Research Article Open Access
Effects of Hyperoxia Exposure on Free Radicals Accumulation in Relation to Ultrastructural Pathological Changes of Diaphragm
Khaldoon Aljerian1,2 and Al-Said Haffor2*1Department of Pathology, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia
2College of Medicine, Dar Aluloom University, Riyadh, Kingdom of Saudi Arabia
- *Corresponding Author:
- Al-Said Haffor
Ph.D., Professor, College of Medicine
Dar Aluloom University, Riyadh
Kingdom of Saudi Arabia115112
Tel: 9660507201601
E-mail: saidhaffor @yahoo.com
Received date: July 17, 2015; Accepted date: August 13, 2015; Published date: August 15, 2015
Citation: Aljerian K, Haffor A (2015) Effects of Hyperoxia Exposure on Free Radicals Accumulation in Relation to Ultrastructural Pathological Changes of Diaphragm. J Clin Exp Pathol 5:247. doi: 10.4172/2161-0681.1000247
Copyright: © 2015 Aljerian K et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
COPD are associated with an increased load on the diaphragm leading to accumulation of reactive oxygen species (ROS) and the subsequent cellular damages and death. The pathological alterations inducted by ROS in the diaphragm during oxygen breathing are not known. The purpose of the present study was to examine the effects of hyperoxia exposure (HP) on free radicals (FR) accumulation in relation to the ultrastructural pathological alterations in the diaphragm. Twenty adult male rats were randomly assigned to two groups; control (C); and hyperoxia (HP). Animals of the HP were breathing 100% O2 for 72 hr continuously. Both serum and diaphragm tissue supernatant analysis showed significantly higher (p<0.05) FR in HP group, as compared with control group. Ultrastructure examinations showed that HP resulted in variety of pathological alterations in the mitochondria and endoplasmic reticulum that were associated with disarrangement of myofibrils, loss of I-banding for myosin, focal myolysis of the myofilaments, complete fragmentation of myosin, tearing of myofilamments from Z plates and tearing of the endothelial cell of the interstitial blood capillaries. Based on the results of the present study, it can be concluded that hyperoxia-induced acceleration ROS formation damaged the contractile apparatuses of the diaphragm and related endomembrane proteins that could involve intracellular calcium channels proteins.
Keywords
Diaphragm; Free radicals; Hyperoxia; Oxygen breathing; Oxidative stress
Introduction
Oxygen is required to sustain life, but continued breathing of 100% oxygen is toxic to the mammalian respiratory system. Oxidant byproducts, such as superoxide anion (O2-), peroxinitrate (ONOO-) and hydrogen peroxide (H2O2), are produced as a consequence of normal aerobic metabolism in skeletal muscle. Exertion-induced muscle injury was attributed to elevated levels of reactive oxygen species [1-17]. The diaphragm is the principal muscle of inspiration. The diaphragm is a skeletal muscle and it is subject to oxidative stress during increased work of breathing. Under normal physiological conditions, reactive O2 species (ROS) production is balanced by an efficient system of antioxidants, molecules that are capable of scavenging ROS and thereby preventing oxidant damage.
Chronic obstructive pulmonary disease (COPD), specifically emphysema, is a highly relevant disorder that inducts respiratory muscle dysfunction mainly due to overinflated lungs [18-20]. Currently, there is no cure for this disorder. Nevertheless, the potential therapeutic strategies focusing on respiratory muscles may overweigh the potential of focusing on restoring destructed multiple lungs' units, acini. Although it is well established that patients with COPD generate less trans-diaphragmatic pressure than healthy subjects [3], the diaphragm weakness has been ascribed to hyperinflation-induced diaphragm shortening, which places the diaphragm at a mechanical disadvantage [21-23]. Ultrastructural pathological alterations are direct reliable markers for the cellular damages inducted by oxidative stress [8,14,22,23,24-32]. Mitochondria inner membrane dependent oxidative stress (MOS) is the origin of cellular oxidative stress [14,27]. There is no previous research provided information related to the impact of O2 breathing on the ultrastructure pathological alterations of the diaphragm myocyte, its mitochondria and its microvasculature in relation to reactive oxygen species by-products (ROS).
In view of the review presented above, the present study was designed to examine the effects 100% O2 breathing on the ultrastrucure of the diaphragm and the associated free radicals accumulation in both serum and diaphragm.
Materials and methods
Animals and experimental design
Twenty adult rats, Rattus norvigicus, matched with age weighing ~196 g, were brought from the university’s animal house, College of Pharmacy, King Saud University (Riyadh, KSA). All rats were kept under the same laboratory conditions of temperature (22 ± 2°C), dark cycle (14 h light: 10 h dark) and were allowed free access to tap water and standard food. Animals were randomly assigned to two groups, ten animals each. The first group served as control (C group) and the animals of the second group (HP group) were exposed to hyperoxia (100% O2, medical grade) for 72 hr continuously. All animal testing procedures and care were conducted according to the principles in the guidelines for the care and use of laboratory animals as described in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by National Institute of Health (NIH publications 86-23 revised 1985) [60] and approved by the King Saud University IACUC panel.
Hyperoxia exposure (HP)
Animals of the experimental group (HP group) were placed in a closed box that has an inlet flow which was connected to 100% O2 tank, medical grade, on which a regulator was connected to maintain flow at 3 litres per minute. The out flow of the regulator passed through a humidifier in order to saturate the inspired air with H2O. The outlet ventilation rate of the box was adjusted at 3 litres per minute to ensure that the concentration of oxygen in the box remains equal to 100% O2 in order to maintain normal flow and maintain normal barometric pressure at 767 mmHg. The temperature inside the box was also maintained at room temperature (22-24°C), throughout the 72 hr of exposure period.
Ultra-structure procedure
Tissue samples from the diaphragm were immersed in buffered 3% glutaraldhyde at 4°C for 4 hr and then post fixed in 1% osmium tetroxide. Fixed tissue samples were dehydrated in graded concentrations of ethyl alcohol, (30%, 50%, 70%, 90% and 100%). Dehydrated tissue samples were then placed in propylene oxide to get rid of ethanol and render the tissues to be penetratable for the embedding media. This step was done at room temperature for 60 min. Tissue samples were then transferred from propylene oxide to a mixture of expoxy resins (Epon/Araldite). First, samples were placed in a mixture of propylene oxide and resins at the ratio of 1:1 for 2 hr and lastly placed in a pure epoxy mixture overnight. Tissue samples were embedded in the epoxy mixture using polyethylene capsules. Polymerization of the resin was done at 60°C for 48 hr. Ultra-thin sections (70 nm) were made and double stained with uranyl acetate and lead citrate and then examined and photographed under transmission electron microscope (JEOL-100 CX) at 80kv.
Tissue preparation
Following the completion 72 hrs of HP period, animals were sacrificed and the diaphragms were isolated and homogenized immediately in 0.9 saline solutions (4:1 ratio) in an iced covet. The homogenates were centrifuged for 10 minutes at 3000 revolution per minute. Supernatant fractions from diaphragm homogenates were separated and used for free radicals (FR) determinations.
Free radical determinations
Free radicals accumulation was determined, using the d-ROMs-4 test kit (Health and Diagnostic, Italy). The test measures the levels of hydroperoxides (R-OOH) which are generated by peroxidation of biological compounds; lipid, amino acids, nucleic acids. This test is based on the principle of the ability of hydrogen peroxides to generate free radicals after reacting with some transitional metals (Fe2+/Fe3+), according to Fenton's reaction as follows:
H2O2 + Fe++ = *OH + OH- + Fe++
Thus, the hydrogen peroxides of biological test sample generate free radicals (alcoxy and peroxyl radicals) after exposure to a transitional metal (F++/Fe+++). In a correctly buffered chromogen substance (N, N-diethyl-phenylendiamine) lead to the reduction of hydrogen peroxides which in turns colored as radical cation. Color intensity was read using spectrophotometer with peak absorbance of 505 nm. In the d-ROMs test results were expressed in CARR units (CARR U), where one CARR unit is equivalent to 0.08 mg H2O2/100 ml (0.08 mg vol%0).
Results
The mean final body weights (± SD) of control and hyperoxia group at the end of the experiment were 197 ± 6, 199 ± 11, and 196 ± 8 g, respectively. There were no significant (p>0.05) changes in body weights prior to the experiment.
Free radicals (FR) in the serum and the diaphragm tissues
The results of paired t-test showed that the diaphragms' tissues and serum means' (± SD) values for free radicals (FR) were significantly (p<0.05) higher in the hyperoxia groups (HP), as compared with their corresponding control means' values (Table 1).
Diaphragm myocyte and mitochondria before O2 breathing
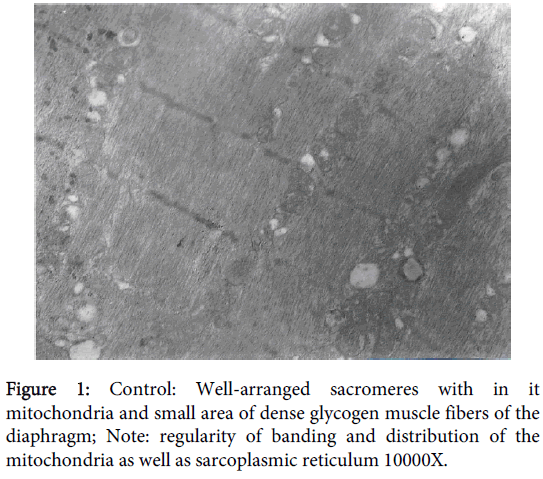
Ultra-structure samples from the control group showed well-arranged sacromeres with normal distribution of the mitochondria and small area of dense glycogen muscle fibers of the diaphragm. Regular arrangement of both, thick (myosin) and thin (actin) filaments, sacrcoplasmic reticulum (T- system) and the regularity of Z-lines that were separating the boundaries at the beginning and the ends of the sarcomeres. Mitochondria distribution was homogenous with normal size and number. Furthermore the locations of the mitochondria were properly sited in close proximity to sarcoplasmic reticulum (Figure 1).
Myo-filaments pathological alterations
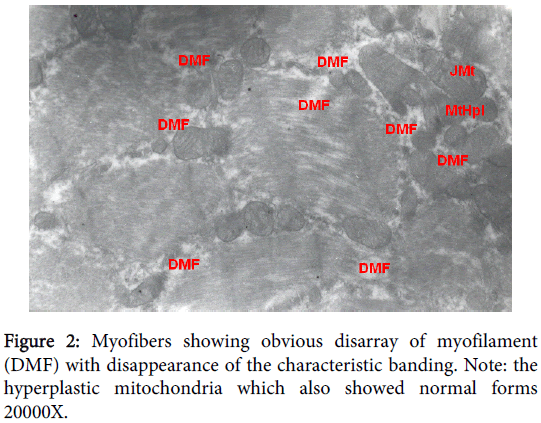
Ultra-structure samples from the diaphragm of the HP group (O2 breathing group) showed irregular arrangement of, thick (myosin) and thin (actin) filaments, dilution and/or disappearnace of sacrcoplasmic reticulum (T-system) and the absence of Z-lines those reflecting distorted sarcomeres units. Myofibers showing obvious disarray of myofilament (DMF) with disappearance of the characteristic banding. Focal necrosis, resulting in indented and split fibers, was observed in a variety of myofibres (Figure 2).
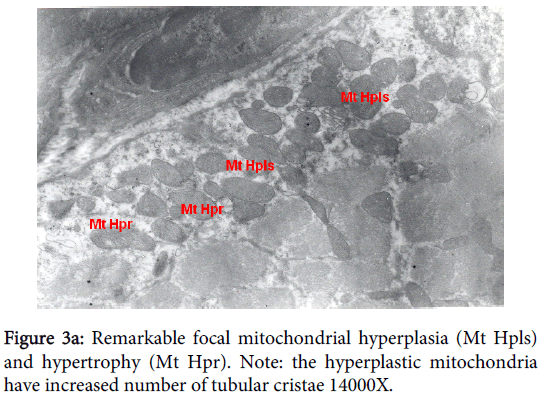
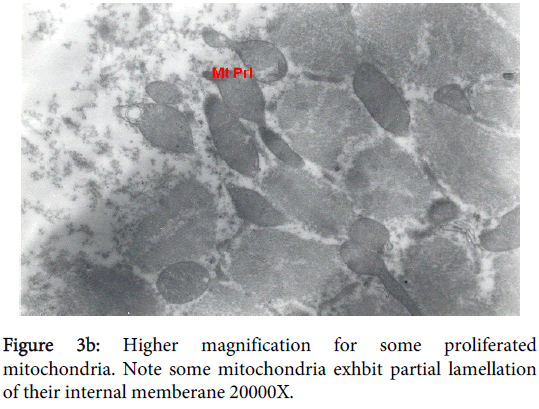
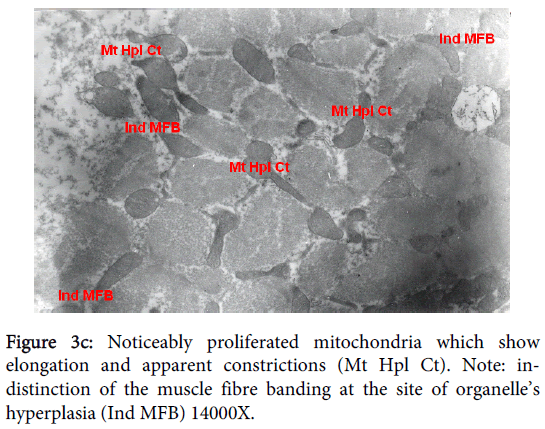
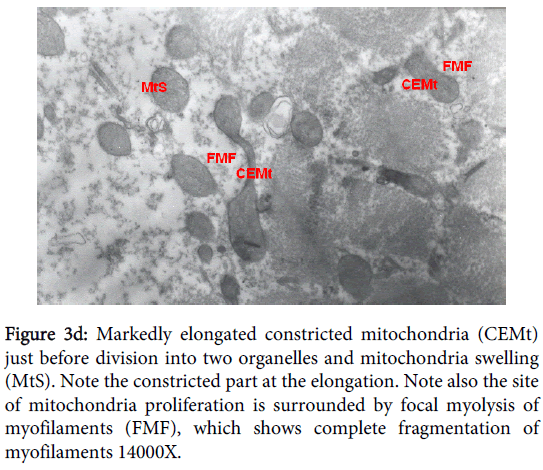
Mitochondria pathological alterations Remarkable Focal Mitochondrial hyperplasia and hypertrophy were clearly observed (Figure 3) the hyperplastic mitochondria showed increased tubular cristae with bizarre (abnormal) forms with partial lamellation of their internal membrane. Markedly elongated constricted mitochondria just before division into two organelles and mitochondria swelling were also observed. The constricted part at the elongation, the site of mitochondria proliferation, was surrounded by focal myolysis of the myofilaments, which exhibited complete fragmentation of myofilaments (Figure 3).
Figure 3d: Markedly elongated constricted mitochondria (CEMt) just before division into two organelles and mitochondria swelling (MtS). Note the constricted part at the elongation. Note also the site of mitochondria proliferation is surrounded by focal myolysis of myofilaments (FMF), which shows complete fragmentation of myofilaments 14000X.
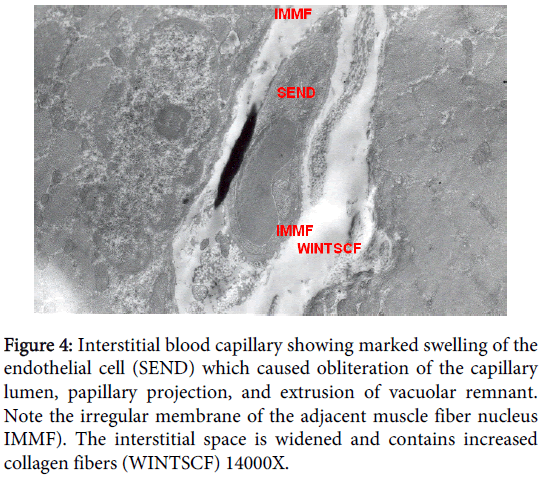
Interstitial blood capillary
Interstitial blood capillary showing marked swelling of the endothelial cell which caused obliteration of the capillary lumen, persistence of basal lamina invaded macrophages were distinctly presented, a long papillary projection and extrusion of vacuolar remnant. The interstitial space was widened and contains increased level of collagen fibers. There was marked swelling of the endothelial cell which caused obliteration of the capillary lumen, papillary projection, and extrusion of vacuolar remnant and invaded macrophages; including pinocytotic cytoplsmic microvesicles. Irregularity of the adjacent membrane of the surrounding muscle fiber nucleus was also observed. The interstitial space was widened and contains increased collagen fibers. Organelles, including mitochondria, in the endothelial cytoplasm were degenerated and there were many pinocytotic cytoplsmic microvesicles (Figure 4).
Figure 4: Interstitial blood capillary showing marked swelling of the endothelial cell (SEND) which caused obliteration of the capillary lumen, papillary projection, and extrusion of vacuolar remnant. Note the irregular membrane of the adjacent muscle fiber nucleus IMMF). The interstitial space is widened and contains increased collagen fibers (WINTSCF) 14000X.
Discussion
The major finding of the present study showed that oxygen breathing resulted in elevated FR production that was associated with variety of pathological alterations and that was interpreted as lack of antioxidants compensatory mechanisms. It had been shown that oxidative stress relate to free radical production [12,13,22,24,25,27,33,34]. During breathing pure oxygen, mixed venous O2 saturation may increase as much as 10% from the ~70% present during normal air breathing. This decreases the content of CO2 carried in the form of the carbamino load and consequently decreases CO2 elimination at the lung capillaries leading to an increase in brain tissue PCO2 that would stimulate respiration via the central chemoreceptors. Another mechanism that could participate in the hyperoxic enhancement of ventilation is the Haldane effect [16]. Normally, 30% of CO2 eliminated in the lungs comes from the carbamino sources carried with the venous blood hemoglobin. Oxygenated hemoglobin has a lower transport capacity for CO2 due to a less reduced state of carbamino bonds and a decreased buffering capacity [2,5-7,9-11,13,27,35-38].
The risk of hyperoxia was reported to increase with exposure period for over 16 hours of hyperoxia [23,39] in diseases conditions, but in a healthy adult risks are rarely seen before 24 hours of exposure [12,35,40]. Previous data from our laboratory showed an increase in the antioxidant enzyme, GPx, following the exposure to hyperoxia for 24 hr [22]. Clearly the oxidative stress induced by oxygen breathing for 72 hr of the present study caused specific morphological pathological changes, apoptosis, in mitochondria and the nucleus that resulted in excess formation of ROS, reduction of ATP, and impairments of antioxidants genes transcriptional. Therefore Oxygen toxicity is believed to occur when the diaphragm antioxidant defenses are overwhelmed by the build-up of ROS [33,41-43]. In addition the mitochondria swelling reported in the present study represents the cellular bases accounted for the impairment of the permeability of both inner and outer mitochondria. The giant and cloudy swelled mitochondria observed in the present study reflected water and hydrogen peroxide accumulation [28,32,36].
Although we did not measure water accumulation but we measured H2O2 based on free radicals production, thus the observed cloudiness and swelling of the present study reflected malfunction of osmotic control secondary to failure of various hydro and lipid peroxidase movement across the inner mitochondria membrane leading to flooding the inner mitochondria membranes. In addition the deteriorations of the cristae were associated with an increase in mitochondria mass, constricted matrix, disarrangement of myofiberils and the loss of I-banding. Myophagia, i.e. the invasion of a myofibre by mononucleated cells, was reported in the emphysematous patients [24,44,45]. There were more signs of streaking, but no differences were found in the presence of vacuoles. Lipofuscin granules were increased and there were more atrophic fibers. Indeed the deterioration of membrane potential of the mitochondria can induct mitochondria oxidative stress (MOS). It was previously shown that hyperoxia induced mitochondrial pathological changes provided critical mitochondrial events responsible for oxidative stress-mediated myocyte death [28].
Oxidative stress is thought to contribute to impairment and reduced regenerating capacity of skeletal muscles in aged and diseased animals [21,22,28,46-49]. Furthermore, elastase-induced emphysema in hamsters is frequently used as an animal model to study morphological and functional changes in the myocytes of diaphragm due to COPD [32,50]. Orozco-Levi et al. [28] showed that sarcomere disruption is present in COPD patients' diaphragmatic myocytes. Abnormalities of the contractile apparatus, like register-shifting and Z-line distortions were consistently found in the diaphragm of emphysematous. In addition, the observed sarcoplasmic masses and focal degeneration indicate various stages of degeneration. Focal as well as segmental necrosis and eventually myonecrosis were found frequently in aged diaphragm [50,51]. Levine and colleagues [23] published a landmark paper showing a fiber-type shift in the diaphragm muscle of patients with severe COPD. This fiber-type shift toward more oxidative type I fibers is regarded as a beneficial adaptive response to increased diaphragm loading, because it renders the diaphragm less susceptible to fatigue. Since then, several investigators have studied the effects of COPD on functional, biochemical, and morphological characteristics of the diaphragm.
Considering the clinical relevance of inspiratory muscle weakness in patients with COPD, counteracting diaphragm weakness is of major importance. Diaphragm wasting appears to play an important role in compromising diaphragm contractile performance in patients with COPD [44,47,52-63]. Because the etiology of diaphragm wasting in COPD is complex and largely unclear, identifying therapeutic targets is extremely difficult. However, downstream in the line of events leading to wasting, increased proteolysis is likely to play a role, presumably through activation of the ubiquitin-proteasome pathway [64]. Hence, agents inhibiting proteasomal activity are of potential therapeutic value. Recently, the proteasome inhibitor bortezomib has been approved for treatment of multiple myeloma in humans [65]. It should be noted, however, that the ubiquitin-proteasome pathway is regarded only to degrade damaged or misfolded proteins. Consequently, inhibiting proteasomal activity might lead to accumulation of damaged proteins, ultimately leading to cell death. Nevertheless, in vivo administration of bortezomib in an animal model of muscle atrophy prevented muscle wasting by approximately 50% [66,67], and in both studies, bortezomib was well tolerated. These findings show promise for the use of proteasome inhibitors in syndromes associated with muscle wasting, such as the diaphragm in COPD. Theoretically, inhibition of E3-ligases rather than the proteasome provides an ideal drug target, because E3-ligases have very high substrate and tissue selectivity. Therefore, a specific inhibitor of, for example, MAFbx should be a highly specific drug, and might prove beneficial in preserving contractile protein content and preventing diaphragm atrophy in COPD diaphragm.
Conclusions
Based on the results of the present study, it can be concluded that hyperoxia-induced-mitochondria acceleration free radical formation impaired the contractile apparatuses of the diaphragm and related endomembrane proteins that could involve intracellular calcium channels proteins. These new findings indicates that mitochondria are a primary source of reactive oxygen species production in the diaphragm during prolonged 100% O2 breathing therapeutic strategies, hence during mechanical ventilation as well. These results could lead to the development of a therapeutic intervention to avoid mechanical ventilation-induced diaphragmatic injury. Perhaps the use of antioxidants, via nasal interventions, should have the potential to help COPD patients to improve the efficacy of their respiratory muscles, hence lowering their conscious awareness of breathlessness, dyspnea.
References
- Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, et al. (2005) Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J RespirCrit Care Med 171: 1116-1124.
- Becker HF, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE (1996) Effect of different levels of hyperoxia on breathing in healthy subjects. J ApplPhysiol (1985) 81: 1683-1690.
- Bégin P, Grassino A (1991) Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis 143: 905-912.
- Cannon JG, St Pierre BA (1998) Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem 179: 159-167.
- Carraway MS, Piantadosi CA (1999) Oxygen toxicity. Respir Care Clin N Am 5: 265-295.
- Cavalcante AG, de Bruin PF (2009) The role of oxidative stress in COPD: current concepts and perspectives. J Bras Pneumol 35: 1227-1237.
- Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G (1997) Muscle weakness is related to utilization of health care resources in COPD patients. EurRespir J 10: 417-423.
- Demchenko IT, Boso AE, Whorton AR, Piantadosi CA (2001) Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions. Brain Res 917: 253-261.
- Engelen MP, Orozco-Levi M, Deutz NE, Barreiro E, Hernández N, et al. (2004) Glutathione and glutamate levels in the diaphragm of patients with chronic obstructive pulmonary disease. EurRespir J 23: 545-551.
- Lenfant C (1966) Arterial-alveolar difference in PCO2 during air and oxygen breathing. J ApplPhysiol 21: 1356-1362.
- Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, et al. (2002) Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J ApplPhysiol (1985) 92: 1205-1213.
- Fridovich I (1998) Oxygen toxicity: a radical explanation. J ExpBiol 201: 1203-1209.
- Haffor ASA, Al-Dokhi OA (2004) Endothelial cell toxicity of cadmium: Transmission Electron Microscopy examination. Pakistan J. of Biological sciences 7: 782-788.
- Haffor ASA, Alttas OS (2010) Effects of Exposure of Rats to Periodic versus Continuous Hyperoxia on Antioxidant Potentials and Free Radical Production in Relation to Ultrastructural Changes in Myocardial Cells. Journal of Inhalation Toxicology 22: 797-804.
- Macgowan NA, Evans KG, Road JD, Reid WD (2001) Diaphragm injury in individuals with airflow obstruction. Am J RespirCrit Care Med 163: 1654-1659.
- Machiels HA, van der Heijden HF, Heunks LM, Dekhuijzen PN (2001) The effect of hypoxia on shortening contractions in rat diaphragm muscle. ActaPhysiolScand 173: 313-321.
- Manning HL, Schwartzstein RM (1995) Pathophysiology of dyspnea. N Engl J Med 333: 1547-1553.
- Levine S, Kaiser L, Leferovich J, Tikunov B (1997) Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 337: 1799-1806.
- Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, et al. (2003) Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J RespirCrit Care Med 168:706-713.
- Marchand E, De LP, Gayan-Ramirez G, Palecek F, Verbeken E, et al. (2002) Effects of lung volume reduction surgery in hamsters with elastase-induced emphysema. EurRespir J 19: 422-428.
- Belda FJ, Aguilera L, García de la Asunción J, Alberti J, Vicente R, et al. (2005) Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA 294: 2035-2042.
- Bin-Jaliah I (2008) Comparison of Glutathione Peroxidase Activity and Free Radicals Production in the Lungs and the Brain of Rats During Graded Hyperoxia. J Med Sci 8: 54-61.
- Bin-Jalia SM, Dallak, Haffor ASA (2009)Effect of Hyperoxia on the Ultrastructural Pathology of Alveolar Epithelium in Relation to Glutathione Peroxidase, Lactate Dehydrogenase Activities, and Free Radical Production in Rats, Rattusnorvigicus. Ultrastructural Pathology 33: 1-11.
- Al-Johany AM, Haffor ASA (2009) Effects of Cadmium Toxicity on Pneumocyte pathological Changes, Leokocyte Count, Activities of Glutathione Peroxidase, Lactate Dehydrogenase in Relation to Free Radicals Production in Uromastixegypyus. Ultrastructural Pathology 33: 1-9.
- Doucet M, Debigaré R, Joanisse DR, Côté C, Leblanc P, et al. (2004) Adaptation of the diaphragm and the vastuslateralis in mild-to-moderate COPD. EurRespir J 24: 971-979.
- Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG (1996) Nutritional status and mortality in chronic obstructive pulmonary disease. Am J RespirCrit Care Med 153: 961-966.
- Haffor AS, Abou-Tarboush FM (2004) Testicular cellular toxicity of cadmium : transmission electron microscopy examination. J Environ Biol 25: 251-258.
- Haffor AS (2010) Effect of Commiphoramolmol on leukocytes proliferation in relation to histological alterations before and during healing from injury. Saudi J BiolSci 17: 139-146.
- Haffor ASA (2004) Effects of O2 Breathing on cardiac mitochondrial, Got, and free radical production. Journal of Medical Sciences 4: 164-169.
- Lambertsen CJ, Dough RH, Cooper DY, EmmelGL,Loeschcke HH, et al. (1953) Oxygen toxicity; effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism. J ApplPhysiol 5: 471-486.
- Mattar EH, Haffor AS (2009) Effect of dobutamine and hyperoxia on free radicals production in relation to the ultrastructural alterations in the endothelial of myocardial capillary in rats, Rattusnorvigicus. UltrastructPathol 33: 209-215.
- MAY P (1957) [Immediate action of oxygen on ventilation in normal man]. HelvPhysiolPharmacolActa 15: 230-240.
- Haffor ASA, Al-Johany AM (2005) Effect of heat stress, hypoxia, hypoxia-hyperoxia on free radical production in mice, MusMusculus. Journal of Medical Sciences 5: 89-94.
- Iben SC, Dreshaj IA, Farver CF, Haxhiu MA, Martin RJ (2000) Role of endogenous nitric oxide in hyperoxia-induced airway hyperreactivity in maturing rats. J ApplPhysiol (1985) 89: 1205-1212.
- Becker H, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE (1995) Ventilatory response to isocapnichyperoxia. J ApplPhysiol (1985) 78: 696-701.
- Comroe J, Dripps R, Dumke P, Deming M (1945) The effect of inhalation of high concentrations of oxygen for 24 hours on normal men at sea level and at a simulated altitude of 18.000 feet. JAMA 128: 710-717.
- Gautier H, Remmers JE, Bartlett D Jr (1973) Control of the duration of expiration. RespirPhysiol 18: 205-221.
- Miller MJ, Tenney SM (1975) Hyperoxic hyperventilation in carotid-deafferented cats. RespirPhysiol 23: 23-30.
- Brown JA, Preul MC, Taha A (1988) Hyperbaric oxygen in the treatment of elevated intracranial pressure after head injury. PediatrNeurosci 14: 286-290.
- Crapo JD (1986) Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 48: 721-731.
- Reid WD, Huang J, Bryson S, Walker DC, Belcastro AN (1994) Diaphragm injury and myofibrillar structure induced by resistive loading. J ApplPhysiol (1985) 76: 176-184.
- Ribera F, N'Guessan B, Zoll J, Fortin D, Serrurier B, et al. (2003) Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J RespirCrit Care Med 167: 873-879.
- Scott A, Wang X, Road JD, Reid WD (2006) Increased injury and intramuscular collagen of the diaphragm in COPD: autopsy observations. EurRespir J 27: 51-59.
- Kapanci Y, Tosco R, Eggermann J, Gould VE (1972) Oxygen pneumonitis in man., Light- and electron-microscopic morphometric studies. Chest 62: 162-169.
- Oliven A, Supinski GS, Kelsen SG (1986) Functional adaptation of diaphragm to chronic hyperinflation in emphysematous hamsters. J ApplPhysiol (1985) 60: 225-231.
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, et al. (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333: 817-822.
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, et al. (2000) Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 320: 1297-1303.
- Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, et al. (1995) Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J ThoracCardiovascSurg 109: 106-116.
- Covey MK, Larson JL, Wirtz SE, Berry JK, Pogue NJ, et al. (2001) High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. J CardiopulmRehabil 21: 231-240.
- Gautier H, Bonora M, Gaudy JH (1986) Ventilatory response of the conscious or anesthetized cat to oxygen breathing. RespirPhysiol 65: 181-196.
- Moore AJ, Stubbings A, Swallow EB, Dusmet M, Goldstraw P, et al. (2006) Passive properties of the diaphragm in COPD. J ApplPhysiol (1985) 101: 1400-1405.
- DRIPPS RD, COMROE JH Jr (1947) The effect of the inhalation of high and low oxygen concentrations on respiration, pulse rate, ballistocardiogram and arterial oxygen saturation (oximeter) of normal individuals. Am J Physiol 149: 277-291.
- Fridovich I (1978) The biology of oxygen radicals. Science 201: 875-880.
- Newell SZ, McKenzie DK, Gandevia SC (1989) Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax 44: 903-912.
- Nguyen T, Rubinstein NA, Vijayasarathy C, Rome LC, Kaiser LR, et al. (2005) Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm. J ApplPhysiol (1985) 98: 2004-2010.
- Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, et al. (2001) Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J RespirCrit Care Med 164: 1734-1739.
- Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, et al. (2006) Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J RespirCrit Care Med 173: 527-534.
- Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, et al. (2005) Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J RespirCrit Care Med 172: 200-205.
- Poole DC, Lieber RL, Mathieu-Costello O (1994) Myosin and actin filament lengths in diaphragms from emphysematous hamsters. J ApplPhysiol (1985) 76: 1220-1225.
- Reid WD, Belcastro AN (1999) Chronic resistive loading induces diaphragm injury and ventilatory failure in the hamster. RespirPhysiol 118: 203-218.
- Reid WD, Belcastro AN (2000) Time course of diaphragm injury and calpain activity during resistive loading. Am J RespirCrit Care Med 162: 1801-1806.
- Similowski T Yan S, Gauthier AP, Macklem PT, Bellemare F (1991) Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 325: 917-923.
- Dal Vecchio L, Polese G, Poggi R, Rossi A (1990) "Intrinsic" positive end-expiratory pressure in stable patients with chronic obstructive pulmonary disease. EurRespir J 3: 74-80.
- Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, et al. (1998) Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol 274: L527-534.
- Scott A, Wang X, Road JD, Reid WD (2006) Increased injury and intramuscular collagen of the diaphragm in COPD: autopsy observations. EurRespir J 27: 51-59.
- Similowski T Yan S, Gauthier AP, Macklem PT, Bellemare F (1991) Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 325: 917-923.
- Barry BE, Crapo JD (1985) Patterns of accumulation of platelets and neutrophils in rat lungs during exposure to 100% and 85% oxygen. Am Rev Respir Dis 132: 548-555.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15316
- [From(publication date):
October-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10701
- PDF downloads : 4615