Research Article Open Access
Effects of HERV-R env Knockdown in Combination with Ionizing Radiation on Apoptosis-Related Gene Expression in A549 Lung Cancer Cells
| Ja-Rang Lee1, Yi-Deun Jung2, Young-Hyun Kim1,3, Sang-Je Park1, Jae-Won Huh1,3* and Heui-Soo Kim4* | |
| 1National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cheongju 363-883, Republic of Korea | |
| 2Stem Cell Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea | |
| 3University of Science and Technology, National Primate Research Center, KRIBB, Cheongju 363-883, Republic of Korea | |
| 4Department of Biological Sciences, College of Natural Sciences, Pusan National University, Busan, Republic of Korea | |
| *Corresponding Authors: | Heui-Soo Kim Department of Biological Sciences College of Natural Sciences Pusan National University Busan 609-735, Korea Tel: +82-51-510-2259 Fax: +82-51-581-2962 E-mail: khs307@pusan.ac.kr |
| Jae-Won Huh National Primate Research Center Korea Research Institute of Bioscience and Biotechnology Cheongju, Korea Tel: +82-43-240-6327 Fax: +82-43-240-6309 E-mail: huhjw@kribb.re.kr |
|
| Received January 07, 2016; Accepted February 23, 2016; Published March 01, 2016 | |
| Citation: Lee JR, Jung YD, Kim YH, Park SJ, Huh JW, et al. (2016) Effects of HERV-R env Knockdown in Combination with Ionizing Radiation on Apoptosis- Related Gene Expression in A549 Lung Cancer Cells. Biochem Physiol 5:200. doi:10.4172/2168-9652.1000200 | |
| Copyright: © 2016 Lee JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Radiotherapy has played a key role in the management of non–small-cell lung cancer (NSCLC). However, the use of radiotherapy in treating NSCLC is limited because of the intrinsic radiation resistance of tumor cells and injury to adjacent normal tissues. Many oncogenes are reported to be involved in radioresistance. Thus, novel moleculartargeting approaches to enhance the radiosensitivity of NSCLC cells are required to improve the therapeutic efficiency of radiotherapy. In this study, we report that expression of the human endogenous retrovirus-R (HERV-R) env gene is greatly elevated in γ-irradiation resistant A549 cells compared with radiation sensitive H460 cells. In addition, the HERV-R env gene was significantly increased in A549 cells after treatment with γ-irradiation. HERV-R env knockdown by siRNA in irradiated A549 cells led to overexpression of TP53 mRNA, followed by significant elevation in the levels of CDKN1A mRNA. Moreover, the expression of the apoptosis-related FAS-1 gene was increased, whereas the expression levels of the anti-apoptotic gene BCL2 were significantly decreased in the A549 cells in which the HERV-R env was suppressed by γ-irradiation. These results suggest that knockdown of HERV-R env with γ-irradiation causes cell cycle disturbances, which in turn induces apoptosis. In conclusion, the combination of HERV-R env knockdown and γ-irradiation has the potential to improve the therapeutic efficiency of radiotherapy for NSCLC.
| Keywords |
| Non–small-cell lung cancer (NSCLC); Radioresistance; HERV-R env; γ-irradiation |
| Introduction |
| Ionizing radiation has been widely used for the treatments of many tumors, either alone or in combination with chemotherapy. However, radiation therapy has limitations because of the intrinsic radioresistance of tumors and the occurrence of injury to adjacent normal tissues, such as the heart, liver, and lung, which are important clinical problems [1]. A number of oncogenes and tumor suppressor genes have been suggested to play important roles in radiosensitivity. For example, expression of oncogenes including Ras, Myc, Raf, and mutated TP53 correlates with radioresistance in many tumor cells [2-6], whereas tumor suppressor genes including wild-type TP53 and p16 are closely linked to radiosensitivity [7,8]. Thus, cancer-related genes may be involved in radioresistance. |
| Human endogenous retroviruses (HERVs) occupy about 8% of the human genome [9] and have been derived from exogenous retroviruses by ancient germ cell infection [10]. Most HERV sequences have defective structures, such as truncations, deletions, and insertions. However, some HERV families, or family members, retain full-length sequences containing a functional open reading frame (ORFs) in the env region [11]. These env genes are expressed in particular physiological contexts and in various human diseases [12,13]. Moreover, proviruses driving the expression of their own retroviral proteins have physiological functions, for example in promoting cell proliferation, cell-cell fusion, anti-apoptotic functions, and immunosuppression [14-17]. A HERV env genes also appear strictly expressed in various cancers, including ovarian, prostate, and breast cancers, and melanoma [18-22]. |
| HERV-R is located on human chromosome 7 and has an intact ORF in the env region, encoding a protein with surface unit (SU) and transmembrane (TM) domains [23,24]. The HERV-R envelope protein contains a putative immunosuppressive domain, and the TM domain may be involved in cell-cell fusion. Therefore, the HERV-R envelope protein could potentially contribute to the formation of syncytiotrophoblast in the placental chorionic villi during gestation [25-27]. Nevertheless, the TM retroviral protein enhances tumorigenicity, and is involved in autoimmune diseases, including type I diabetes. Three env-containing mRNAs are transcribed from HERV-R: a 3.5- kb transcript comprising only proviral sequences and 7.3-kb and 9-kb transcripts that extend downstream of the provirus [23]. HERV-R mRNAs are expressed in normal tissues in the brain, prostate, testis, kidney, placenta, thymus, and uterus [28]. HERV-R env is regulated by various endogenous and exogenous stimulants, for example cytokines, steroids, drugs, or ionizing radiations [29-32]. In particular, γ-irradiation induces upregulation of HERV-R env expression in normal human cell lines [32]. The regulation of expression via γ-irradiation is based on epigenetic control mechanisms, including histone modification. Expression of HERV-R env is also elevated in liver and lung cancers compared to that in adjacent non-tumor tissues [33]. Thus, HERV-R env is a potential biomarker for liver and lung cancers; however, the role of HERV-R env in the cancers is yet to be elucidated. |
| In this study, the differential expression of HERV-R env in fractionated, γ-irradiation–treated lung cancer cell lines was observed, and the role of HERV-R env in cell radioresistance was investigated using knockdown experiments. HERV-R env knockdown in fractionated, γ-irradiated cells was observed to induce upregulation of apoptosisrelated genes. |
| Materials and Methods |
| Cell culture and ionizing radiation |
| A549 and H460 cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin and maintained in a humidified, 5%-CO2 atmosphere at 37°C. For irradiation, cells were seeded in 60-mm cell culture dishes, allowed to grow to 60–80% confluence, and then irradiated with 2 Gy per day. Total gamma-irradiation (Cs-137) doses were 2 Gy, 4 Gy, and 6 Gy at a dose of 0.81 Gy/min. Experiments were performed in triplicate. All irradiations were performed using a Gamma Cell 1000 Elan (Nordion International, Inc., Ontario, Canada). Dishes containing control cells were taken to the irradiation chamber, but were not exposed to radiation. |
| in vitro siRNA treatment |
| siRNA targeting HERV-R env (5’-AGG CAU AAC UAU AGG AGA U-3’) and negative siRNA were purchased from RNAi Co. (Bioneer, Korea). Cells were transfected with 100 nM siRNA by using the Lipofectamine 2000 transfection reagent (Invitrogen). The cells were incubated for 24 h after transfection, and then HERV-R env expression was determined by means of real-time reverse transcription polymerase chain reaction (RT-PCR). |
| RNA isolation and cDNA synthesis |
| A549 and H460 cells were trypsinized, washed with phosphatebuffered saline (PBS), followed by total RNA extraction using a High Pure RNA isolation kit (Roche). For cDNA synthesis, 1 μg of pure mRNA was reverse-transcribed in a 20 μL reaction volume containing 10 pmol oligo-dT, M-MLV reverse transcriptase, and the corresponding buffer. The reaction was carried out at 42°C for 90 min and terminated by heating for 2 min at 95°C. |
| Real-time RT-PCR and statistical analyses |
| HERV-R env, radioresistance, and apoptosis-related genes were analysed by means of real-time RT-PCR amplification. We also performed real-time RT-PCR amplification on pure mRNA samples without a reverse transcription reaction to confirm that the prepared mRNA samples did not contain genomic DNA. All the primers used in this study and their sequences are listed in Table 1. For normalization, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified. The amplification efficiencies and correlation coefficients (R2) of the env gene were determined from the slopes of the standard curves that were obtained using a serial dilution series. Each primer pair exhibited a single, sharp peak, indicating that the primers amplified only one specific PCR product. Primer dimers were not observed. The sample was added to a 19 μL reaction mixture containing 7 μL H2O, 10 μL QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA), and 1 μL each of the forward and reverse primers. Real-time RT-PCR was performed in a Rotor Gene 3000 (Corbett Research, Australia) for 50 cycles of 94°C for 10 s, 58°C for 15 s, and 72°C for 15 s. Melting curve analyses were carried out for 30 s at 55–99°C. All samples were amplified in triplicate. The statistical significance of the differences between two groups was determined using two-tailed Student’s t-test. P < 0.05 was considered to indicate a statistically significant difference. |
| Results |
| Differential expression of radioresistance-related genes in 2 NSCLC cell lines |
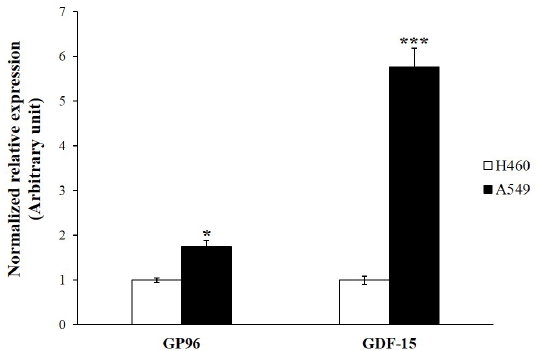
| A549 lung adenocarcinoma cancer cells tend to be a more radioresistant phenotype than H460 lung cancer cells. Previous work has shown that A549 are more radioresistant than H460 cells [34,35]. We amplified the genes reported to contribute to radioresistance by using real-time RT-PCR [36,37]. GP96 and GDF-15 gene expressions were significantly increased in A549 cells compared to those in H460 cells (Figure 1). These data add weight to previous work that showed that A549 cells are more radioresistant than H460 cells. |
| Differential expression of HERV-R env in γ-irradiated lung cancer cells |
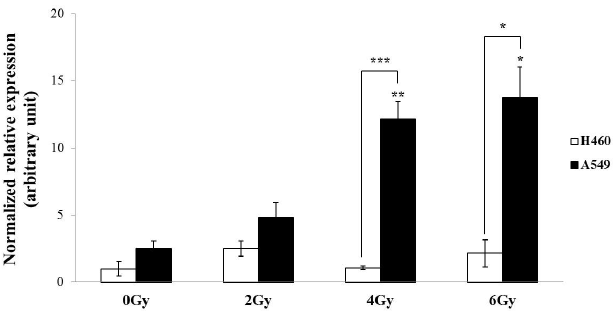
| To investigate differential expression of HERV-R env in γ-irradiated A549 and H460 cells, real-time RT-PCR analyses were performed using cells subjected to γ-irradiation at doses of 0 Gy, 2 Gy, 4 Gy, and 6 Gy (Figure 2). The mRNA levels of HERV-R env were significantly increased at 4 Gy and 6 Gy of γ-irradiation in A549 cells, but H460 cells did not show any trends of differential expression. We further observed that HERV-R env expression was upregulated by 4.8-fold and 5.4-fold in A549 cells after exposure to 4 Gy and 6 Gy of γ-radiation, respectively, compared to non-irradiated A549 cells. Furthermore, HERV-R env expression level was higher in A549 than in H460 cells after exposure to 4 Gy and 6 Gy of γ-radiation. These results suggest that HERV-R env expression in radioresistant cells is significantly increased by γ-irradiation. |
| Effect of siRNA transfection on HERV-R env expression |
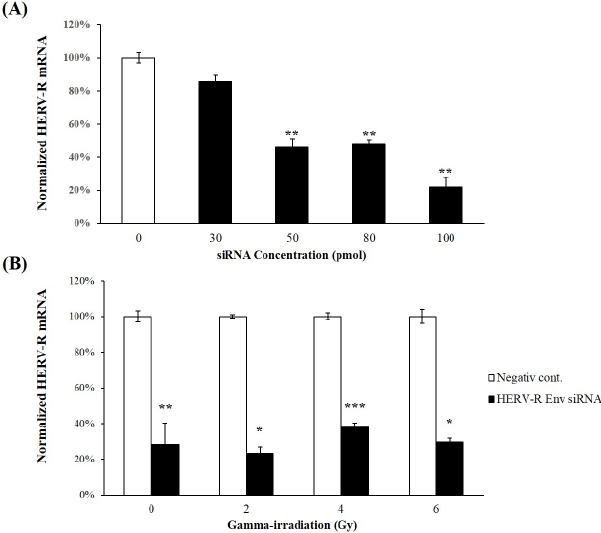
| The knockdown efficiency of HERV-R env expression by HERV-R siRNAs was evaluated in A549 cells. Using real-time RT-PCR, HERV-R env expression was found to be significantly reduced to 46.0 ± 5.33%, 47.6 ± 3.14%, and 21.8 ± 6.32% in mock transfected control cells in A549 cells transfected with 50 pmol, 80 pmol, and 100 pmol of siRNA, respectively (Figure 3A). The 100 pmol siRNA transfection generated the highest knockdown efficiency compared to the other concentrations. In addition, HERV-R env expression remained significantly decreased after HERV-R env siRNA treatment in γ-irradiated A549 cells (Figure 3B). HERV-R env expression was significantly reduced to 23.3 ± 3.64%, 38.4 ± 1.88%, and 29.6 ± 2.18%, respectively, in HERV-R env siRNAtransfected A549 cells treated with 2 Gy, 4 Gy, and 6 Gy of fractioned γ-radiation. |
| HERV-R env knockdown induces upregulation of apoptosis related genes and downregulation of anti-apoptotic gene in radioresistant cells after γ-irradiation |
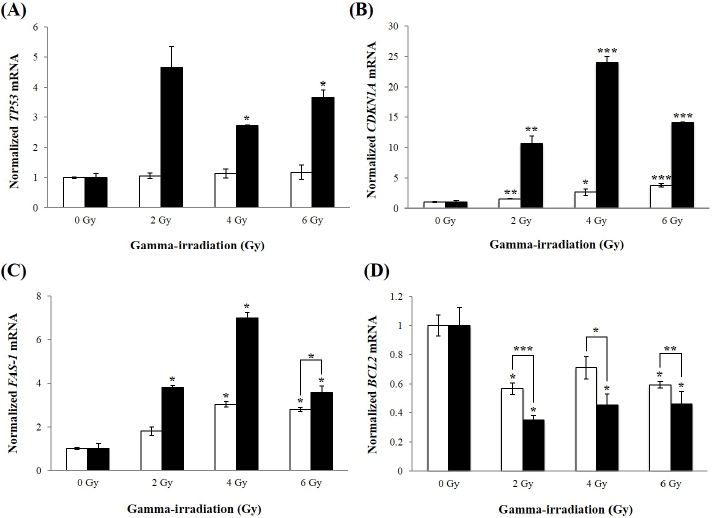
| To determine whether inhibition of HERV-R env sensitizes A549 cells to radiation, expression levels of apoptosis-related mRNAs were determined using real-time RT-PCR analysis. Levels of the TP53 mRNA were significantly increased at 4 and 6 Gy of γ-irradiation in HERV-R env siRNA transfected A549 cells (Figure 4A). The CDKN1A mRNA was also upregulated by 10.7-, 24.0-, and 14.1-fold in HERV-R env siRNA transfected A549 cells after exposure to 2, 4, and 6 Gy of γ-irradiation, compared to non-irradiated A549 cells (Figure 4B). In addition, levels of FAS-1 were increased at 2, 4, and 6 Gy of γ-irradiation, compared to non-irradiated A549 cells (Figure 4C). By contrast, expression of the BCL2 gene was significantly decreased at 2, 4, and 6 Gy of γ-irradiation in HERV-R env siRNA transfected A549 cells (Figure 4D). These results suggest that knockdown of HERV-R env led to increased transcription of pro-apoptotic molecules, and decreased levels of anti-apoptotic markers in γ-irradiated A549 cells. |
| Discussion |
| Many lung cancers, in particular non-small cell lung cancer (NSCLC), are highly aggressive tumors with very poor prognoses. One major treatment for lung cancer is radiotherapy; however, NSCLCs with intrinsic radio- and drug-resistance have been reported [38]. In addition, the response of NSCLCs with the same histology to radiations can vary substantially [34]. Therefore, it is important to improve the efficiency of radiotherapy, which could be achieved by adopting novel molecular-targeting approaches to increase the radiosensitivity of NSCLC cells [39,40]. In particular, A549 lung adenocarcinoma cancer cells have been reported to tend towards a more radioresistant phenotype compared with H460 lung cancer cells [34,35]. In this study, we investigated whether the HERV-R env gene is involved in radioresistance by comparing 2 NSCLC cell lines, A549 and H460. Our results confirmed the differences in the basal expression levels of radioresistance-related genes in response to radiation between A549 and H460 cells. |
| The radioresistance of cancer cells has been postulated to be under the control of various oncogenes [2-6]. Previous studies have provided evidence that the activity of retroelements, including HERV families, plays a distinct role in tumorigenesis and tumor progression via the activation of oncogenes or the expression of functional proteins, including immunosuppressive proteins [12,41]. In particular, the TM cytoplasmic YXXM motif in the jaagsiekte sheep retrovirus envelope protein has been shown to be tumorigenic both in vitro and in vivo [42]. In addition, the fusogenic HERV envelope protein exerts anti-apoptotic function, and downregulates active caspase-3 expression [15]. HERV-R has been described as an active provirus containing a long ORF that includes the TM and SU domains in the env region that encodes viral envelope proteins. These env transcripts are often activated by environmental stress such as ionizing radiations, as well as by cytokines, steroids, and drugs [29-32]. Moreover, HERV-R env is overexpressed in lung and liver cancers compared with their adjacent non-tumor tissues [33]. However, whether the upregulation of HERV-R env by γ-irradiation plays a specific role in cancer is not yet known. Thus, we first investigated whether HERV-R env expression changed in response to γ-irradiation in A549 cells. In these cells, the expression of HERV-R env was significantly increased in a dose-dependent manner 24 h after fractioned γ-irradiation with 4 Gy and 6 Gy (Figure 2). However, no unusual expression of HERV-R env was observed in H460 cells. A notable increase in HERV-R env expression is triggered by exposure to ionizing radiation in radioresistant cells. This finding is of special interest, the results of the present study suggested for the first time that HERV-R env is potentially involved in the regulation of radioresistance in lung cancer cells. |
| Human TP53 encodes the p53 nuclear protein, which is a transcription factor with tumor suppressor functions [43]. As a key regulator of cell growth and programmed cell death, DNA-damaging agents, including UV, ionizing radiations, and chemical agents, stimulate the expression levels of TP53. Activated p53 can induce cell cycle arrest at the G1/S DNA damage recognition regulation point [44,45]. CDKN1A encodes the p21 nuclear protein, also known as cyclin-dependent kinase inhibitor 1, or CDK-interacting protein 1. CDKN1A expression is controlled by the p53 protein, and p21 regulates the p53-dependent cell cycle G1 phase arrest in response to DNA damage stimulants. In addition, p21 can prevent cell division after exposure to DNA-damaging agents, resulting in cell growth arrest [46]. TP53 and CDKN1A genes are, therefore, representative apoptosisrelated genes. Furthermore, the FAS gene encodes a member of the TNF-receptor superfamily and contains a death domain. This gene has been reported to play a key role in programmed cell death [47]. In this study, to demonstrate the radiosensitizing effects caused by HERV-R env knockdown, the abovementioned genes were used as apoptosis markers. Quantitative RT-PCR showed high levels of TP53, CDKN1A, and FAS-1 mRNA expression in HERV-R env siRNA-transfected A549 cells that were subjected to γ-irradiation, compared to non-irradiated cells (Figure 4). These results demonstrate that HERV-R env knockdown cells subjected to γ-irradiation upregulate TP53 expression in a dosedependent manner, followed by induction of CDKN1A expression. Our observations suggest that the cell cycle arrest induced by HERV-R env knockdown in γ-irradiated cells might be mediated via functionally activated p53. Furthermore, we observed that genes positively regulating apoptosis, such as TP53, CDKN1A, and FAS-1 were upregulated, but anti-apoptotic genes, including BCL2, were downregulated in HERV-R env siRNA-transfected A549 cells subjected to γ-irradiation; this was not the case in non-irradiated cells. These data suggest that HERV-R env knockdown may induces radiosensitizing effects via apoptosis and cell cycle arrest; however, further study will be necessary to confirm the phenotype and establish definitively the signalling pathway by which HERV-R env knockdown induces radiosensitization and programmed cell death. |
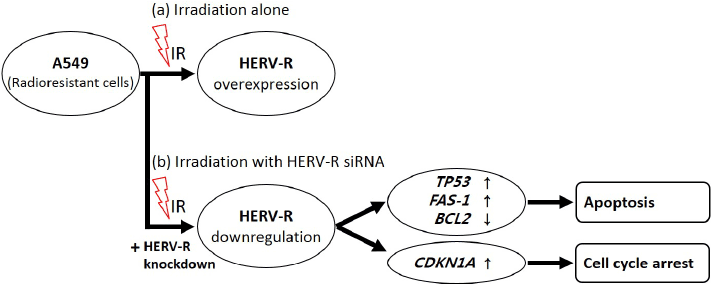
| In conclusion, this is the first study to demonstrate that HERV-R env may be involved in the radioresistance phenotype of NSCLC A549 cells. This study showed that HERV-R env mRNA levels increase after irradiation in A549 cells but not in H460 cells and that knockdown of HERV-R env mRNA increased TP53, CDKN1A and FAS-1 mRNA while decreasing BCL2 mRNA. These results suggests the radiosensitizing effects of HERV-R env, which is the target of the ionizing radiations, and could be of great use in understanding the role of active HERV elements in radioresistance (Figure 5). Taken together, these results indicating a possible role of HERV-R env in mediating radiation resistance and as such may be a useful target for novel anticancer strategies. |
| Acknowledgment |
| This research was supported by a grant from the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4241642). |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 10971
- [From(publication date):
March-2016 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 10076
- PDF downloads : 895
