Effects of Foliar Application Pure Amino Acid and Amino Acid Containing Fertilizer on Broccoli (Brassica oleracea L. var. italica) Transplants
Received: 18-Apr-2017 / Accepted Date: 24-Apr-2017 / Published Date: 28-Apr-2017 DOI: 10.4172/2329-8863.1000280
Abstract
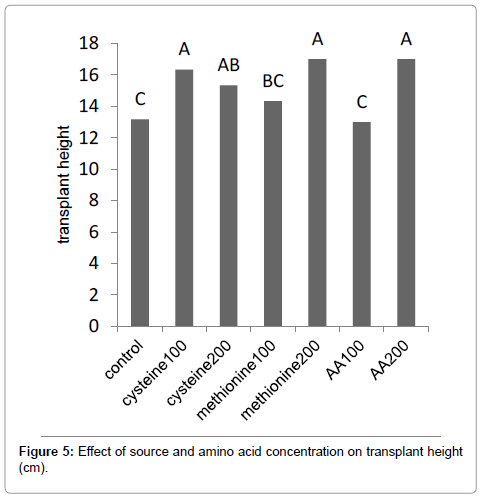
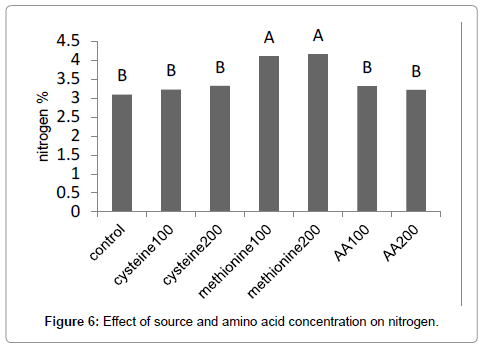
The greenhouse experiments were carried out to study the effects foliar application of L-cysteine, L-methionine and Aminoacid fertilizer (AA) on Broccoli (Brassica oleracea var. italica) transplants. L-cysteine, L-methionine and AA was applied at three concentrations, 0, 100 and 200 mg L-1 commencing from 20 days after the sowing. Physical and chemical properties of the transplants determined according to the methods described by Tabatabaee. Results showed that L-cysteine at concentrations of 100 and 200 mg L-1 increased the leaf area by 53% and 48%, while methionine and the amino-acid-bearing fertilizer, both at 200 mg L-1, increased the leaf area by 59% and 48% respectively. These percentages are expressed in comparison to the control. L-cysteine at concentrations of 100 and 200 mg L-1 increased the fresh weight of shoots by 50% and 55%, respectively, while methionine at 100 and 200 mg L-1 increased the fresh weight of shoots by 55% and 60% respectively. The amino-acid-bearing fertilizer treatments at a concentration of 200 mg L-1 increased the shoot dry weight of Broccoli seedlings by 38%, L-cysteine at concentrations of 100 and 200 mg L-1 increased the root dry weight by 26% and 60%, methionine at 100 and 200 mg L-1 increased the root dry weight by 31% and 177%. The results showed that methionine at 200 mg L-1 increased chlorophyll by 61%. The amino-acid-bearing fertilizer at a concentration of 200 mg L-1 managed to increase the length of transplants by 30%, compared to the control. Methionine at 100 and 200 mg.L-1 increased the amount of nitrogen in transplanted Broccoli by 36% and 38%.
Keywords: Broccoli transplant; Chlorophyll; Fertilizer; L-cysteine; L-methionine; Nitrogen
406897Introduction
The Broccoli (Brassica oleracea var. italica) is a species of the Crucifer family which is of high nutritional value. It contributes to an ideal diet because of its nutritional content. Roughly 100 g Broccoli has 40 calories, 5 g protein, 1 g fat, 0.14 mg B1, 0.3 mg B2, 1.2 mg Niacin, 50 mg calcium carbohydrate, 3880 IU vitamin A and 1.7 mg iron. One of the most appealing characteristics of Broccoli is its high vitamin C content which has been reported to be 82-140 mg [1]. Plant Growth regulating substances (PGRs) were shown to enhance the biosynthesis of certain chemical constituents in plants. In this respect, the amino acids which have a high integrity with different metabolic pools in plants were used to promote plant growth. PGRs also play important roles in plant adaptation to stressful environments. Their common role is to serve as building blocks of proteins, which exert manifold functions in plant metabolism, and as metabolites and precursors wherein they are involved regarding plant defense, the biosynthesis of vitamins, nucleotides and hormones, and as precursors of a huge variety of secondary compounds. One way or the other, as active catalysts or as precursors, amino acids are essentially involved in all metabolic, regulatory and physiological aspects of plant metabolism. Amino acids can serve as the sole source of nitrogen, which can be taken up more rapidly than inorganic nitrogen. Nonetheless, exogenous amino acids reduce both ammonium influx and transporter transcript in the root tissue. Sulfur is a macronutrient that is essential for plant growth and development. The most abundant form of sulfur in nature and the source of sulfur for plants is sulfate; this form is reduced and assimilated into Cysteine. In addition to its role as an amino acid in proteins, cysteine functions as a precursor for a huge number of essential bio-molecules, such as vitamins and cofactors [2] and antioxidants like glutathione, which is decisively involved in cellular redox homeostasis and the synthesis of many defense compounds [3]. All of these bio-molecules contain sulfur moieties that act as functional groups and are derived from cysteine in plants. Cysteine biosynthesis plays a central role in fixing inorganic sulfur from the environment and provides the only metabolic sulfide donor for the creation of methionine, glutathione, phytochelatins, iron-sulfur clusters, vitamin cofactors and multiple secondary metabolites. O-acetyl-serine-sulfhydrylase (OASS) catalyzes the final step of cysteine biosynthesis, the pyridoxal 5-phosphate (PLP)-dependent conversion of O-acetylserine into cysteine. Cysteine proteinases are potentially responsible for exhibiting resistance against both low temperatures and drought [4]. Cysteine is the metabolic precursor of vital bio-molecules such as vitamins, cofactors, antioxidants and many defense compounds. The last step of cysteine metabolism is catalyzed by O-acetyl-serine (thiol) lyase (OASTL), which incorporates reduced sulfur into O-acetylserine to produce cysteine. In Arabidopsis thaliana, the main OASTL isoform OAS-A1 and the cytosolic desulfhydrase DES1, which degrades cysteine, contribute to the cytosolic cysteine homeostasis. In addition to its structural role in proteins, cysteine functions as a precursor to essential bio-molecules, such as vitamins, cofactors and antioxidants such as glutathione and many defense compounds, such as glucosinolates, thionins or phytoalexins. Cysteine is synthesized in plants in the cytosol, plastids and mitochondria by the sequential action of the enzymes serine acetyltransferase which synthesizes the intermediary product O-acetylserine (OAS), and O-acetylserine (thiol) lyase which combines a sulfide molecule with an OAS molecule to produce cysteine [5]. Maxwell and Kieber indicated the relevance of methionine to the biosynthesis of growth regulating substances, e.g., cytokinins, auxins and brassinosteroids in plants [6]. Broccoli (Brassica oleracea var. italica) crops require high supplies of N for optimum yield [7]. It is known that fertilization is critical for successful Broccoli production. The aim of the present study was to investigate the effect of different sources of amino acids on growth, yield and some physical and chemical properties of the Broccoli plant. Amino acids can be directly absorbed by the roots and display positive interactions between amino acids and micronutrients in the rhizosphere which facilitate absorption by the roots. Spraying with amino acids led to an increase in the absorption and a decrease in leaching of nitrogen from the soil. The use of amino acids alanine, serine, tyrosine and phenylalanine through the hydroponic culture of tomatoes increases the concentration of calcium, potassium, magnesium, iron, copper and manganese in the plant. By removing amino acids, nutrient absorption is facilitated from the roots. This corresponds to a change in the amino acid which conforms it to enter the cell membrane elements [8]. The objective of this research was to determine the effects of replacement of NPK with different amino acids on Broccoli growth and some chemical properties of Broccoli transplant.
Materials and Methods
The experiments were conducted at Shiraz University in 2013. The ‘Green Parasol’ cultivar seeds were purchased from Takii Seed Company, Kyoto, Japan and were used as experimental material. The cultivar is early maturing, with uniform, dark green heads. L-Cysteine and L- methionine were supplied from Merck Company. The amino acid- bearing fertilizer was supplied by Bazargankala. The seeds were sown in pots containing peat and perlite (60:40). Seven treatments were arranged in a completely randomized design with three replications and 24 plants were used for each replication. The data were analyzed through analysis of variance technique using SAS 9.1.3 portable by SAS in situ procedures. The variables were subjected to separation of the mean values using the LSD test (p < 0.05). Physical and chemical properties of the transplants were measured during the 4-leaf-stage of seedlings which corresponded to the end of the fifth week after sowing. Experiment included 7 treatments with 3 replicates [9]. Chlorophyll and nitrogen contents of the plant determined according to the methods described by. Plant samples were taken randomly to measure growth criteria such as leaf area, fresh and dry weights of the root and shoot and chlorophyll and nitrogen contents of the plant. The samples were taken 35 days after planting.
Transplant treatments
1- Foliar application of cysteine at 100 mg L−1.
2- Foliar application of cysteine at 200 mg L−1.
3- Foliar application of methionine at 100 mg L−1.
4- Foliar application of methionine at 200 mg L−1.
5- Plus amino-acid-bearing fertilizer at 100 mg L−1.
6- Plus aminoacid- bearing fertilizer at 200 mg L−1.
7- Not foliar application (control).
Results and Discussion
The amino acid treatment increased all of the measured parameters. Amino acids contribute to the synthesis of growth hormones; therefore, it can be concluded that an increase in cell division and cell enlargement is the reason behind enhanced growth parameters. The biosynthesis of chlorophyll and proteins necessitates more nitrogen either from amino acids or via enhanced nitrogen uptake from the soil. Amino acid increase the dry weight of onion plants [10]. Amino acids may play an important role in plant metabolism and protein assimilation which is necessary for cell formation and consequently increase the fresh and dry matter [11]. This could be attributed to the fact that organic matter is vital for the production of plant dry matter, and the same goes for many amino acid compounds which regulate photosynthesis and plant production [12]. Amino acids are involved in the process of chlorophyll synthesis [13]. Results show that leaf area was increased by all treatments, and amino acids had a significant positive effect in this respect. The positive effect of amino acids can be explained by several reasons. Firstly, the role of amino acids in maintaining the structure of proteins required for cell division. Secondly, amino acids help plant cells to divide and enlarge by entering the hormone structures and, thirdly, the ability of amino acids to be converted to polyamines which are effective in cell division, differentiation and growth [14]. Amino acids can be effective by entering biological cycles like citric acid [15]. The use of putrescine and glutathione increased leaf area in the onion plant [13]. Phenylalanine and tryptophan increases leaf area in Philodendron [16]. Some amino acids are known as pre-factors of phytohormones. Furthermore, amino acids participate in making amines, alkaloids, enzymes and vitamins. They stimulate cell growth and serve as a buffer, and can also be sources of carbon and energy [10]. Amino acids can be converted to polyamines during cell division, differentiation and growth [14]. Methionine is the precursor of growth regulators such as cytokinin, auxin and brassinosteroids [6]. Auxin increases rooting [17]. Methionine increased the dry weight of shoots in corn [18]. Spraying with proline and tyrosine increased the length and dry weight of beetroot [19]. Cysteine plays a vital role in the cytosol and the mitochondria of plant cells. Its presence is required for the development of hairy roots [20]. Amino acids are involved in the biosynthesis of chlorophyll [21]. The increase in photosynthetic pigments may be due to the incorporation of amino acids in anabolic processes and also due to enhancements of metabolic efficiencies in the plant [22]. The spraying of ornithine, phenylalanine and proline on chamomile increased plant height [23]. This effect of amino acids may be due to the stimulation of gibberellins biosynthesis. Foliar applications of amino acid increases the protein, nitrogen, copper and manganese in the tissues of the garlic plant [11]. Treatment with methionine increases the absorption of nitrogen, phosphorus and potassium in corn [18]. Since methionine plays a role in the synthesis of auxin and since auxin increases the initiation of roots, it can help to absorb more nutrients by the plant. The assimilation of nitrate requires the activity of several specific proteins: a membrane-bound transporter, nitrate reductase (NR), nitrite reductase (NiR), glutamine synthase (GS), glutamine oxoglutarate aminotransferase (GOGAT) and a number of trans-aminases involved in the synthesis of further amino acids. Amino acids that move freely in the cytosol partake in nitrogen metabolism, whereby nitrogen is transferred between cells and organs. In addition to their structural role in proteins, amino acids are the starting point for the synthesis of pyrimidines (aspartate), purines (glutamine and glycine), growth regulators (tryptophan, methionine) and many secondary metabolites. Amino acids also engage in osmotic regulation and provide a link between the nitrogen and carbon metabolic pathways [24]. Amino acid can increase the uptake of nitrogen, phosphorus and potassium. Research has demonstrated that plants can absorb and use amino acids [25]. Gibberellic acid and indole acetic acid both increase in their amounts when amino acids are sprayed on the plant [26].
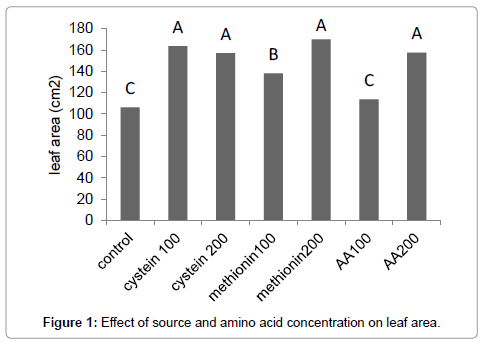
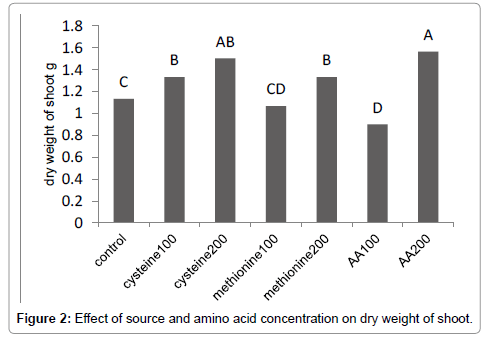
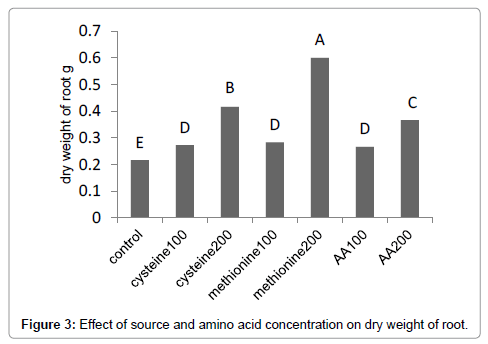
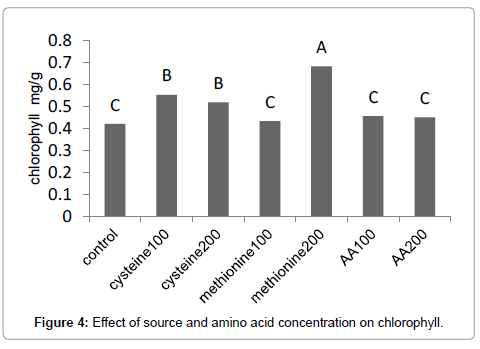
Results (Figure 1) showed that methionine at 200 mg L-1, increased the leaf area by 59%. The amino-acid-bearing fertilizer treatments at a concentration of 200 mg L-1 increased the shoot dry weight of Broccoli seedlings by 38% (Figure 2). Results (Figure 3) showed that methionine at 200 mg L-1 increased the root dry weight by 177%. L-methionine at the concentration of 200 mg/L established the highest chlorophyll content in Broccoli seedlings so much so that a 61% increase in total chlorophyll content was observed (Figure 4). The amino-acid-bearing fertilizer at a concentration of 200 mg L-1 was observed to increase the length of transplants by 30%, compared to the control (Figure 5). The results (Figure 6) showed that, among the treatments, L-methionine at concentrations of 100 and 200 mg/L increased the amount of nitrogen by 36% and 38% respectively.
Conclusions
The obtained results from the present research indicated that foliar application of amino acid, at suitable concentrations, had positive effects on the physical and chemical properties of the Broccoli transplants. These effects strengthened the role of the applied amino acids in the metabolism of broccoli plants.
References
- Sermenli T, Mavi K, Yilmaz S (2011) Determination of transplanting dates of Broccoli (Brassica oleracea L var italica plenck) under Antakya conditions. Journal of Animal and Plant Sciences 21:638-641.
- Mohamed E, Mohamed S (2012) Improvement the Growth and Quality of Green Onion (Allium cepa L.) Plants by Some Bioregulators in the New Reclaimed Area at Nobaria Region, Egypt. New York Science Journal 5:114-120.
- Hell R, Dahl C, Knaff D, Leustek T (2008) Sulfur metabolism in phototrophic organisms. Biologia Plantarum 53: 538.
- Bonner ER, Cahoon RE, Knapke SM, Jez JM (2005) Molecular basis of cysteine biosynthesis in plants structural and functional analysis of o-acetylserine sulfhydrylase from Arabidopsis thaliana. Journal of Biological Chemistry 280: 38803-38813.
- Ãlvarez C, Ãngeles BM, Romero L, Gotor C, GarcÃa I (2012) Cysteine homeostasis plays an essential role in plant immunity. New Phytologist 193: 165-177.
- El-Awadi M, El-Bassiony A, Fawzy Z, El-Nemr M (2011) Response of Snap Bean (Phaseolus vulgaris L) plants to nitrogen fertilizer and foliar application with methionine and tryptophan. Nature and Science 9:87-94.
- Diniz E, Santos R, Urquiaga S, Peternelli L, Barrella T, et al. (2007) Green manure incorporation timing for organically grown Broccoli. Pesquisa Agropecuária Brasileira 42:199-206.
- Garcia A, Madrid R, Gimeno V, Rodriguez-Ortega W, Nicolas N, et al. (2011) The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Spanish Journal of Agricultural Research 9: 852-861.
- Tabatabaee(2013) Mineral Nutrition of Plants. Tabriz University Press, 1: 544.
- Shafeek M, Helmy MS (2012) Response of onion plants to foliar application of sources and levels of some amino acid under sandy soil conditions. Journal of Applied Sciences Research 8: 5521.
- Fawzy Z, El-Shal Z, Yunsheng L, Zhu O, Sawan O (2012) Response of Garlic (Allium sativum L) Plants To Foliar Spraying of Some Bio-Stimulants Under Sandy Soil Condition. Journal of Applied Sciences Research 8: 770.
- Khalilzadeh R, Tajbakhsh M, Jalilian J (2012) Effect of foliar application of bio-organic fertilizers and urea on yield and yield components characteristics of mung bean. International Journal of Agriculture: Research and Review 2:639-645.
- Amin A, Gharib F, El-Awadi M, Rashad E (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Scientia Horticulturae 129: 353-360.
- Kakkar R, Nagar P, Ahuja P, Rai V (2000) Polyamines and plant morphogenesis. Biologia Plantarum 43:1-11.
- Heldt HW, Piechulla B (2010). Plant Biochemistry. Academic Press: San Diego, CA, USA.
- Dahab T, El-Aziz N(2006) Physiological effect of diphenylamin and tryptophan on the growth and chemical constituents of Philodendron erubescens plants. World Journal of Agricultural Sciences 2:75-81.
- Taiz L, Zeiger E(1991) Assimilation of mineral nutrients. Plant Physiology, pp: 292-317.
- Chen M, Cheng B, Zhang Q, Ding Y, Yang Z, et al. (2005) Effects of applying L-methionine, L-phenylalanine and L-tryptophan on Zea mays growth and its nutrient uptake. The Journal of Applied Ecology 16:1033-1037.
- El-Sherbeny MR, Teixeira DJA (2013) Foliar treatment with proline and tyrosine affect the growth and yield of beetroot and some pigments in beetroot leaves. Journal of Horticultural Research 21:95-99.
- Romero I, Téllez J, Yamanaka L, Steindel M, Romanha A, et al. (2014) Transsulfuration is an active pathway for cysteine biosynthesis in Trypanosoma rangeli. Parasites & Vectors 7: 197.
- Iida K (2013) Quantitative evaluation of the biosynthetic pathways leading to δ-aminolevulinic acid from the Shemin precursor glycine via the C5 pathway in Arthrobacter hyalinus by analysis of 13C-labeled coproporphyrinogen III biosynthesized from [2-13C] glycine,[1-13C] acetate, and [2-13C] acetate using 13C NMR spectroscopy. Journal of Radioanalytical and Nuclear Chemistry 295:1819-1827.
- Bahari A, Pirdashti H, Yaghubi M (2013) The effects of amino acid fertilizers spraying on photosynthetic pigments and antioxidant enzymes of wheat (Triticum aestivum L.) under salinity stress. International Journal of Agronomy and Plant Production 4:787-793.
- El-Din K, El-Wahed M (2005) Effect of some amino acids on growth and essential oil content of chamomile plant. International Journal of Agriculture And Biology, pp: 376-380.
- Padgett P, Leonard R (1996) Free amino acid levels and the regulation of nitrate uptake in maize cell suspension cultures. Journal of Experimental Botany 47:871-883.
- Hua-Jing W, Liang-Huan W, Min-Yan W, Yuan-Hong Z, Qin-Nan T, et al (2007) Effects of amino acids replacing nitrate on growth, nitrate accumulation, and macroelement concentrations in pak-choi (Brassica chinensis L.). Pedosphere 17: 595-600.
- Talaat I, Bekheta M, Mahgoub M (2005) Physiological response of periwinkle plants (Catharanthus roseus L.) to tryptophan and putrescine. International Journal of Agriculture and Biology 7:210-213.
Citation: Shekari G, Javanmardi J (2017) Effects of Foliar Application Pure Amino Acid and Amino Acid Containing Fertilizer on Broccoli (Brassica oleracea L. var. italica) Transplant. Adv Crop Sci Tech 5: 280. DOI: 10.4172/2329-8863.1000280
Copyright: © 2017 Shekari G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 10974
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 9476
- PDF downloads: 1498