blood samples of three fish in each group were collected by vein (≥2 mL), and preserved samples. The white muscle, the red muscle and the liver were isolated from nine fish in each group, and stored at -20°C for the analysis of tissue nutrient components [19]. The liver was isolated from three fish in each group for tissue section.
Determination of biochemical components
The water content of white muscle, red muscle and liver was determined by atmospheric pressure drying method (105°C); the content of crude ash was determined by the method of high temperature burning in muffle furnace (550°C); and the content of crude protein was determined by automatic Kjeldahl Kjeltec (Denmark Foss Kjeltec TM2300). Crude fat content was determined by Soxel extraction system (Foss Soxtec TM2055) and glycogen was determined by anthrone colorimetry.
Liver tissue section
After dissection, the liver tissue was fixed in 10% neutral formalin, embedded in paraffin, and made into 5μm thick sections stained with H. E., observed and photographed under microscope.
Statistical analysis
SPSS Statistics 17.0 (IBM, Somers, NY, USA) was used for data analysis. The data for all parameters were presented as mean ± SE. The differences between the experimental groups were analyzed with one-way analysis of variance and two-way analysis of variance (ANOVA), followed by a Duncan’s test to identify significantly different means (p<0.05).
The indicators are based on the following formula:
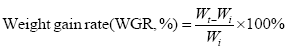
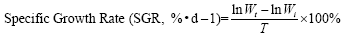
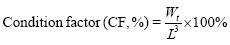

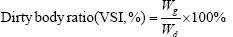
In the formula, Wi and Wt represent the initial body mass (g) and the final body mass (g); T represents the actual number of days of feeding (d); L denotes the sample body length (cm); Wd denotes the sample body mass (g); Wl denotes the sample fish liver weight (g); Wg denotes the sample fish visceral weight (g).
Results
Effects of intermittent feeding and exercise training on growth
Table 2 shows the growth of tilapia in each combination. The WGR and SGR of group 0A were significantly lower than those of group 1.5A, group 0B and group 1.5B (P>0.05). For the same feeding mode, group 1.5A was significantly higher than group 0A (P<0.05). There was no significant difference between group 0B and group 1.5B (P>0.05). For the same exercise pattern, group 0B was significantly higher than group 0A (P<0.05). There was no significant difference between group 1.5A and group 1.5B (P>0.05). The HIS and CF of group 0B and group 1.5B were significantly higher than group 0A and group 1.5A (P<0.05).
| Items | Experimental combinations | |||
|---|---|---|---|---|
| 0C | 1.5C | 0E | 1.5E | |
| IBW/g | 41.72±5.48 | 41.10±5.90 | 41.82±3.86 | 42.48±6.30 |
| FBW/g | 96.10±4.01c | 113.98±12.59b | 124.00±10.09ab | 136.11±6.90a |
| WGR/% | 130.37±20.97b | 177.32±30.63a | 196.45±24.13a | 220.40±16.24a |
| SGR/%·d-1 | 1.65±0.21b | 2.04±0.23a | 2.18±0.17a | 2.35±0.10a |
| HSI/% | 1.77±0.19c | 2.10±0.10b | 2.37±0.18a | 2.31±0.10a |
| VSI/% | 6.28±0.37a | 6.16±0.41a | 5.28±0.39b | 6.64±0.34a |
| CF/% | 4.44±0.02b | 4.46±0.06b | 4.61±0.09a | 4.68±0.10a |
Note: Values with different superscripts are significantly different (P<0.05).
Table 2: Effects of exercise training and intermittent feeding on the growth of O. niloticus.
According to Two-way analysis of variance, intermittent feeding and exercise training had significant synergistic effects on HSI and VSI of each experimental group (P<0.05).
Effects of intermittent feeding and exercise training on body nutrition
Table 3 shows the effects of intermittent feeding and exercise training on the nutritional components of the white muscle, red muscle and liver.
| Tissue | Items | Experimental combinations | |||
|---|---|---|---|---|---|
| 0A | 1.5A | 0B | 1.5B | ||
| White muscle | Moisture | 21.88 ±0.68b | 21.95 ± 0.80b | 22.60 ± 0.58a | 21.91 ± 0.50b |
| Ash | 7.62 ± 1.03 | 7.08 ± 0.91 | 7.57 ± 0.47 | 7.05 ± 1.21 | |
| Crude protein | 84.72 ± 1.71b | 88.51 ± 1.21a | 86.48 ± 2.11b | 88.67 ± 0.73a | |
| Crude lipid | 19.48 ± 1.67a | 16.18 ± 2.63b | 16.31 ± 1.03b | 14.85 ± 1.04b | |
| Red muscle | Moisture | 23.46 ± 1.14ab | 22.34 ± 1.25b | 23.92 ± 1.14a | 22.70 ± 1.06b |
| Ash | 4.99 ± 0.27 | 4.20 ± 0.98 | 4.42 ± 0.51 | 4.54 ± 0.88 | |
| Crude protein | 75.93 ± 2.58b | 79.68 ± 0.53a | 76.99 ± 070b | 80.34 ± 2.05a | |
| Crude lipid | 29.01 ± 3.05b | 33.04 ± 1.82a | 24.62 ± 1.70c | 27.24 ± 1.22b | |
| Liver | Moisture | 32.27 ± 1.99a | 27.59 ± 1.71c | 32.70 ± 1.88a | 29.53 ± 1.48b |
| Ash | 3.71 ± 0.51 | 3.54 ± 0.35 | 3.25 ± 0.66 | 3.14 ± 0.64 | |
| Crude protein | 34.61 ± 2.24b | 40.23 ± 1.48a | 36.00 ± 0.60b | 41.82 ± 3.12a | |
| Crude lipid | 40.40 ± 1.52a | 36.78 ± 2.09b | 38.85 ± 1.47ab | 35.25 ± 0.92b | |
Note: Values with different superscripts are significantly different (P<0.05).
Table 3: Effects of intermittent feeding and exercise training on proximate composition in tissues in O. niloticus.
Intermittent feeding and exercise training had no significant effect on crude ash content of white muscle, red muscle and liver (P>0.05).
For crude protein in all components, the contents of group 1.5A, group 0B and group 1.5B were significantly higher than that of group 0A. For the same feeding mode, group 1.5A was significantly higher than that of group 0A (P<0.05), and group 1.5B was significantly higher than that of group 0B (P<0.05). For the same exercise mode, there was no significant difference between group 1.5A and group 1.5B (P>0.05).
For crude fat in white muscle and liver, the contents of group 0B, group 1.5B and group 1.5A were significantly lower than those in group 0A. For the same feeding mode, group 1.5A was significantly lower than group 0A (P < 0.05). There was no significant difference between group 0 B and group 1.5 B (P>0.05). For the same exercise mode, group 0B was significantly lower than group 0A (P<0.05).
For crude fat in red muscle, the contents of group 0B and group 1.5B were significantly lower than those of group 0A. For the same feeding mode, group 1.5A was significantly higher than group 0A (P<0.05), and group 1.5B was significantly higher than group 0B (P<0.05), and for the same exercise mode, group 0A was significantly higher than group 0B (P<0.05).
According to Two-way analysis of variance, for the contents of crude protein and fat in white muscle, red muscle and liver, there was no significant interaction between intermittent feeding and exercise training (P>0.05).
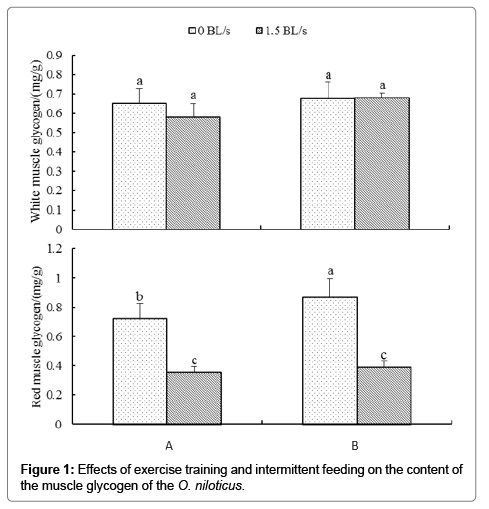
Effects of intermittent feeding and exercise training on muscle glycogen and liver glycogen
Figure 1 shows the effect of intermittent feeding and exercise training on the content of muscle glycogen. There was no significant difference in the content of glycogen in the white muscle of each group (P>0.05). For the content of glycogen in red muscle, group 0B was significantly higher than that of group 0A, group 1.5A and group 1.5B (P<0.05), and group 0A was significantly higher than group 1.5A and group 1.5B (P<0.05). For the same feeding mode, group 1.5A was significantly lower than group 0A (P<0.05) and group B was significantly lower than group 0B (P<0.05). For the same exercise mode, the content of group 0B was significantly higher than group 0A (P<0.05), and there was no significant difference between group 1.5A and group 1.5B (P>0.05).
According to Two-way analysis of variance, for the contents of glycogen in white and red muscles, there was no significant interaction between intermittent feeding and exercise training (P>0.05).
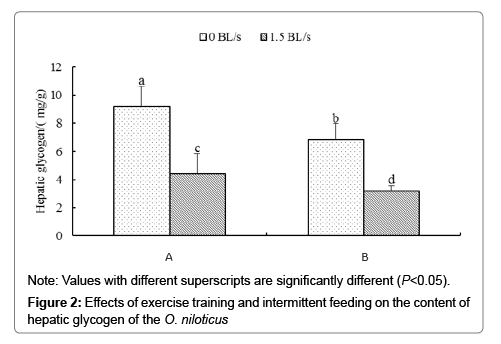
Figure 2 shows the effect of intermittent feeding and exercise training on liver glycogen content. The order of liver glycogen content from high to low is: group 0A>group 0B>group 1.5A>group 1.5B, and there is significant difference among all experimental groups (P<0.05).
According to Two-way analysis of variance, for the content of glycogen in liver, there was no significant interaction between intermittent feeding and exercise training (P>0.05).
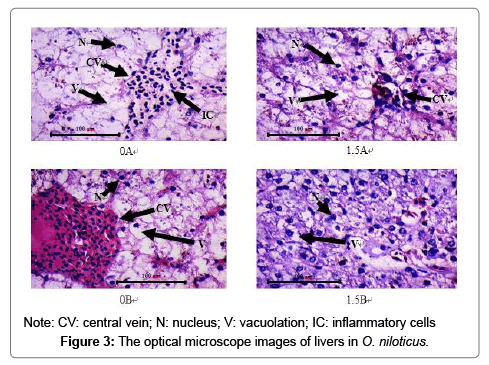
Effect of intermittent feeding and exercise training on liver tissue
Figure 3 shows the liver tissue morphology of the various experimental combinations. This is a 400-fold optical microscope photograph of the liver tissue section. In group 0A and group 0B, the hepatocytes were swollen, a large number of cells were filled with lipid droplets, the nucleus was squeezed to one side, and even broken. Inflammatory cell infiltration was found in the central vein of group 0A. The cells of group 1.5A were obviously swollen, the cell volume was enlarged, the nucleus was skewed obviously, the cytoplasm was granule like degeneration, but there was no nuclear fragmentation and inflammatory cells. However, there was only slight vacuolation and nuclear deviation in the hepatocytes of group 1.5B.
Discussion
Effects of intermittent feeding and exercise training on growth
In this study, a diet with a carbohydrate level of 45% was used. The results show that, for WGR and SGR, continuous feeding swimming group (1.5A), intermittent feeding resting group (0B) and intermittent feeding swimming group (1.5B) were significantly higher than those of continuous feeding resting group (0A). There was no significant difference among the three combinations, which indicated that exercise training, intermittent feeding and synergistic effects could reduce the negative effects of high sugar diet on the growth of tilapia, and improve the utilization rate of carbohydrate in fish.
For the body index, both resting group and swimming group, the intermittent feeding of HIS, VSI and CF were higher than those of the continuous feeding group. The results showed that HSI was influenced by the synergistic effect of exercise training and intermittent feeding. The HSI of group 1.5A, group 0B and group 1.5B were significantly higher than that of group 0A, which may be due to the increase of liver fat content induced by high carbohydrate diet. This may also be due to low fat density or hepatocyte necrosis resulting in a decrease in HIS [20]. In this experiment, intermittent feeding and exercise training improved the utilization efficiency of carbohydrate to some extent, increased the consumption of carbohydrate, reduced the lipid conversion of carbohydrate, improved the liver structure, and the corresponding ratio of liver to body increased.
Effects of intermittent feeding and exercise training on muscle tissue composition
For the content of glycogen in red muscle, group 0B was significantly higher than that in group 0A. The reason may be that intermittent feeding not only promotes the recovery of muscle glycogen consumed during starvation [21], but also induces adaptive response of experimental fish, and stores more energy substances to protect against hunger in the next cycle [22]. However, under exercise conditions, there was no significant difference in the glycogen of red muscle between the two feeding modes. The reason may be that the glycogen of red muscle was used to supply energy during exercise, and the experimental fish fed intermittently was trained during starvation, which may aggravate the consumption of glycogen. This study shows that no matter under any feeding mode, exercise training can effectively improve the utilization rate of glycogen in red muscle and reduce glycogen deposition. But the content of glycogen in the white muscle was not affected by it.
In the aspect of lipid, the content of crude fat decreased significantly in exercise mode, but there was no significant difference between the two feeding modes. However, intermittent feeding mode in still water can significantly reduce crude fat content. It can be seen that although intermittent feeding and exercise training have significant effects on adipose catabolism of red muscle, exercise training seems to have a more significant effect on the fat catabolism of red muscle. It has been pointed out that under the condition of continuous movement, fish mainly rely on fat oxidation for energy supply, and the structure and biochemical characteristics of red muscle can meet the requirement of rapid fat oxidation. Therefore, red muscle is the most important place for oxidation energy supply in this process [23-25].
In summary, in high carbohydrate diet, exercise training significantly reduced the content of glycogen and fat in fish muscles, and improved the tolerance of fish.
Effects of intermittent feeding and exercise training on liver tissue composition
In a hungry state, the metabolic function of fish will change to adapt to different nutritional conditions, often using their own energy storage substances to provide the necessary energy for life [26]. However, in most fish, liver glycogen is usually decomposed into glucose to provide energy, and it is also used to maintain the dynamic balance of blood glucose concentration [27,28]. Therefore, the level of liver glycogen can reflect the body's glucose metabolism to some extent. In this study, intermittent feeding and exercise training had significant effects on liver glycogen content, but there was no significant synergistic effect between them. The order of liver glycogen content from high to low was: group 0A>group 0B>group 1.5A>group 1.5B. Moreover, there were significant differences among the experimental combinations. Both intermittent feeding and exercise training could enhance the activity of glucose catabolism in the liver, mobilize the stored liver glycogen to provide energy, and reduce excessive hepatic glycogen deposition caused by high glucose, thus increasing the tolerance to high sugar feed.
Effects of intermittent feeding and exercise training on liver morphology
Fish eat excessive carbohydrate, which can lead to excessive accumulation of fat and glycogen in the liver. This can also cause swelling and vacuolation of liver cells and damage liver tissue, thus affecting the metabolic ability of fish [29-32]. In this study, the liver cells of group 0A had obvious symptoms of fatty liver, but there were no inflammatory cells infiltrating in liver tissue of group 0B. While the hepatocytes of group 1.5A had obvious swelling, nuclear deviation and other fatty liver symptoms, but their nuclear staining substances were more than those of the former two groups. The symptom of fatty liver is relieved to some extent. In the end, only slight vacuolization and nuclear bias occurred in group 1.5B. The results showed that the synergistic effect of intermittent feeding and exercise training could significantly alleviate the symptoms of fatty liver caused by high glucose, which coincided with the decrease of crude fat content and glycogen content in the liver of the combination.
Conclusion
In this experiment, Oreochromis niloticus was used as the research object, and were fed with a diet of 45% carbohydrate level. Through intermittent feeding and exercise training, the effects of both and their synergistic effects on the metabolic changes of carbohydrates and their control on fatty liver caused by carbohydrate sugar feed were discussed. The main conclusions were as follows:
1) Under the condition of high carbohydrate diet, the growth rate of intermittent feeding swimming group was significantly higher than that of continuous feeding resting group.
2) Exercise training and intermittent feeding had no significant effect on the accumulation of white muscle glycogen, but significantly decreased the fat content. Intermittent feeding promotes glycogen accumulation in red muscle and inhibits fat accumulation. Exercise training inhibits glycogen accumulation in the red muscle and promotes fat accumulation.
3) According to the observation results of liver fat, glycogen content and tissue section, intermittent feeding or exercise training could alleviate the symptoms of fatty liver, but the effect of exercise training was better than that of simple intermittent feeding. In particular, the synergistic effect of exercise training and intermittent feeding can significantly alleviate the symptoms of fatty liver in Oreochromis niloticus.
Acknowledgements
This work was supported by the Ocean and Fishery Special Fund Project of Guangdong Province for Technology Extension (2017A0010).
References
- Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124: 67-80.
- Wu CL, Ye JY, Gao J, Chen L, Lu Z, et al. (2016) The effects of dietary carbohydrate on the growth, antioxidant capacities, innate immune responses and pathogen resistance of juvenile Black carp Mylopharyngodon piceus. Fish Shellfish Immunol 49: 132-142.
- Metón I, Fernández F, Baanante IV (2003) Short-and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparusaurata). Aquaculture 225: 99-107.
- Jiang LH, Wu HY, Huang K, Ma YQ, Yang Q, et al.(2013)Effects of dietary carbohydrate levels on growth performance andliver metabolism functions of juvenile tilapia (Oreochromis niloticus)37: 245-255.
- Kumar S, Sahu NP, Pal AK (2005) Effect of dietary carbohydrate on hematology, respiratory burst activity and histologoical changes in L.rohita juveniles. Fish & Shellfish Immunology 19: 331-344.
- Jobling M, Christiansen B,Meløy OH, Santos JD (1994) The compensatory growth response of the Atlantic cod: effects of nutrition history. Aquaculture Internationa l2: 75-90.
- Xie XJ, Deng L, Zhang B (1998) Advances and studies on ecophysiological effects of starvation on fish. Acta Hydrobiolgica Sinica 22:181-188.
- Ali MZ, Jauncey K (2004) Evaluation of mixed feeding schedules with respect to compensatory growth and body composition in African catfish Clariasgarie pinus. Aquaculture Nutrition 10: 39-45.
- Bélanger F, Blier PU, Dutil JD (2002) Digestive capacity and compensatory growth in Atlantic cod (Gadusmorhua). Fish Physiology and Biochemistry 26: 121-128.
- Cui Z, Wang Y, Qin J (2006) Compensatory growth of group-held gibel carp, following food deprivation. Aquacult Res 37: 313-318.
- Hayward RS, Noltie DB, Wang N (1997) Use of compensatory growth to double hybrid sunfish growth rates. Transactions of the American Fisheries Society 126: 316-322.
- Deng DF, Refstie S, Hemre G, Crocker CE, Chen HY, et al. (2000) A new technique of feeding, repeated sampling of blood and continuous collection of urine in white sturgeon. Fish Physiology and Biochemistry 22: 191-197.
- Moreira IS, Peres H, Couto A, Oliva-Teles A, Enes P (2008) Temperature and dietary carbohydrate level effects on performance and metabolic utilization of diets in European sea bass (Dicentrarchuslabrax) juveniles. Aquaculture 274: 153-160.
- Zhu XR, Shen WY (2002) Effect of starvation and refeeding on glyeogen metabolism in grass carp (ctenopharyngodonidellus) fingeling. Journal of Shaoxing University (Natural Science) 22: 23-25.
- He YH, Zhang HY, Gong ZH(2009) Analysis on breed and development of tilapia culture in china. Journal of Aquaculture:12-14.
- LiD, WeiXL, LinXT, XuZN, MuXP (2015) Effects of exercise training on carbohydrate and lipid catabolism in the swimming muscles of Nile tilapia (Oreochromisniloticus). J Anim Physiol Anim Nutr (Berl) 99: 893-898.
- Song BL, Lin XT, Xu ZN (2012) Effects of upstream exercise training on feeding efficiency,growth and nutritional components of juvenile tinfoil barbs(Barbodes schwanenfeldi). Journal of Fisheries 36:106-114.
- Zhu Z, Song B, LinX, XuZ (2016) Effect of sustained training on glycolysis and fatty acids oxidation in swimming muscles and liver in juvenile tinfoil barb Barbonymusschwanen feldii (Bleeker, 1854). Fish Physiol Biochem: 1-11.
- Cheng HL, Xia DQ, Wu TT (2006) Fatty liver and regulation of lipids metabolism in fish. Chinese Journal of Animal Nutrition 18: 294-298.
- Wei XL (2015) Physiological Mechanism of Exercise Training Affecting Protein and Sugar Metabolism and Nutritional Requirements of Oreochromis niloticus 1-67.
- Mu XP, Lin XT, Zhu ZM (2014) Effect of upstream and replacement of dietary fish oil by sunflower seed oil on growth and body composition of GIFT Oncorhynchus mykiss. South China Fisheries Science 10: 27-35.
- Johnston IA, Moon TW(1980b) Exercise training in skeletal muscle of brook trout(Salvelinus fontinalis). J Exp Bio l45: 177-194.
- Room LC (1998) Some advances in integrative muscle physiology. Comp Biochem Physiol B Biochem Mol Bio l120: 51-72.
- Richards JG, Mercado AJ, Clayton CA, Heigenhauser GJF, Wood CM (2002a) Substrate utilization during graded exercise in rainbow trout. J Exp Biol 205: 2067-2077.
- Machado CR, Garofalo MAR, Miglioroni RH (1988) Effects of starvation, refeeding, and insulin on energy-linked metabolic processes in catfish (Rhamdiahilarii) adapted to a carbohydrate-rich diet. Gen Comp Endocrinol 71: 429-437.
- Blasco J, Fernà ndez-Borràs J, Marimon I, Requena A (1995) Plasma glucose kinetics and tissue uptake in brown trout in vivo: effect of an intravascular glucose load. J Comp Physiol B 165: 534-541.
- Fan GY, Zhao CY, Li YW (2010) Effects of starvation and refeeding on metabolism of glucose in juvenile parabramispekinensis. Journal of Chongqing Normal University (Natural Science) 27: 9-22.
- Cheng C, Xia XJ, Luo YP (2007) Effect of dietary carbohydrate level on histology of liver, pancreas and kidney in the southern catfish (silurus meridionalis) juveniles. J. Southwest China (Natural Science Edition)29: 103-108.
- Miao LH, Liu B, Ge XP, Jun X, Ru-li C, et al. (2011) Effect of high carbohydrate levels in the dietary on growth performance, immunity and transmission electron microscopy (TEM) on hepatic cell of allogynogenetic crucian carp (Carassius auratus gibelio). J Fish China 35: 221-230.
- Jiang LH, Wu HY, Huang K, Yanqun MA, Lingxiang Z, et al. (2013)Effects of dietary carbohydrate levels on growth performance andliver metabolism functions of juvenile tilapia (Oreochromis niloticus). J Fish China 37:245-255.
- Kumar S, Sahu NP, Pal AK(2005) Effect of dietary carbohydrate on hematology, respiratory burst activity and histologoical changes in L.rohita juveniles. Fish Shellfish Immunol 19: 331-344.
- Song BL, Lin XT, Xu ZN ( 2012) Effects of upstream locomotion training on metabolism and norfloxacin hydrochloride residues of juvenile tinfoil barbs Barbodes schwanenfeldi. Freshwater Fisheries 42:3-7.
Citation: Lingyun LI, Qingting Z, Qian TU, Yonghui YU, Xiaotao L (2018) Effects of Intermittent Feeding and Exercise Training on the Carbohydrate Utilization of Oreochromis niloticus. J Fisheries Livest Prod 6: 266. DOI: 10.4172/2332-2608.1000266
Copyright: © 2018 Lingyun LI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4096
- [From(publication date): 0-2018 - Jul 11, 2025]
- Breakdown by view type
- HTML page views: 3216
- PDF downloads: 880