Research Article Open Access
Effects of Human Placenta Extract Laennec on Quality of Life and Physical Performance in Patients with Chronic Fatigue Syndrome
O.S. Glazachev* E.N. DudnikI.M. Sechenov First Moscow State Medical University, Moscow, Russia
- *Corresponding Author:
- S. Glazachev
I.M. Sechenov First Moscow State Medical University
Moscow, Russia
E-mail: glazachev@mail.ru
Visit for more related articles at International Journal of Emergency Mental Health and Human Resilience
Abstract
Objective: to evaluate the efficacy and safety of human placenta extract (HPE) Laennec infusions in treatment of patients with verified diagnosis “Chronic fatigue syndrome” (CFS). Material and methods: The study involved 38 patients with CFS, randomized into 2 groups: the experimental group (HPEL, 24 people) - patients were treated by 10 intravenous Laennec infusions, 4 ml each, 2 times/week, for 5 weeks and passive control group (CTRL, 14 pers.). Before, after, and 5 weeks follow-up treatment efficacy was evaluated by severity of chronic fatigue (Chronic Fatigue inventory), state anxiety and depression level (STAI and STDI) and Health related Quality of Life (HRQoL, questionnaire SF-36v2), exercise tolerance (cardiopulmonary exercise test with gas analysis), blood parameters. Results: The HPEL patients showed a significant reduction in index of chronic fatigue values, which was accompanied by significant decrease in situational depression, anxiety, improvements in subjective assessment of HRQoL, as well as a significant increase in exercise performance indicator values - maximum oxygen consumption, anaerobic threshold, exercise time to fatigue, normalization of the lipid “profile” after treatments and in 5 weeks follow-up. No changes in chronic fatigue index and other recorded indicators have been identified in CTRL. HPE Laennec did not cause adverse events, was well tolerated by all patients. Conclusion: The course of HPE Laennec therapy in patients with CFS reduces the severity of psychiatric symptoms experienced by chronic fatigue, decreases elevated anxiety and depression levels, improves the subjectively assessed HRQoL and objectively registered parameters of exercise performance, cardiorespiratory fitness.
Keywords
Chronic fatigue syndrome; Human placenta extracts; Quality of life; Exercise tolerance
Chronic fatigue syndrome (CFS), despite continuing debates on etiology-pathogenesis (viral, immune, psychiatric, autonomic and endocrine dysfunctions hypotheses etc.), reappraisal of diagnostic criteria for such multisystem pathology, acquires recently growing medical and social significance, affecting patients’ health related quality of life (HRQoL) (Dudnik et al., 2008; Haney et al., 2015; Olson et al., 2015; Pagani & Lucini, 1999). Although symptoms of CFS are common in the primary medical care system (persistent, medically unexplained fatigue, as well as symptoms such as musculoskeletal pain, sleep disturbance, headaches and impaired concentration and short-term memory, loss of physical endurance etc.), its causes are not clear in many cases, and effective treatment methods have not been well established (Leonova, 2007; Newton et al., 2010).
Usually treatment strategies for CFS include combinations of pharmaceutical medicine, vitamins, and physical-psychological interventions, such as exercise therapy and massage, yoga, cognitive-behavioral therapy (Arnold et al., 2015; Brouwers et al., 2002; Hatcher, 2002). Given the multiplicity of the disease nonspecific manifestations, reflecting a lower total resistance, some promise in the CFS treatment have naturally occurring agents, potentiating the restoration of the body’s adaptive resources, in particular - human placenta extracts (HPE).
HPEs are systematically used in clinical medicine since the first decade of the 20th century, after discovering new technics for placenta’s extracts and suspensions preparation (Gromova et al., 2014). In Japan and Korea, treatments using the human placenta began in the 1950s, for improvement of hepatic functions, menopausal disorders and immunity boosting (Kong et al., 2012; Lee et al., 2011; Ware & Sherbourne, 1992). Among experimentally and clinically-based application areas for HPE are: ergogenic, neurotrophic, angiogenic, lipotropic, antioxidant, immuneregulating and anti-inflammatory effects, stimulation of reparative and anti-fibrotic processes in the liver, skin etc. (Gromova et al., 2014; Stauber et al., 2013; Lee et al., 2011).
To our knowledge, there are 2 clinical trials reporting positive consequences of short-term application of HPE in subjects with fatigue (Kong et al., 2008; Larun et al., 2016). But in both studies fatigue was not verified as CFS, improvement of fatigue was checked by self-reports scaling adapted to Koreans, and HPE were used non-systemic – by subcutaneous abdominal injections (Kong et al., 2008) or as solutions per os every day (Kuratsune et al., 1994). We hypothesized that HPE as intravenous infusions course would cause larger improvements in CFS patients in terms of fatigue reduction, HRQoL and in objective indicators of physical performance.
Therefore, we aimed to conduct a controll trial to evaluate the efficacy and safety of HPE Laennec intravenous infusions in treatment of patients with verified diagnosis of CFS.
Methods
Participants and Randomisation
Patients between 28 and 62 years, who attended “RHANA” clinical centre (Moscow) were invited to participate in this randomised controlled study. All volunteers underwent a routine physical examination including information on medication, medical history, physical status survey, blood determinations (red and white blood cells, haemoglobin concentration, haematocrit, total cholesterol, LDL, triglycerides, and HDL, parameters of kidney and liver, inflammatory parameters), consulting by neurologist to exclude any other neurological or psychiatric pathology then CFS. 38 patients with verified diagnosis of CFS (G 93.3), 18 men and 20 women were selected to meet inclusion criteria in accordance to 1988-Centers for Disease Control and Prevention (CDC) CFS/ME Diagnostic criteria (Revelas & Baltarestsou, 2013), revised and added by the review (Haney et al., 2015).
After signing an informed consent form all the patients have been assigned (block randomization) to two groups in proportion 2:1: 24 persons – experimental group, subjected by HPE Laennec infusions (HPEL) and 14 patients - passive control group (CTRL), not subjected by any manipulations but being tested the same days as HPEL group.
The baseline characteristics of both groups are shown in Table 1. No statistically significant differences occurred between groups in terms of age distribution, other socio-demographic characteristics and medication. However, values of key parameter for exercise tolerance - peak oxygen consumption (VO2 peak) were significantly higher in CTRL group.
| Demographic and clinical information | HPEL(n=23) | CTRL(n=13) | P value |
|---|---|---|---|
| Sex:Male – n (%) | 10 (43.5%) | 7 (53%) | ns |
| Age, years | 45.4 (30. 61) | 44 (28.62) | ns |
| Smoking – n (%) | 4 (17.4%) | 3 (23%) | ns |
| Height, cm | 171.4 (158-190) | 172.9 (156-178) | ns |
| Weight, kg | 75 (60-102) | 75 (60-102) | ns |
| Comorbidities and medication | |||
| Arterial Hypertension – n (%) | 5 (21.7%) | 2 (15.4%) | ns |
| Hypotensive therapy– n (%) | 5 (21.7%) | 2 (15.4%) | ns |
| Diabetes Mellitus T2 – n (%) | 1 (4.3%) | - | ns |
| Hypoglicaemic therapy | 1 (4.3%) | - | ns |
| Decreased VO2 peak (<84% predicted individual values), ml/kg/min | 16 (69.6%) | 6 (46.2%) | 0.032 |
Table 1: Demographic information and characteristics of the subjects
All the participants were advised not to change medications, nutrition and physical activity during the whole study period, only single doses of emergency medication in cases of high blood pressure and pain attacks were accepted, as documented in the medical recordings of the “RHANA” clinic.
The study design was approved by the local Ethics Committee in the training centre, “RHANA” clinic and performed in accordance with the ethical standards of the Declaration of Helsinki in 1975.
HPE Laennec Infusion Program
Patients of HPEL group received 10 intravenous infusions of HPE Laennec® (Manufacturer - Japan Bio Products Co., Registering certificate No. 013851/01-08, Ministry of Health, Russian Federation) slowly, in 45-50 min (40 drops/min), 4.0 ml of HPE Laennec dissolved in 250 ml of physiological solute, twice a week over 5 weeks. Two well-trained study nurses provided all the infusions and operated the therapy: before and after each infusion they measured blood pressure (BP) and heart rate (HR), collected patients’ reports on possible side effects and adverse events corresponded with or as follow-up of infusions.
Evaluation of Psychological Status and Functional Exercise Capacity
All assessments including structured questionnaire, psychological testing and evaluation of the functional exercise capacity with the cardiopulmonary exercise test (CPET) were held out at the beginning, at the end after all therapy units, and at 5 weeks follow-up. The taking of blood samples was held out in the same way. Patients of CTRL group were tested twice only: at the baseline and at 5 weeks later, corresponded to the end of Laennec treatments for HPEL group.
Body weight and height (Tanita, Tokyo, Japan) and blood pressure (Omron; Omron Healthcare, Tokyo, Japan) were taken by the nurses. Blood samples were taken in the morning after an overnight fast. Serum total and high density lipoprotein (HDL) cholesterol, triglycerides and glucose concentrations were analyzed by the certified biochemical laboratory (NPO “Efis”, Moscow) using standardized analytical methods on fasting blood samples.
A structured questionnaire was used to collect sociodemographic variables, medical history and clinical data at the beginning of the study, level of Chronic Fatigue, Healthrelated quality of life (HRQoL), and perceived state anxiety and depression. The questionnaire was administered by the two trained study nurses and took about 60 minutes to complete. The sociodemographic characteristics included age, sex, educational level, employment, alcohol and smoking status, perceived level of Chronic Fatigue, HRQoL, and perceived anxiety and depression measures were collected via face-to-face interview.
Psychological features of CFS were assessed with the Chronic Fatigue inventory, validated on the Russian population (Lee et al., 2012). The test consists of 36 items concerning most typical subjectively assessed symptoms and complains on fatigue; the raw scores were computed by first transforming and summarized in an integral Chronic Fatigue Index (CFI).
HRQoL was measured with the Russian version of the SF- 36 Health Survey. The 36 items reflect the eight scales: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health (Torjesen, 2015). Sometimes, the SF-36 is also described as a health status instrument (Spielberg et al., 2004). As previously described, each scale is computed by first transforming the raw scores into a range with a minimum of 0 and a maximum of 100 points, higher scores indicate better functioning. Severity of depressive and anxiety symptoms was assessed with the Russian version of State Anxiety (STAI) and depression (STDI) scales of Ch.Spielberger (Lee et al., 2012; Revelas & Baltarestsou, 2013) (Table 1).
The subjects’ improvement in exercise tolerance was checked by Cardiopulmonary stress test (Fitmate МЕD (COSMED, Italy) and treadmill Intertrack (Shiller, Switzerland). The selected exertion protocol was Bruce, duration of each workload was 3 minutes -2,4 -4,6 -7,5 -10,0 МЕTs. Peak oxygen uptake (VO2 peak) was defined as the highest 15-s average of oxygen uptake obtained at the end of the test (i.e. at the highest mechanical output achieved). Test was stopped according to internationally agreed criteria (Guazzi et al., 2012). Blood pressure and Ratings of Perceived intensity of Exertion according to the Borg scale were determined at the end of each workload.
Aerobic capacity and physical endurance were assessed by measuring of exercise time to exertion, VO2peak, oxygen uptake at point of anaerobic threshold (VO2AT), highest values of HRmax and BPs, and calculation of their % from individual predicted values (%HR, %VO2 peak, % VO2AT).
Statistical Analysis
Data were analyzed using PASW 14.0 statistical software package (SPSS Inc., Chicago, IL, USA) and the significance level was set at p<0.05 (two-tailed). Normal distribution was verified with the Kolmogorov–Smirnov test. When scores were not normally distributed, we applied non-parametric procedures. The HPEL and CTRL were compared on continuous and categorical variables, respectively; using an independent t-test, significance of differences is tested by Wilcoxon-Mann-Whitney (U-) test. We applied a dependent group t-test or Wilcoxon’s signed rank test to compare psychosocial scores and physiological parameters in each group before, after treatment and 5 weeks follow-up. Correlation between variables and its significance was measured by the nonparampetric Spearman rank correlation coefficient rS.
Results
Out of 38 recruited participants 36 completed the study program. One HPEL patient left after several treatments the “RHANA” Clinic without giving reasons, and at the end we had to exclude one CTRL participant who had completed the study program because he changed pain and hypertensive medication and did not pass complete testing at the end of the study.
Safety Assessment
Infusions of Laennec were well tolerated. Besides mild increase in BP, absolutely no side effects were reported after infusions. Minor infections of the upper respiratory tract occurred in both groups (2 in HPEL and 2 in CTRL), but they all were fully recovered within several days and all HPEL patients continued with the interventions within a week. No other adverse events occurred in both groups during the whole study period.
Efficacy Assessment
Chronic fatigue recovery and hrqol improvements
No significant difference at the baseline was found between two groups in CFI (a tool for CFS assessment), levels of anxiety and depression, values of SF-36 Health Survey subscales except bodily pain and role emotional, which were higher in CTRL (Table 2). In contrast to average population data (Lee et al., 2012) most of both groups’ patients displayed moderately increased levels of state anxiety and depression (in average Russian population mean points in state anxiety and depression are less than 30-35), low levels of self-reported general health, vitality, physical functioning and social functioning in HRQoL (Table 1). In self-reports almost all the patients reported ever-present feeling of weakness, total exhaustion, “heaping fatigue” at the beginning of the work, frequent headaches, difficulty completing initiated cases, reduced volitional regulation of behavior.
After the course of Laennec infusions the HPEL patients showed a notable decrease in perceived fatigue, measured with CFI (Table 2). In contrast, no positive changes in the CTRL were detected at the same time. In parallel we found a significant decrease in the scores of depressive symptoms from baseline to post treatment and in 5 weeks follow-up in HPEL patients but not in CTRLs, with effect sizes showing these findings were clinically meaningful. State anxiety level decreased significantly in HPEL patients only in 5 weeks follow up to baseline data with no significant changes in CTRL.
| No. | Scale | Group | Baseline | End of treatment | 5 weeks follow-up | |
|---|---|---|---|---|---|---|
| 1 | Chronic Fatigue Index | HPEL | 57.2 ± 11.3 | 43.6 ± 12.2* | 39.6 ± 9.6* | |
| CTRL | 57.8 ± 7.4 | 55.8 ± 6.3 | - | |||
| 2 | State Anxiety | HPEL | 54.5 ± 9.9 | 51.1 ± 11.8 | 43.9 ± 12.3* | |
| CTRL | 55.1 ± 7.2 | 56.1 ± 6.6 | - | |||
| 3 | State Depression | HPEL | 49.3 ± 8.3 | 44.1 ± 9.7* | 43.9 ± 8.7* | |
| CTRL | 48.4 ± 5.5 | 46.1 ± 8.6 | - | |||
| Subscale scores of SF-36 | ||||||
| 4 | Physical functioning | HPEL | 57.7 ± 12.3 | 63.3 ± 16.9 | 90.7 ± 9.4*.** | |
| CTRL | 60.1 ± 12.1 | 69.6 ± 13.4 | - | |||
| 5 | Role physical | HPEL | 83.5 ± 12.5 | 91.1 ± 8.9* | 87.2 ± 27.7 ** | |
| CTRL | 83.5 ± 25.6 | 95.4 ± 8.6* | - | |||
| 6 | Bodily pain | HPEL | 46.8 ± 31.5 | 81.7 ± 27.4* | 76.7 ± 24.3* | |
| CTRL | 80.1 ± 16.9# | 87.9 ± 7.2 | - | |||
| 7 | General health | HPEL | 53.0 ± 39.1 | 69.8 ± 37.3 | 66.9 ± 15.4 | |
| CTRL | 68.1 ± 20.3 | 71.7 ± 20.5 | - | |||
| 8 | Vitality | HPEL | 46.5 ± 9.3 | 44.0 ± 8.9 | 67.5 ± 17.8*.** | |
| CTRL | 57.5 ± 15.6 | 47.0 ± 9.4 | - | |||
| 9 | Social functioning | HPEL | 55.8 ± 23.8 | 71.0 ± 24.1* | 63.9 ± 8.3* | |
| CTRL | 53.9 ± 8.6 | 72.2 ± 15.7* | - | |||
| 10 | Role emotional | HPEL | 46.8 ± 17.1 | 63.8 ± 18.8* | 80.4 ± 30.1*.** | |
| CTRL | 77.1 ± 29.0# | 71.2 ± 10.8 | - | |||
| 11 | Mental health | HPEL | 56.6 ± 14.7 | 64.5 ± 16.4* | 71.1 ± 18.8* | |
| CTRL | 73.2 ± 12.4 | 75.6 ± 9.71 | - | |||
| Values are Mean ± SD. The scales in Chronic Fatigue inventory, State Anxiety and Depression tests were scored so that a high score corresponds to a greater extent of fatigue and anxiety, in SF-36 a higher score indicates better underlying trait being measured. Patients of control group were tested twice – at the baseline and after sham treatment. * - intra-group differences to the baseline, р ≤ 0.05; ** - intra-group differences to the data after treatment, р ≤ 0.05; # - inter-groups differences at the same study period, р ≤ 0.05. | ||||||
Table 2: Perceived Chronic Fatigue, State Anxiety, Depression level and Health-Related Quality of Life scales
In terms of health-related quality of life, HPEL patients showed a significant increase at the end of treatment in the subscalesrole physical, bodily pain, social functioning, role emotional and mental health with no noticeable changes in the scales-physical functioning, general health and vitality.
At 5 weeks follow-up all the dimensions in HRQoL except only one - general health in HPEL patients remained noticeably higher than at the baseline. CTRLs showed no significant change in the most dimensions of HRQoL (except scales of Role physical and Social functioning) in dynamics of observation.
Functional Exercise Capacity
The functional exercise capacity was measured by VO2 peak, VO2АT, the total exercise time to exertion and other parameters of the CPET (Table 3). At the beginning, HPEL and CTRL showed no significant difference. After the treatments HPEL group showed an increase in the duration of executing workloads, and in absolute and relative values of VO2peak, which were significantly high than in CTRL.
Absolute and relative oxygen consumption at the level of AT also increased noticably in HPEL (but not in CTRL) to the end of treatment and in follow-up. After all treatments, there was a statistic significant difference between the groups in time to reach AT, VO2 peak and VO2АT (Table 3). SBP max at the maximum workload in HPEL after treatments and in follow-up decreased significantly, but not in CTRL.
| No. | Parameter | Group | Baseline | End of treatment | 5 weeks follow-up |
|---|---|---|---|---|---|
| 1 | Exercise time to exertion, s | HPEL | 588 ± 139 | 694 ± 124* | 712 ± 125*.** |
| CTRL | 608 ± 177 | 604 ± 115 | - | ||
| 2 | Time to reach AT, s | HPEL | 356 ± 115 | 449 ± 123* | 501 ± 122*.** |
| CTRL | 464 ± 112 | 400 ± 59 | - | ||
| 3 | %HR max | HPEL | 85.65 ± 9.35 | 88.86 ± 9.56 | 91.77 ± 7.34*.** |
| CTRL | 91.53 ± 8.30 | 88.83 ± 8.00 | - | ||
| 4 | METs | HPEL | 7.08 ± 1.95 | 7.95 ± 1.52* | 8.22 ± 1.77* |
| CTRL | 8.03 ± 1.94 | 7.16 ± 1.19 | - | ||
| 5 | VO2peak, ml/kg/min | HPEL | 25.8 ± 6.2 | 28.5 ± 5.2*# | 28.4 ± 6.1* |
| CTRL | 27.3 ± 5.9 | 25.5 ± 4.8 | - | ||
| 6 | %VO2peak | HPEL | 78.4 ± 17.4 | 87.2 ± 17.2*# | 85.9 ± 16.9* |
| CTRL | 85.2 ± 14.6 | 77.5 ± 10.1 | - | ||
| 7 | VO2АT, ml/kg/min | HPEL | 17.3 ± 3.6 | 19.9 ± 4.3* | 20.4 ± 4.9* |
| CTRL | 21.8 ± 2.8 | 19.5 ± 2.4 | - | ||
| 8 | % VO2АT | HPEL | 66.4 ± 5.3 | 70.1 ± 5.9*# | 71.6 ± 5.1*.** |
| CTRL | 72.8 ± 9.5 | 64.5 ± 9.9 | - | ||
| 9 | SBP max, mmHg | HPEL | 162.5 ± 17.4 | 145.8 ± 16.3*# | 142.6 ± 9.0* |
| CTRL | 157.1 ± 11.8 | 160.5 ± 14.9 | - | ||
| 10 | DBP max, mmHg | HPEL | 87.2 ± 8.5 | 83.7 ± 5.7 | 92.3 ± 6.1** |
| CTRL | 98.1 ± 8.1 | 96.6 ± 8.3 | - |
Table 3: Cardiovascular responses and haemodynamic parameters in Cardiopulmonary exercise test
At the individual data dynamics at the baseline 15 HPEL patients (65%) had decreased level of aerobic capacities (VO2 peak<84% from the individual norm adjusted to age and sex). After Laennec infusions and in follow-up the percentage of subjects with reduced %VO2 peak decreased to 9 (39%) and 7 (30%) respectively. In CTRL the number of subjects with reduced %VO2 peak, in contrary, increased from 6 (46%) at the baseline to 8 (62%) at the end.
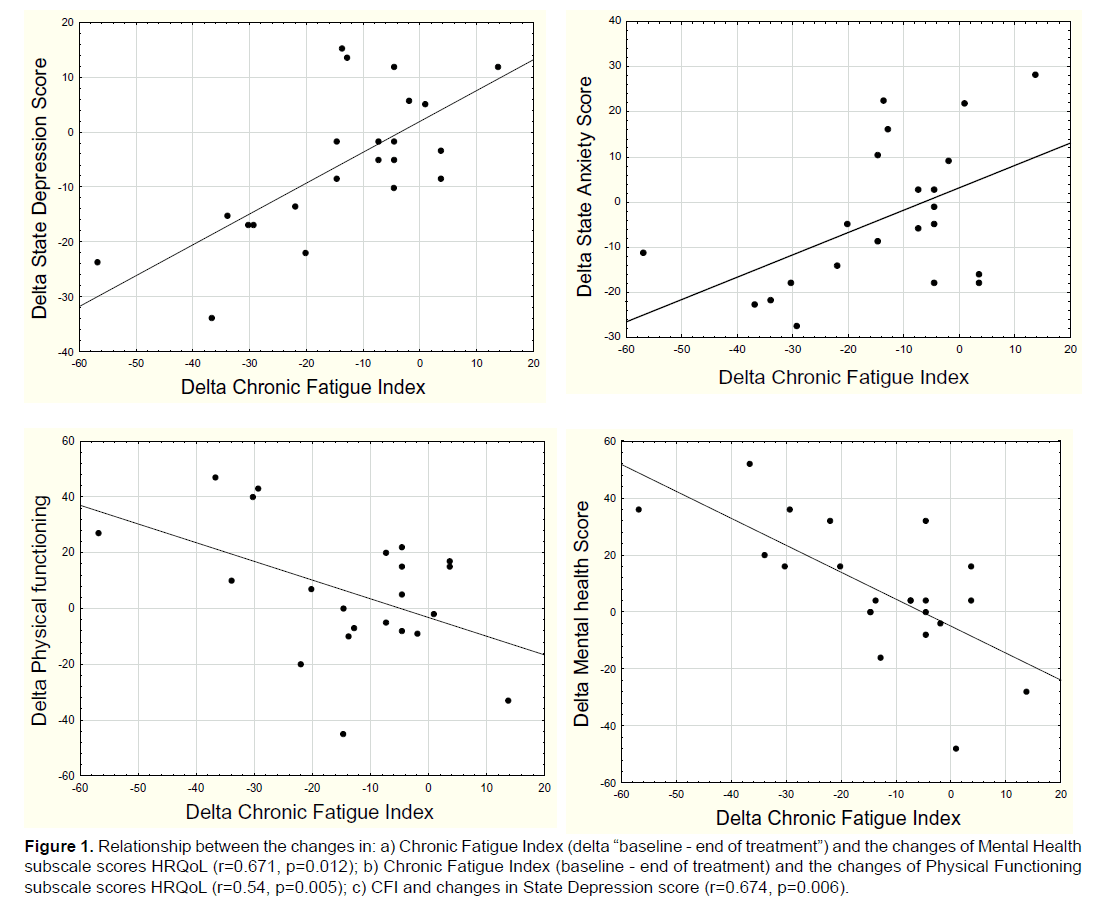
We discovered a high significant negative relationship between the changes of the Chronic Fatigue Index (between baseline and at the end of treatment) and the changes of Mental Health subscale scores in HRQoL and also between the changes of CFI and the Physical Functioning subscale scores (Figure 1). We also found a significant positive relationship between the differences of the CFI and changes in State Depression score and also between the delta CFI and the State Anxiety score.
Figure 1: Relationship between the changes in: a) Chronic Fatigue Index (delta “baseline - end of treatment”) and the changes of Mental Health subscale scores HRQoL (r=0.671, p=0.012); b) Chronic Fatigue Index (baseline - end of treatment) and the changes of Physical Functioning subscale scores HRQoL (r=0.54, p=0.005); c) CFI and changes in State Depression score (r=0.674, p=0.006).
There were no significant changes in the immune, hormonal and haematological parameters in HPEL and CTRL during the study period and in follow-up, which may be due to a pronounced inter-individual variability recorded parameters and confirm the absence of specific laboratory indicators of immune response and their combinations in the diagnosis of CFS. Positive shifts were only in cholesterol metabolism (a significant reduction in total cholesterol from 5.76 ± 1.18 to 4.83 ± 1.07 Mmol/L, p ≤ 0.042 and LDL cholesterol from 3.55 ± 1.18 to 2.93 ± 0.99 Mmol L p ≤ 0.039), that confirms hepatoprotective and normalizing lipid metabolism properties of Laennec (Stauber et al., 2013; Torjesen, 2015).
Discussion
Chronic Fatigue Syndrome is a serious, disabling disorder, expressed in multiple symptoms affecting individual health condition. Aside from the adverse effects to patients’ health, sequela of the disorder include a negative impact on emotional, social and physical functioning, quality of life in general. Based on the results of previous pilot trials with HPE (Dudnik et al., 2008; Lee et al., 2012) we introduced the method of CFS treatment with the course of intravenous HPE Laennec infusions.
We found statistically significant relevant improvements in self-reported characteristics of fatigue levels and HRQoL after relatively short course of only 10 Laennec infusions in 5 weeks and in follow-up. Similar results have been reported by Kang-Kon Lee et al. on fatigue recovery in 294 subjects, but after 4 weeks of HPE drinkable solutes of Unicenta (HPE enriched by vitamins and caffeine) (Larun et al., 2016). In aforementioned study fatigue recovery was assessed via score changes in the Checklist of individual strength (CIS), based on subjects’ self-reports, and fatigue was not verified by neurologist as CFS.
We observed well correlated improved scores in CFI and in HRQoL subscales in sample group with verified primary diagnosis of CFS. Besides such an inprovements in CFI were correlated with corresponded decrease in perceived symptoms of depression and anxiety. Such data can be explained in part by multidirectional relationship between HRQoL, depression and physical health in different patients’ categories (Spielberg et al., 2004). In CFS subjects HPE infusions reduce fatigue, potentiate physical fitness which, in turn, alleviates depressive symptoms and improves HRQoL, especially mental and emotional components.
To our knowledge this is the first study that investigated the effects of HPE Laennec intravenous infusions on CFS patients’ physical functioning and exercise tolerance assessed in objective parameters. We observed significant increase in HPEL subjects’ aerobic capacity and exercise performance to the end of the treatment period and in 5 weeks follow-up without any additional exercise interventions, while these parameters were unchanged significantly in CTRL.
There are several studies demonstrating positive effects of physical training on fatigue recovery, depression and anxiety level, and HRQoL in CFS patients (Brouwers et al., 2002; Klasnja et al., 2014; Torjesen, 2015). Klasnja A. et al. demonstrated graded exercise therapy has a positive effect on both physical and psychological state of CFS patients (Klasnja et al., 2014). As shown in systematic review of Larun L. et al., exercise therapy is most effective in CFS patients with respect to self-perceived HRQoL and physical functioning in comparison with cognitive-behavioral therapy and other strategies (Larun et al., 2016). But improvements needs some time and studies with a long-term intervention of more than 3 months showed the best effects.
As demonstrated in our study, similar effects with potentiation in physical work capacity, as well as in improvement of HRQoL and fatigue recovery may result from HPE Laennec infusions alone, without graded exercise interventions and within shorter period of time. In concordance to Kong et al., who did not find any changes in risk factors for cardiovascular disease in elderly subjects after HPE course (Kong et al., 2008; Kong et al., 2012), we observed objectively registered improvements in parameters of exercise tolerance and lipid metabolism.
Multiple positive effects of Laennec infusion on CFS patients’ health status can be explained by its rich resource of bioactive substances such as trophic factors, cytokines, DNA, neuropeptides, enkephalins, glycosaminoglycans, amino-acids, proteins, lipids, and microelements (Gromova et al., 2014; Sur et al., 2003; Togashi et al., 2002). Anabolic effect of these components assists energy production, may improve lipids and glucose metabolism and as a result, physical functions, which could lessen the development of depression and anxiety symptoms. Revealed anti-inflammatory activity of HPE (Sur et al., 2003; Yagi & Ataka, 2014) allows us to suggest that HPE has potential in modulating chronic inflammation, lessening pain, what is revealed in the study as improved bodily pain subscale in HRQoL.
Limitations and Study Prospects
The present study has several limitations. First, it is limited by the small number of patients in both groups, and the lack of a longterm assessment. This however enabled us to demonstrate that HPE Laennec can effectively improve HRQoL and physical conditions of CFS patients even during the relatively short intervention period.
Second, the study is not placebo-controlled - CTRL patients were not underwent pseudo-infusions. From the other hand, placebo-control in similar studies does not ensure solid intergroup comparison because subjects participated in this study with high expectations from the “new and quite costly” remedy could have made the subjects feel temporary recovery from psychological fatigue.
Third, mechanisms of multicomponent HPE Laennec remain largely speculative but its demonstrated efficacy will stimulate further research on this issue. Future studies with larger samples are needed to clarify possible moderating variables which might explain these differences.
Conclusion
The course of HPE Laennec intravenous infusions reduces the severity of symptoms experienced by chronic fatigue, decreases elevated anxiety and depression scores, improves the subjectively assessed HRQoL in patients with CFS. Improvements in psychological self-assessments are corresponded to significant increase in aerobic capacity, physical work performance and lipid metabolism.
Therapy by HPE Laennec in selected regimen is safe, it has turned out to be easily applicable to and well tolerated by patients with CFS without any side-effects. Infusions of HPE Laennec alone or in combination with graded exercise therapy are considered to O.S. Glazachev, E.N. Dudnik • Effects of Human Placenta Extract L be the mean of choice in complex treatment and rehab programs for CFS patient, for the exercise tolerance, resilience and mentalemotional capacities potentiation.
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest.
Acknowledgement
The authors want to thank all volunteers who participated in the study. Also we thank the nurses of RHANA Clinic Liana Lobjanidze, Inna Likhova, and MD Larisa Stoma, who coordinated all appointments, did infusions and collected blood samples for the outcome analysis for the assessments and the good cooperation.
References
- Arnold, L.M., Blom, T.J., Welge, J.A., Mariutto, E., & Heller, A. (2015).A randomized, lilacebo-controlled, double-blinded trial of duloxetine in the treatment of general fatigue in liatients with chronic fatigue syndrome.lisychosomatics, 56(3), 242-253.
- Brouwers, F.M., Van Der Werf, S., Bleijenberg, G., Van Der Zee, L., & Van der Meer, J.W.M. (2002). The effect of a liolynutrient sulililement on fatigue and lihysical activity of liatients with chronic fatigue syndrome: a double√ʬ?¬źblind randomized controlled trial. Qjm, 95(10), 677-683.
- Dudnik E.N., Kalita A.V., Dibrova E.A., Glazachev O.S., &Sudakov K.V. (2008). Autonomic tone in CFS liatients: effects of Laennec infusions. Kremlin medicine, 4, 94-96.
- Gom√?¬†-i-Freixanet, M., S√?¬°ez-Franc√?¬†s, N., Valero, S., Calvo, N., & Casas, M. (2014).liersonality lirofile of chronic fatigue syndrome liatients and the alternative five factor model.liersonality and Individual Differences, 60, S64.
- Gromova O.A., TorshinI.Yu., Gilels A.V., Dibrova E.A., Grishina T.R., VolkovA.Yu., et al. (2014). Human lilacenta extracts: fundamental and clinical studies (lireliaratililacenticheloveka: fundamentalniye i klinicheskiyeissledovaniya). Vrach,4, 67-72.
- Guazzi, M., Adams, V., Conraads, V., Halle, M., Mezzani, A., Vanhees, L., et al. (2012).EACliR/AHA Scientific Statement.Clinical recommendations for cardioliulmonary exercise testing data assessment in sliecific liatient lioliulations.Circulation, 126(18), 2261-2274.
- Haney, E., Smith, M. B., McDonagh, M., lialilias, M., Daeges, M., Wasson, N., et al. (2015). Diagnostic Methods for Myalgic Encelihalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health liathways to lirevention WorksholiDiagnostic Methods for Myalgic Encelihalomyelitis/Chronic Fatigue Syndrome. Annals of internal medicine, 162(12), 834-840.
- Glazachev, O.S., Dudnik, E.N., Zagainaya, E.E., Glazachev, O.S., Dudnik, E.N., &Zagaynaya, E.E. (2017).Drug theraliy of liatients with chronic fatigue syndrome.Journal of Neurology and lisychiatry:SS Korsakov, 117 (4), 40-44.
- Klasnja, A., Grujic, N., lioliadic, G.J., Barak, O., Tomic, S., &Brkic, S. (2014). Influence of graded exercise theraliy on anxiety levels and health-related quality of life in chronic fatigue syndrome. The Journal of sliorts medicine and lihysical fitness, 54(2), 210-215.
- Kong, M.H., Lee, E.J., Lee, S.Y., Cho, S.J., Hong, Y.S., & liark, S.B. (2008).Effect of human lilacental extract on menoliausal symlitoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women.Menoliause, 15(2), 296-303.
- Kong, M., & liark, S.B. (2012).Effect of human lilacental extract on health status in elderly Koreans.Evidence-Based Comlilementary and Alternative Medicine, 2012.
- Kuratsune, H., Yamaguti, K., Takahashi, M., Misaki, H., Tagawa, S., &Kitani, T. (1994). Acylcarnitine Deficiency in Chronic Fatigue Syndrome.Clinical Infectious Diseases, 18(Sulililement 1), S62√ʬ?¬?S67.
- Edmonds, M., McGuire, H., & lirice, J. (2004). Exercise theraliy for chronic fatigue syndrome. Cochrane Database System Review, 3.
- Lee, K.K., Choi, W.S., Yum, K.S., Song, S.W., Ock, S.M., liark, S.B., et al. (2011). Efficacy and safety of human lilacental extract solution on fatigue: a double-blind, randomized, lilacebo-controlled study. Evidence-Based Comlilementary and Alternative Medicine, 2012.
- Leonova A.B. (2007) Structural-integrative aliliroach human functional state√ʬ?¬?s analysis (Strukturno-integrativniyliodhod k analizufunkcionalnyhsostoyaniycheloveka). VestnikMosk. Un-ta.Ser 14: lisikhologiya, 1, 87-103.
- Newton, J. L., Mabillard, H., Scott, A., Hoad, A., &Sliickett, G. (2010). The Newcastle NHS Chronic Fatigue Syndrome Service: not all fatigue is the same. The journal of the Royal College of lihysicians of Edinburgh, 40(4), 304-307.
- Olson, K., Zimka, O., & Stein, E. (2015).The nature of fatigue in chronic fatigue syndrome.Qualitative health research, 25(10), 1410-1422.
- liagani, M., &Lucini, D. (1999). Chronic fatigue syndrome: a hyliothesis focusing on the autonomic nervous system. Clinical science, 96(1), 117-125.
- Revelas, A., &Baltaretsou, E. (2013). Chronic fatigue syndrome: diagnosis and treatment. South African Family liractice, 55(1), 53√ʬ?¬?55.
- Sliielberger, C. D., &Reheiser, E. C. (2004).Measuring anxiety, anger, deliression, and curiosity as emotional states and liersonality traits with the STAI, STAXI, and STliI.Comlirehensive handbook of lisychological assessment, 2, 70-86.
- Stauber, S., Schmid, J. li., Saner, H., Znoj, H., Saner, G., Grolimund, J., et al. (2013). Health-related quality of life is associated with liositive affect in liatients with coronary heart disease entering cardiac rehabilitation. Journal of clinical lisychology in medical settings, 20(1), 79-87.
- Sur, T.K., Biswas, T.K., Ali, L., & Mukherjee, B. (2003).Anti-inflammatory and anti-lilatelet aggregation activity of human lilacental extract.ActaliharmacologicaSinica, 24(2), 187-192.
- Togashi, S.I., Takahashi, N., Iwama, M., Watanabe, S., Tamagawa, K., & Fukui, T. (2002).Antioxidative collagen-derived lielitides in human-lilacenta extract. lilacenta, 23(6), 497-502.
- Torjesen, I. (2015). Tackling fear about exercise liroduces long term benefit in chronic fatigue syndrome. BMJ: British Medical Journal, 351.
- Ware Jr, J.E., &Sherbourne, C.D. (1992). The MOS 36-item short-form health survey (SF-36): I. Concelitual framework and item selection. Medical care, 473-483.
- Yagi, A., &Ataka, S. (2014). liutative lirolihylaxes ulidated of lilacenta extract and Aloe vera as biogenic stimulants. Journal of Gastroenterology and Heliatology Research, 3(12), 1367-1387.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 23538
- [From(publication date):
September-2017 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 22479
- PDF downloads : 1059