Effects of Ganoderma Lucidum on Pain in Women with Fibromyalgia
Received: 29-Mar-2017 / Accepted Date: 17-Apr-2017 / Published Date: 23-Apr-2017
Abstract
Objective: Fibromyalgia is a chronic syndrome of an unknown etiology characterized by widespread musculoskeletal pain and several others symptoms like poor sleep, fatigue and decreased physical fitness. These symptoms can lead to decreased Health Related Quality of Life and a high level of impediments. Ganoderma lucidum (Curt.: Fr.) P. Karst is a natural treatment widely used in traditional medicine for beneficial properties. It can improve pain, the quality and the quantity of sleep, increase physical performance and raise the defence levels of the organism, between other potential benefits. Despite its potential benefits on fibromyalgia symptoms, to our knowledge, and to date there are no studies aimed at the assessment of the effects of the Ganoderma lucidum on pain within the fibromyalgic population. The purpose of this study was to evaluate the effects of Ganoderma lucidum on self-reported pain levels in women with fibromyalgia. Secondly, to compare the effects of both Ganoderma lucidum and the active placebo Ceratonia siliqua on the impact of fibromyalgia and health-related quality of life.
Methods: A double-blind randomized trial was carried out. One group took 6 g/day of Ganoderma lucidum powder for 6 weeks, while the second group took the same dosage of the active placebo Ceratonia siliqua flour. Pain levels were self-reported every morning over 72 days after the treatment started. Fibromyalgia Impact Questionnaire (FIQ), its revised version (FIQ-R) and 15D questionnaire were used. Efficacy analysis was performed with a sample of 50 women.
Results: Ganoderma lucidum reduced the impact of fibromyalgia assessed using the FIQ. It also improved two dimensions of the 15D questionnaire: "Move" and "Sleep" compared to Ceratonia siliqua. Finally, Ganoderma lucidum could lead a reduction of up to 20-30% in pain levels. On the other hand, Ceratonia siliqua seemed to be ineffective in improving almost all analyzed variables.
Conclusion: Ours findings showed that six-weeks of the Ganoderma lucidum treatment could reduce the impact of fibromyalgia and pain levels, while increasing the health-related quality of life. Further studies will be necessary in order to identify the most adequate dose of Ganoderma lucidum and the optimal duration of the treatment. The current study lays down the foundation for future research, focused on Ganoderma lucidum as a complement for the treatment of FM.
Keywords: Alternative medicine; Ceratonia siliqua; Chronic disease; Clinical trial; Mushroom; Quality of life; Reishi
4752Introduction
Fibromyalgia (FM) is a chronic disease characterized by multiple painful regions and specific symptoms, such as fatigue, non-restorative sleep, impaired cognition, poor physical fitness, intestinal function problems, and depression [1,2]. It is also related with poor health quality of life (HRQoL) [3]. The estimated overall prevalence of FM oscillates from 2.9% to 4.7% [4]. Social costs of FM are very high due to healthcare costs and the inability of patients to work [5,6].
Up to date, the most accepted treatment for FM consists on a multidisciplinary approach. It should include pharmacologic and nonpharmacological approaches. Among pharmacologic treatments, what is recommended is tricyclic antidepressants amitriptyline and nortriptyline, cyclobenzaprine, tramadol, duloxetine, milnacipran, pregabalin and gabapentin [7]. Several non-pharmacologic treatments may be effective as a treatment of FM, including physical exercise, sleep hygiene, patient education, and cognitive behavioral therapy [8]. In addition, complementary and alternative medicine could be helpful in FM subjects.
Ganoderma lucidum (GL), also known as "Reishi" or "Lingzhi", is a type of mushroom commonly used in traditional Chinese medicine. It is used due to its potential therapeutic effects for the prevention and treatment of cancer, diabetes, Human Immuno Virus, or Parkinson´s disease. The reported benefits of GL also included the improvement of sleep duration, anti-inflammatory, hepatoprotective, antiproliferative, antioxidant, neuroprotective, hypotensive and immunomodulatory effects [9]. Given that the main symptom of FM is pain, the antinociceptive effect [10-12] of GL may be extremely helpful.
However, many of the studies on the effects of GL were not developed with human samples. Thus, there is a lack of studies that assess the GL effects on specific human samples.
Ceratonia siliqua (CS) has been widely used in traditional mediterranean medicine. CS flour is a high fibre food [13], with a rich source of carbohydrates, proteins, and minerals. Among the minerals, calcium, potassium, magnesium, sodium and phosphorus are abundant [14]. Several studies investigated the effects of CS on blood cholesterol [15,16] and blood glucose levels [17]. It was suggested that CS may reduce the hypercholesterolemia and improve intestinal functions [18]. Antidepressant activity mediated by dopamine and noradrenaline was observed in mice [19]. However, to our knowledge these effects on depression have not been studied in humans. An antioxidant effect was also reported [20]. According to our knowledge, Ceratonia siliqua does not have any known effects on pain, but could have effects on the secondary variable assessed in this study. For this reason, it represents an active placebo [21] for the pain evaluation, which was the main objective of this investigation.
To date, there is only one known research that has studied both GL and CS effects on physical fitness in patients with fibromyalgia [22]. However to our knowledge there is no data on the GL and CS effects on the outcome we determined in this study.
The main objective of the current research was to assess and to compare the effects of a 6-week GL treatment with those of a placebo (CS treatment) on self-reported pain level. The second objective was to evaluate the effects on HRQoL and impact of FM.
Materials and Methods
Subjects
All participants were recruited from three FM associations. The following inclusion criteria were set: a) be diagnosed with FM by a rheumatologist, b) to be able to communicate effectively with the study staff, and c) age of more than 18 years. The following exclusion criteria was applied: a) pregnancy b) change their usual care therapies during the 6 weeks of treatment, c) be taking immunosuppressive, d) be suffering from diabetes, e) be participating in other studies, f) be taking C vitamin supplementation, g) be taking anticoagulants, and h) have taken micromilled GL before. Diagnosis of FM was checked using an algometer. Participants who did not feel an acute painful response in at least 11 of 18 specified tender points, when these points were digitally palpated with a pressure of 4 kg/cm2 were also excluded.
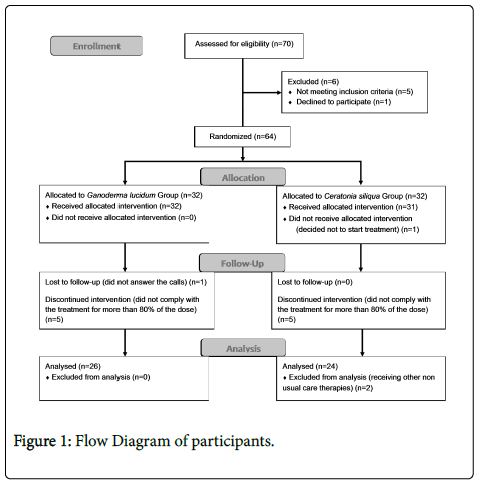
As can be seen in Figure 1, a total of 70 subjects were initially recruited from associations (67 women and 3 men). Of these, one participant declined to participate in the study, one had not been diagnosed with FM, another one was excluded because of the criteria "feel an acute painful response in at least 11 of 18 specified tender points when these points are digitally palpated with a pressure of 4 kg/cm2", and three participants were excluded because they fulfilled the exclusion criteria "be suffering from diabetes". A total of 64 women with FM participated in the study and provided written informed consent in accordance with the Declaration of Helsinki.
Patients could abandon the treatment if the following circumstances occurred: a) they withdrew informed consent, b) the researcher or their general practitioner felt that they should withdraw from the study for reasons of safety, and c) the patient did not comply with 80% of the treatment.
Design
The current study is a randomized double blind clinical trial with an active placebo. The trial has been approved by the Committee of Bioethics of the University of Extremadura and it was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR), ID: ACTRN12614001201662.
Participants were randomly allocated into one of the two groups. The first group was "Ganoderma lucidum group" (GLG), while the placebo group was "Ceratonia siliqua group" (CSG). Due to 1:1 allocation, both groups had a sample of 32 women. All participants knew that there were two groups and they could take GL or CS. However, they could not know the group to which they belonged to. Randomization and allocation processes were performed by one researcher using random code numbers. In order to preserve the double blind factor, this researcher did not take part in the acquisition or statistical analysis of data.
The active placebo
According to our knowledge, GL and CS do not have any secondary effects that might be associated to one or the other group, but both could have effects on masking the improvement of some associated FM symptoms that are not the principal object of this investigation, like depression [19], intestinal functions [18] and physical fitness [22]. For this reason, it represents an active placebo [21] for pain evaluation, which was the main objective of this investigation.
We used Ceratonia siliqua because it was the only product on the market that complied at the same time with the methodological set up we would carry out and with the budget of the research for the following reason:
- It had no effects on pain, that was the principal outcome we would test.
- In order to maintain the blindness of the trial, CS flour was chosen because it was the most similar product to GL powder available on the market in order to permit the auto-administration of the treatment. Encapsulation was not feasible because it was not possible to find a company able to encapsulate the product according to the measurements timetable and the available budget. We did not want the study to overlap with the Christmas period where drastic change in eating habits could alter the results of the analysis.
Procedure
Participants were given 252 g of micromilled (less than 35 μm) GL powder or CS flour (6 g daily, divided in dosis of 3 g each for 6 weeks). The GLG took 3 g of GL twice a day: one dosis during or immediately after breakfast and one during or immediately after dinner. The GL had to be dissolved in warm water and orally ingested. The CSG took CS flour instead of GL. The administration was exactly the same. GL and CS were provided by the company “Mundo Reishi”.
Data collection
General HRQoL and impact of FM were assessed at baseline and after a 6-week treatment. Pain was also measured during treatment. Participants were asked to report their pain level on a Visual Analog Scale (VAS) every morning when they woke up. In order to observe how pain levels changed when the treatment stopped, participants were asked to report their daily pain level for 4 weeks after the treatment ceased.
General HRQoL was measured using the 15D questionnaire [23]. It is a generic instrument for measuring HRQoL among adults. It comprises 15 dimensions, namely mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. The general score and the score of each dimension can range from 0 (worst) to 1 (best). It is a reliable and sensitive questionnaire that has been used widely in several populations.
Impact of FM was assessed using both the FM Impact Questionnaire (FIQ) and the Revised FM Impact Questionnaire (FIQR). FIQ is a 10-item, self-administered instrument. The 10 items are: physical impairment, feel good, work missed, do job, pain, fatigue, rested, stiffness, anxiety, and depression. A consensus version for Spanish population was developed by Esteve-Vives, Rivera Redondo, Isabel Salvat Salvat, de Gracia Blanco and de Miquel [24]. FIQ was revised in 2009, originating a new questionnaire called FIQ-R. It has three domains: function, overall impact, and symptoms. It differs from FIQ in some points [25]; first, the dimension “function” was reduced from 10 to 9 items and modified; second, the overall impact domain was completely revised to better reflect the overall impact on functional ability and on the perception of reduced function; finally, four additional items were added to the dimension “symptoms”, namely tenderness, memory, balance and environmental sensitivity. The validation of the Spanish version of FIQ-R was developed by Salgueiro, Garcia-Leiva, Ballesteros, Hidalgo, Molina and Calandre [26].
The FIQ-R item "pain" was assessed separately because pain is the main symptom of FM. This question can range from 0 (no pain) to 10 (unbearable pain).
Statistical analysis
Student´s t test for independent samples was used in order to calculate differences between groups at baseline. The Kolmogorov- Smirnov test was utilized to determine distribution of data.
Student´s t test for independent samples was also used to compare the self-reported pain during treatment between groups. Paired t test was calculated in order to estimate the changes of both groups comparing from baseline.
The analysis of variance (ANOVA) for repeated measures was used to calculate the effects of the treatment on pain, 15D, FIQ and FIQ-R. It was also utilized for every dimension of 15D and FIQ.
Two different analyses were performed. The first was the efficacy analysis (n=50) and it comprised the sample that fulfilled all inclusion and exclusion criteria, taking at least 80% of the dose. The second analysis was the intent-to-treat analysis. This analysis comprised the 64 initially randomized participants. Data of all participants that came the post-treatment-measurement´s day were utilized. Post-treatment data of the remainder of the sample (n=4) was imputed according to the mean change of their group.
The level of significance was set at p
Results
Descriptive statistics are shown in Table 1. As it can be observed, no statistically significant differences were observed between the two groups at the baseline.
| GLG (n=26) | CSG (n=24) | P* | |
|---|---|---|---|
| Age (years) | 56.19 (7.97) | 53.74 (11.50) | 0.382 |
| Date when fibromyalgia symptoms started | 1994 (11.86) | 1992 (12.56) | 0.601 |
| Date of diagnosis | 2006 (6.56) | 2003 (7.02) | 0.935 |
| Height (cm) | 157.08 (4.55) | 156.29 (6.08) | 0.541 |
| Weight (kg) | 64.26 (9.67) | 61.30 (13.24) | 0.411 |
| Muscle mass (%) | 61.97 (7.17) | 64.82 (8.58) | 0.245 |
| Fat mass (%) | 34.81 (7.51) | 32.24 (7.75) | 0.285 |
| BMI (kg/m2) | 26.05 (3.75) | 25.06 (4.75) | 0.522 |
| 15D total score (0 worst; 1 best) | 0.64 (0.12) | 0.69 (0.09) | 0.092 |
| FIQ score (0 best; 100 worst) | 63.64 (21.77) | 59.47 (13.74) | 0.427 |
| FIQ-R score (0 best; 100 worst) | 56.37 (18.64) | 53.69 (16.54) | 0.594 |
Table 1: Characteristics of women with FM from both groups at baseline.
Results of the efficacy analysis can be seen in Table 2.
| GLG (n=26) | CSG (n=24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean(SD) at baseline | Mean(SD) after treatment | Pa | Mean(SD) at baseline | Mean(SD) after treatment | Pa | Pb | Effect size | Treatment effect (95% CI) | |
| 15D total score | 0.64 (0.12) | 0.75 (0.11) | <0.01 | 0.69 (0.09) | 0.76 (0.10) | <0.01 | 0.130 | 0.445 | 0.04 (-0.01 to 0.09) |
| FIQ total score | 63.64 (21.77) | 40.21 (21.92) | <0.01 | 59.47 (13.74) | 47.69 (16.25) | <0.01 | 0.046 | 0.593 | -11.65 (-23.04 to -0.24) |
| FIQ-R total score | 56.37 (18.64) | 39.67 (20.28) | <0.01 | 53.69 (16.55) | 43.51 (13.17) | <0.01 | 0.195 | 0.379 | -6.52 (-2.82 to 0.02) |
| Function domain | 14.26 (6.05) | 10.58 (6.60) | <0.01 | 13.71 (6.25) | 11.90 (6.92) | 0.276 | 0.347 | 0.274 | -1.87 (-16.50 to 3.46) |
| Overall impact domain | 9.85 (6.16) | 6.31 (5.66) | 0.011 | 9.21 (5.76) | 6.42 (4.09) | 0.059 | 0.696 | 0.114 | -0.75 (-5.84 to 2.09) |
| Symptom domain | 32.27 (8.71) | 22.79 (10.08) | <0.01 | 30.77 (7.55) | 25.19 (5.85) | <0.01 | 0.094 | 0.493 | -3.9 (-4.56 to 3.07) |
| Pain assessed by FIQ-R | 7.15 (2.09) | 4.92 (2.62) | <0.01 | 7.17 (1.6) | 6.33 (2.10) | 0.084 | 0.053 | 0.212 | -1.39 (-8.49 to 0.69) |
| Pain reported during treatment* | 54.39 (20.93) | 43.91 (26.92) | 0.034 | 59.32 (26.83) | 48.77 (27.27) | 0.052 | 0.993 | <0.001 | 0.07 (-15.50 to 15.64) |
Table 2: Effects of 6 weeks of treatment on HRQoL, impact of FM and pain, Efficacy analysis.
After the 6-week treatment, no statistically significant differences were observed in the pain levels between the two groups, both for selfreported pain and FIQ-R dimension. A statistically significant difference in favor of GLG in the FIQ score (p=.046) was observed. The treatment effect of this measure was -11.65, which means an improvement higher than 18%. The GLG intra-group analysis revealed an improvement higher than 37% (p<.01). Table 2 also shows that GLG significantly improved every assessed outcome compared to the baseline. On the other hand, CSG did not significantly improve the pain levels.
Intent-to-treat analysis is shown in Table 3. A statistically significant difference was observed in the overall domain of FIQ-R (p=.048), in favor to GLG. An intra-group improvement was observed for GLG in all variables except in self-reported pain, during the treatment. Similar results were found for the CSG, improving all variables except for the function domain of the FIQ-R compared to the baseline.
| GLG (n=32) | CSG (n=32) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean(SD) at baseline | Mean(SD) after treatment | Pa | Mean(SD) at baseline | Mean(SD) after treatment | Pa | Pb | Effect size | Treatment effect (95% CI) | |
| 15D total score | 0.63 (0.11) | 0.74 (0.11) | <0.01 | 0.69 (0.09) | 0.75 (0.09) | <0.01 | 0.413 | 0.109 | 0.05 (-0.01 to 0.09) |
| FIQ total score | 65.49 (20.03) | 43.11 (21.50) | <0.01 | 59.60 (13.68) | 46.68 (16.41) | <0.01 | 0.499 | 0.054 | -9.46 (-19.10 to 0.17) |
| FIQ-R total score | 58.49 (17.78) | 42.67 (20.03) | <0.01 | 51.87 (16.95) | 42.31 (14.24) | <0.01 | 0.383 | 0.136 | -6.26 (-14.56 to 2.04) |
| Function domain | 15.20 (5.95) | 11.66 (6.51) | <0.01 | 13.60 (5.98) | 11.87 (7.00) | 0.191 | 0.283 | 0.269 | -1.81 (-5.02 to 1.42) |
| Overall impact domain | 10.03 (5.84) | 7.14 (6.05) | 0.013 | 8.69 (5.49) | 6.09 (4.47) | 0.027 | 0.048 | 0.853 | -0.29 (-3.43 to 2.84) |
| Symptom domain | 33.27 (8.39) | 23.88 (9.64) | <0.01 | 29.58 (8.27) | 24.35 (6.18) | <0.01 | 0.539 | 0.038 | -4.16 (-8.07 to -0.24) |
| Pain assessed by FIQ-R | 7.44 (2.00) | 5.22(2.48) | <0.01 | 6.84(1.67) | 5.81 (2.22) | <0.01 | 0.539 | 0.038 | -1.19 (-10.05 to 15.25) |
| Pain reported during treatment | 52.40 (18.50) | 44.88 (27.22) | 0.109 | 54.19 (26.66) | 44.06 (24.59) | 0.028 | 0.104 | 0.683 | 2.61 (-2.31 to -0.07) |
Table 3: Effects of 6 weeks of treatment on HRQoL, impact of FM and pain, Intent-to-treat analysis.
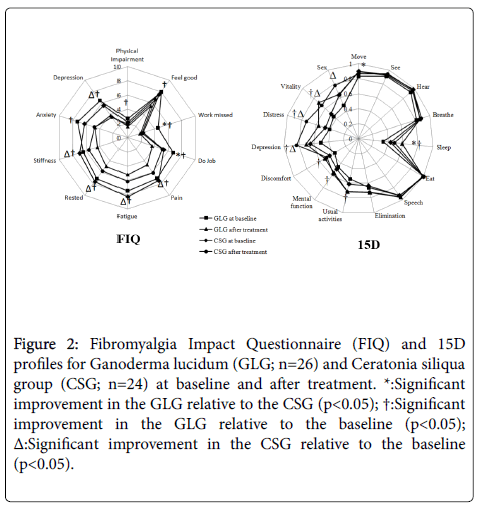
Figure 2 shows the score for each of the 10 FIQ items of both groups at baseline and after treatment. Significant improvement in the GLG in relation to CSG was observed for "do Job" and "work missed". Withingroup analysis revealed a significant change from baseline to aftertreatment in all 10 items for GLG, while CSG significantly improved "pain", "fatigue", "rested", "stiffness", and "depression".
Figure 2: Fibromyalgia Impact Questionnaire (FIQ) and 15D profiles for Ganoderma lucidum (GLG; n=26) and Ceratonia siliqua group (CSG; n=24) at baseline and after treatment. *: Significant improvement in the GLG relative to the CSG (p<0.05); †: Significant improvement in the GLG relative to the baseline (p<0.05); Δ: Significant improvement in the CSG relative to the baseline (p<0.05).
The 15D profiles for both groups at baseline and after treatment can be seen in Figure 2. Significant improvement between GLG and CSG groups was observed in dimensions "move" and "sleep". Significant improvement in the GLG relative to baseline was observed in 6 of the 15 dimensions, namely "sleep", "usual activities", "mental function", "discomfort", "depression", "distress", and "vitality"; while CSG significantly changed in 4 of the 15 dimensions, i.e. "depression", "distress", "vitality", and "sex".
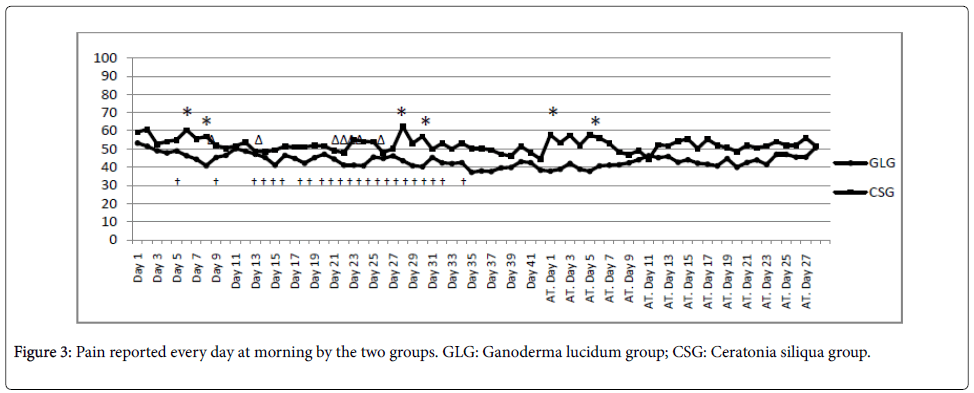
Evolution of self-reported pain every day in the morning can be seen in Figure 3. Statistically significant differences were observed in 6 particular days (4 days during treatment, namely, 6th, 8th, 28th, and 30th; and 2 days after treatment, i.e. 1st and 5th).
AT: After the end of the treatment. *: Significant improvement in the GLG relative to the CSG (p<0.05); †: Significant improvement in the GLG relative to the baseline (p<0.05); Δ: Significant improvement in the CSG relative to the baseline (p<0.05).
A total of 52 participants took at least 80% of the treatment, which means 81% of the initial sample. Number of participants who decided to stop treatment was 5 for both groups (16%). Two participants completed the treatment, but were excluded because they began to receive other non-usual care therapies during the treatment.
Discussion
The main finding was that 6 g of GL per day for 6 weeks reduced the impact of FM. The treatment effect on the total FIQ score was higher than 18% compared with CSG, while the GLG-improvement relative to the baseline was close to 37%. FIQ dimension analysis proved that differences between groups were placed at dimensions "work missed" and "do Job". Implications of these findings are extremely relevant given the social costs of FM [5].
Although no significant differences between the groups were found in the 15D total score used for the assessment of HRQoL, data showed an enhancement in sleep quality and move dimensions in the GLG. A significant correlation was observed between GLG improvement in the 15D dimension "move" and the improvement in the total score of FIQ (p<.05 and R=-0.46). Thus, improvement in physical functioning could lead a global improvement in the impact of FM. This result was supported by subsequent regression analysis (p=0.017).
Detailed information about self-reported pain during and after treatment is shown in Figure 3. GLG experienced a reduction of pain in the first 8 days. After that, the effects were reduced in the second treatment week. From the third week until the end of the treatment, GLG self-reported pain levels remained low (close to 40 over 100). When the treatment stopped, GLG experienced slow enhancement of pain levels, always lower than baseline values. On the other hand, CSG experienced fast reduction of pain levels in the first 3 days, followed by an increment that led pain levels to initial levels of day 6. After that, CSG reported another reduction of pain which ended at day 13, where a significant difference in respect to the baseline was observed. From day 13 and until the start of the 3rd week, pain levels were stabilized. At the end of the treatment, significant lower values relative to CSG baseline were found. When the treatment stopped, CSG experienced a fast and wide increment of pain levels (pain levels the last treatment day and pain reported the first day after treatment were significantly different). Behaviour of CSG may be related with a placebo effect, because large fast changes were found when the treatment started to when the treatment stopped [27]. However, GLG experienced a more stable and slower improvement in pain relative to baseline.
Pain levels were also assessed using FIQ-R. There were significant differences in GLG when comparing levels at baseline and immediately after treatment. This improvement was not observed in CSG. Treatment effect was near to 20% and change from baseline was 31% in GLG. These results are far from the improvement of around 15% reported as placebo effect [28]. However, CSG reported and improvement around 12%, which supports the hypothesis of CSGrelated effects on pain are related to the placebo effect. Comparing pain levels changes, reported by both groups in the FIQ-R, the p-value was . 053, which represents a non-statistically significant difference in spite of being so close to the significant threshold.
The current study started in October and the treatment ended in December. According to the Statal Agency of Meteorology (AEMET), the average climatological temperature of Spain in October was 18.7ºC, in November was 12.5ºC, and in December was 7.8ºC. Although there is no consensus about the effects of weather and temperature in health status and pain of FM patients, these changes may lead to an increment of reported pain levels [29] and other associated symptoms, like stiffness [30] or depression [31].Women suffering from FM usually comment that they feel worse when the weather is cold and wet. Therefore, the effects of both treatments reported in the current paper may be partially masked by weather changes (if the weather had remained stable throughout treatment, changes might have been greater).
The current study supports findings of a previous study that reported an antidepressant effect of CS in mice [19]. To our knowledge, this is the first study that assesses the effects of CS on depression in human samples, assessed by the dimension "depression" of the 15D. CSG significantly improved in this dimension, relative to the baseline. However, this result has to be treated with caution. Further studies using specific depression questionnaires are needed. On the other hand, CS did not improve the score of dimension "elimination", as could be expected according with previous studies [18].
The current study had some limitations. First, the lack of a placebo group for the evaluation of the secondary variables. In this case we made a comparison between two possible active substances. Secondly, the small sample size could cause that changes in various relevant outcomes reported as "no statistically significant" were actually significant, namely "pain assessed using FIQ-R" (p=.053), "Symptom domain of FIQ-R" (p=.094), or 15D total score (p=.13). The third limitation could be the lack of references about the most adequate doses of both GL and CS. In order to keep double-blind, the same dose of both substances was provided so that both groups received the same indications. Fourth, the duration of the study was 6 weeks. This length could be insufficient for different outcomes like 15D dimensions "sex", "discomfort", or "usual activities".
Further studies are necessary in order to identify the most adequate dose of both GL and CS in FM patients. At the same time, optimal duration of the treatment should be studied deeply. Although all these factors remains unclear, the current study lays the foundation for future research focused on GL as a treatment of FM.
In conclusion, results showed that 6 weeks of GL treatment reduced the impact of FM. Relevant improvement on sleep quality and moving were also found in GLG. Self-reported pain was analysed at baseline, after treatment, and every morning until day 72 after the treatment started. Results indicated that GL could lead a reduction of 20-30% in pain levels.
Conflict of Interest Statement
The study was promoted and sponsored by MundoReishi Salud S.L. Spanish Company dedicated to the production and sale of the fungus Ganoderma lucidum. The corresponding author of the research is a scientific collaborator of the Company MundoReishi Salud S.L.
Acknowledgments
The authors acknowledge the assistance of the local associations of Palencia, Salamanca and Chipiona and Laura de Ynigo Mojado.
Financial Supporters
The present research was financed by MundoReishi Salud S.L.
References
- Wolfe F (2015) Editorial: the status of fibromyalgia criteria. Arthritis Rheumatol 67: 330-333.
- egura-Jimenez V, Alvarez-Gallardo IC, Carbonell-Baeza A, Aparicio VA, Ortega FB, et al. (2015) Fibromyalgia has a larger impact on physical health than on psychological health, yet both are markedly affected: The al-Andalus project. Semin Arthritis Rheum 44: 563-570.
- Burckhardt CS, Clark SR, Bennett RM (1993) Fibromyalgia and quality of life: a comparative analysis. J Rheumatol 20: 475-479.
- Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, et al. (2010) Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 39: 448-453.
- Wolfe F, Walitt BT, Katz RS, Hauser W (2014) Social security work disability and its predictors in patients with fibromyalgia. Arthritis Care Res 66: 1354-1363
- White LA, Birnbaum HG, Kaltenboeck A, Tang J, Mallett D et al. (2008) Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med 50: 13-24.
- Skaer TL (2014) Fibromyalgia: disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics 32: 457-466
- Arnold LM, Clauw DJ, Dunegan LJ, Turk DC (2012) Fibro Collaborative. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc 87: 488-496.
- Wachtel-Galor S, Yuen J, Buswell JA, Benzie IFF (2011) Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In: Herbal Medicine: Biomolecular and Clinical Aspects, Benzie IFF, Wachtel-Galor S Eds., Boca Raton (FL).
- Koyama K, Imaizumi T, Akiba M, Kinoshita K, Takahashi K, et al. (1997) Antinociceptive components of Ganoderma lucidum. Planta Med 63: 224-227.
- Hijikata Y, Yamada S (1998) Effect of Ganoderma lucidum on postherpetic neuralgia. Am J Chin Med 26: 375-381
- Li EK, Tam LS, Wong CK, Li WC, Lam CW, et al. (2007) Safety and efficacy of Ganoderma lucidum (lingzhi) and San Miao San supplementation in patients with rheumatoid arthritis: a double-blind, randomized, placebo-controlled pilot trial. Arthritis Rheum 57: 1143-1150.
- Milek L, Tomzack L, Fuganti L, Ramos M, Carneiro C (2014) Glycemic Response to Carob (Ceratonia Siliqua L) in Healthy Subjects and with the in Vitro Hydrolysis Index. Nutr Hosp 31: 482-487.
- Ozcan MM, Arslan D, Gokcalik H (2007) Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int J Food Sci Nutr 58: 652-658
- Ruiz-Roso B, Quintela JC, de la Fuente E, Haya J, Perez-Olleros L (2010) Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant Foods Hum Nutr 65: 50-56.
- Zunft HJ, Luder W, Harde A, Haber B, Graubaum HJ, et al. (2003) Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur J Nutr 42: 235-242.
- Feldman N, Norenberg C, Voet H, Manor E, Berner Y, et al. (1995). Enrichment of an Israeli ethnic food with fibres and their effects on the glycaemic and insulinaemic responses in subjects with non-insulin-dependent diabetes mellitus. Br J Nutr 74: 681-688.
- Martinez-Rodriguez R, Navarro-Alarcon M, Rodriguez-Martinez C, Fonolla-Joya J (2013) Effects on the lipid profile in humans of a polyphenol-rich carob (Ceratonia siliqua L.) extract in a dairy matrix like a functional food; a pilot study. Nutr Hosp 28: 2107-2114.
- Agrawal A, Mohan M, Kasture S, Foddis C, Frau MA, et al. (2011) Antidepressant activity of Ceratonia siliqua L. fruit extract, a source of polyphenols. Nat Prod Res 25: 450-456.
- Custodio L, Patarra J, Albericio F, Neng NR, Nogueira JM, et al. (2015) In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, alpha-amylase and alpha-glucosidase. Nat Prod Res 1-5.
- Park LC, Covi L (1965) Nonblind placebo trial: an exploration of neurotic patients' responses to placebo when its inert content is disclosed. Arch Gen Psychiatry 12: 336-345.
- Collado D, Pazzi F, Dominguez FJ, MartÃn JP, Olivares PR, et al. Ganoderma lucidum improves physical fitness in women with fibromyalgia. Nutr Hosp 32: 2126-2135
- Sintonen H (2001). The 15D instrument of health-related quality of life: properties and applications. Ann Med 33: 328-336.
- Esteve-Vives J, Rivera Redondo J, Isabel Salvat Salvat M, de Gracia Blanco M, de Miquel CA (2007) Proposal for a consensus version of the Fibromyalgia Impact Questionnaire (FIQ) for the Spanish population. Reumatol Clin 3: 21-24.
- Williams DA, Arnold LM (2011) Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arthritis Care Res 63: 86-97.
- Salgueiro M, Garcia-Leiva JM, Ballesteros J, Hidalgo J, Molina R, et al. (2013) Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health and quality of life outcomes 11: 132.
- Savvas SM, Zelencich LM, Gibson SJ (2014). Should placebo be used routinely for chronic pain in older people? Maturitas 79: 389-400.
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, et al. (2014) Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med 6: 218ra5
- Macfarlane TV, McBeth J, Jones GT, Nicholl B, Macfarlane GJ (2010) Whether the weather influences pain? Results from the EpiFunD study in North West England. Rheumatology 49: 1513-1520.
- Miranda LC, Parente M, Silva C, Clemente-Coelho P, Santos H, et al. (2007) Perceived pain and weather changes in rheumatic patients. Acta Reumatol Port 32: 351-361.
- Mirzakhani L, Poursafa P (2014) The Association between Depression and Climatic Conditions in the Iran Way to Preventive of Depression. Int J Prev Med 5: 947-951.
Citation: Pazzi F, Fabero RF (2017) Effects of Ganoderma Lucidum on Pain in Women with Fibromyalgia. Fibrom Open Access 2: 115.
Copyright: © 2017 Pazzi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 11820
- [From(publication date): 0-2017 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 11036
- PDF downloads: 784