Research Article Open Access
Effects of Fe Deficiency on Organic Acid Metabolism in Pisum sativum Roots
| Nahida Jelali1*, Marc El Beyrouthy2l, Marta Dell’orto3, Mohamed Gharsallil and Wissem Mnif4 | ||
| 1Laboratoire Plant Adaptation to Abiotic Stress, Centre for Biotechnology, Borj-Cedria Technopark (CBBC), BP 901, 2050 Hammam-Lif, Tunisia | ||
| 2Faculty of Agricultural and Food Sciences, Holy Spirit University of Kaslik, P.O. Box 446, Jounieh, Lebanon | ||
| 3Dipartimento of Agricultural and Environmental Sciences-Production, Land, Agroenergia, University of Milan, Via Celoria 2, I-20133 Milan, Italy | ||
| 4LR11-ES31 Biotechnology and Bio-Enhancement of Geo Resources, Higher Institute of Biotechnology of Sidi Thabet Sidi Thabet BiotechPole, 2020, Tunisia Manouba University, Tunisia | ||
| Corresponding Author : | Dr. Nahida Jelali Laboratoire Plant Adaptation to Abiotic Stress Centre for Biotechnology, Borj-Cedria Technopark (CBBC) BP 901, 2050 Hammam-Lif, Tunisia E-mail: nahidajelali@yahoo.fr |
|
| Received January 31, 2013; Accepted February 28, 2013; Published March 06, 2013 | ||
| Citation: Jelali N, Beyrouthy MEl, Dell’orto M, Gharsalli M, Mnif W (2013) Effects of Fe Deficiency on Organic Acid Metabolism in Pisum sativum Roots. Adv Crop Sci Tech 1:102. doi:10.4172/2329-8863.1000102 | ||
| Copyright: © 2013 Jelali N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Iron deficiency induces several responses to iron shortage in plants. Metabolic changes occur to sustain the increased iron uptake capacity of Fe-deficient plants. The aim of this work was to investigate the impact of Fe deficiency on the organic acid metabolism in Pisum sativum roots. For this purpose, seedlings of Pisum sativum (cv. Douce) were grown under controlled conditions, in the presence of iron sufficient (C) or deficient (D) mediums. Our results showed that PEPC activity increased by 290% in root extracts of Fe deficient plants, compared to the control. Citrate concentration increased in Fe deficient Pisum roots (114% of the control). As well, MDH, CS and ICDH activities showed a marked increase in roots subjected to D treatment. However, the extent of stimulation was especially important for MDH and ICDH activities (99% and 150% of the control, respectively). These data suggest that the capacity of Douce cultivar to increase organic acid metabolism enzyme activities under iron deficiency is related to its better Fe-use efficiency, which indicate the tolerance level of this cultivar to iron chlorosis.
| Keywords | |
| Iron deficiency; Pea; PEPC activity, Organic acids, Metabolic responses, Pisum sativum | |
| Introduction | |
| Iron deficiency is a yield-limiting factor, and a worldwide problem in crop production of many agricultural regions, particularly in calcareous soils [1]. Theoretically, total soil-Fe content would be sufficient to meet Fe needs of plants; however, most of the Fe in the soil is present as inorganic forms, poorly available for root, thus exposing the plant to severe deficiency of this nutrient [2,3]. Since Fe is a vital element for plants, as it is essential for the proper functioning of metabolic processes related to electron transport, such as respiration and photosynthesis, and for chlorophyll biosynthesis [4,5]; plants have evolved different adaptive mechanisms to mobilize and increase the availability of Fe from soil [6]. | |
| Under such tricky environmental conditions, dicots and nongraminaceous monocots termed Strategy I plants induce a set of physiological and biochemical responses [7]. As anticipated by Zocchi [8], metabolic responses were shown to be deeply involved in the response to iron deficiency, with particular regard to the central role played by Phosphoenolpyruvate Carboxylase (PEPC). As well, several enzymes of Krebs cycle, especially those involved in the organic acid metabolism, such as Citrate Synthase (CS), Isocitrate Dehydrogenase (ICDH), Fumarase and Aconitase has been assumed to be important for the whole response to Fe deficiency [9], and it has been shown to occur in roots of Strategy I plant species [10]. For this reason, research on carboxylate exudation (e.g. citrate, malate, etc.) has gained interest globally, as carboxylate release into the rhizosphere enhances the availability of Fe, by accessing soluble forms of this nutrient to the plant [11]. It has been shown that root PEPC activity increased in Fe-deficient plants, and correlates with an enhancement of organic compounds accumulation in roots (e.g. organic acids, phenols and amino acids) [10]. In this perspective, organic acids were shown to be the source for H+ released by the roots [12]. Citrate and malate are the main carboxylate anions accumulating in the root tissues and exudates, in response to Fe deficiency [13,14]. | |
| Previous studies investigating the morpho-physiological responses to Fe deficiency in pea showed that Douce cultivar has a tolerant behavior to Fe deficiency [15]. In the present work, using 18-d-old Fedeficient pea plants (Pisum sativum L., cv ‘Douce’), we studied the effect of Fe-deficiency on organic acids and phenols concentrations in roots and exudates, together with the activities of some root enzymes related to the organic acids metabolism (PEPC, MDH, CS and ICDH). | |
| Materials and Methods | |
| Plant material and growth conditions | |
| Pea seeds (cv. Douce) obtained from the Tunisian National Institute of Agronomic Research (INRAT) were sterilized in a saturated solution of calcium hypochlorite (30%) for 2 min, and then abundantly rinsed in distilled water. Seeds were germinated for 6 days on filter paper constantly moistened with 0.1 mM CaSO4 and then grown for 12 days in a continuously aerated nutrient solution (pH adjusted at 6.0 with 1 M KOH), being exposed to 30 μM Fe (Fe-EDTA); thereafter, the plants were transferred for a further week to a Fe-free nutrient solution (Fe-deficient). The nutrient solution was renewed every three days. The composition of the nutrient solution was: (mM) 1.25 Ca (NO3)2, 1.25 KNO3, 0.5 MgSO4, 0.25 KH2PO4 and (μM) 10 H3BO3, 1 MnSO4, 0.5 ZnSO4, 0.05 (NH4)6MO7O24 and 0.4 CuSO4. Two treatments were established as follows: control (presence of 30 μM Fe(III)-EDTA: C); Fe deficiency (D). The pH was adjusted to 6.0 with NaOH, for both C and D treatments. NaHCO3 and CaCO3 were added to the nutrient solution to simulate the effect of a calcareous soil on Fe availability. Aerated hydroponic cultures were maintained in a growth chamber, with a day/ night regime of 16/8 h, 24C/18C regime, PPFD of 200 μmol m-2 s-1 at the plant level, and a relative humidity of 60% in the dark and 80% in the light. | |
| Determination of plant dry weight | |
| To determine the dry weight (DW) of roots, twelve plants from each treatment (n=12) were sampled at the end of the treatment. Roots were dried at 65°C, and dry weight was recorded as grams per plant. | |
| Soluble protein extraction and cytosolic enzyme assays | |
| Soluble protein extraction for measuring enzyme activities was performed, as reported by De Nisi and Zocchi [16]. Roots were homogenized in a mortar 2-4°C in one volume of a buffer containing 50 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 10% (v/v) glycerol, 1 mM EDTA, 14 mM μ-mercaptoethanol and 1 mM PMSF. To avoid or minimize proteolysis, 10 μg Ml-1 leupeptin were added. The homogenate was centrifuged at 13000×g for 15 min, and the supernatant was again centrifuged at 100,000×g for 30 min. The extracted soluble proteins were dialysed against the same homogenization buffer and used for enzyme activity assays directly, or after storing in liquid N2. | |
| Phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) was determined, as reported by De Nisi and Zocchi [16]. Reaction was started by adding aliquots of protein extracts, and the enzymatic assay was performed at 25°C in 1.5 ml final volume. Oxidation of NADH was followed spectrophotometrically at 340 nm. | |
| Malate dehydrogenase (MDH; EC 1.1.1.37) activity was determined with oxaloacetate as a substrate, by measuring the decrease in absorbance at 340 nm due to the enzymatic oxidation of NADH, as reported by López-Millán et al. [17], with some modifications. The reaction was carried out with 5 μL of extract in 0.2 mM NADH, 0.5 mM oxaloacetate and 94 mM phosphate buffer (pH 7.5). The enzyme assay was performed at 26°C and in 1 mL final volume. | |
| Citrate synthase (CS; EC 4.1.3.7) was assayed spectrophotometrically, by monitoring the reduction of acetyl CoA to CoA with 5-50-dithiobis- 2-nitrobenzoic (DTNB) acid at 412 nm. The reaction was carried out with 50 μl of extract in 0.1 mM DTNB, 0.36 mM acetyl CoA, 0.5 mM oxalacetate and 100 mM Tris–HCl, pH 8.1 [17]. | |
| Isocitrate dehydrogenase (ICDH; EC 1.1.1.42) activity was determined by monitoring the reduction of NADP+ at 340 nm, with 50 μL of extract in a reaction mixture containing 3.5 mM MgCl2, 0.41 mM NADP+, 0.55 mM isocitrate and 88 mM imidazole buffer, pH 8.0 [17]. | |
| Root exudate collection | |
| Root exudates were collected, as described by Zocchi et al. [7]. Five plants from each treatment, with four replicates, were transferred to vessels containing 250 ml of distilled water, and root exudates were collected for a period of 4 h. After collection, Micropur was added to the exudates to prevent decomposition of organic matter by microorganisms. The samples were then cooled to 0°C and filtered through filter paper. Solutions containing root exudates were thereafter concentrated by evaporation, and again filtered on a 0.22 μm cellulose acetate filters. This final solution was used for quantification of citrate, malate, and phenols. | |
| Quantification of malate and citrate | |
| At the end of the treatment, entire root systems of 18-day-old plants were excised, rinsed in distilled water, homogenized in the presence of 5 mL of 10% (v/v) perchloric acid and centrifuged for 15 min at 10,000×g. Supernatant pH was brought to 7.5 with 0.5 M K2CO3, to neutralize the acidity and to precipitate the perchlorate. The extract was clarified with another centrifugation at 15,000×g for 15 min. Citric and malic acid contents were determined enzymatically, according to Rabotti et al. [18]. The recovery of both organic acids was more than 90%, as determined by the use of an internal standard. | |
| Statistical analysis | |
| Variance analysis of data (TWO-WAY ANOVA) was performed using the SPSS 10.0 program, and means were separated, according to Duncan’s test at p ≤ 0.05. Data shown are means of twelve (DW), and five (organic acids concentrations and enzyme activities) replicates for each treatment. | |
| Results | |
| Root dry weight | |
| Although grown under Fe-deficient conditions for 7 days, Pisum sativum root DW was not affected by such constraint (Figure 1); no significant decrease was recorded for this plant organ. | |
| PEPC, MDH, CS and ICDH activities | |
| Since the accumulation of organic acids in Fe-deficient plants is attributable to the stimulation of PEPC activity [10], this metabolic enzyme and others with a key role in the organic acid synthesis, were assayed in Douce cultivar roots grown under Fe deficiency conditions. | |
| As shown in table 1, the (D) treatment resulted in a significant increase of PEPC activity in root extracts of Douce cultivar (1.9-fold increase), compared to the control. In addition, the D treatment led to a significant increase of root MDH, CS and ICDH activities in P. sativum plants. The extent of stimulation was especially important for MDH and ICDH activities (99% and 150% of the control, respectively). | |
| Concentration of organic acids in roots and exudates | |
| Under Fe deficiency, the root concentration of citrate and malate underwent a significant increase in Douce cultivar (Table 2). The values recorded were larger for citrate (114% of the control) than malate (90% of the control). As well, citrate and malate concentrations were stimulated in root exudates in Fe deficient P. sativum roots (Table 2). | |
| Discussion | |
| As shown above in our results, Fe deficiency elicited an increase in PEPC activity in P. sativum plants. These results suggest an important role of this enzyme in the adaptation of plant to environmental changes. Similar results were described in other plant species, such as pepper, kiwifruit and sugar beet [13,19,20]. In addition, all the organic acid related enzymes determined in our work (MDH, CS and ICDH) showed a significant increase in their activities, under Fe deficiency conditions in P. sativum. | |
| The extent of stimulation was especially important for MDH and ICDH activities (Table 1). In accordance with our findings, López- Millán et al. [17] reported that the increase in PEPC activity in Fedeficient roots of sugar beet would lead to carbon fixation, accumulation of organic acids, and in turn, to increased activities of ICDH, MDH, and G6PDH enzymes. The extent of stimulation of all enzymes measured was markedly lower than that reported in sugar beet [17], and tomato [21]. These observations could be ascribed to the metabolic differences of these species that may be due to their different Fe efficiency. Iron deficiency has been generally shown to cause increase in the organic acid concentrations in roots, leaves and exudates of different plant species [10]. The current study revealed that Fe deficiency led to an increase of citrate and malate concentration in P. sativum roots (Table 2). Similar results were found in other species, such as barley, sorghum and maize [22]. It has been shown that citrate could be used as a biochemical marker of Fe-chlorosis tolerance, as suggested by Ollat et al. [14] and Rombolà et al. [13]. | |
| Moreover, in the present study, we found small amounts of citrate and malate exuded from P. sativum roots under Fe deficiency conditions (Table 2), compared to the concentration of these organic compounds in roots. Based on model calculations, Jones et al. [23] suggested that even the low exudation of citrate in roots might be sufficient for Fe(III) solubilization, at a rate which would be high enough to cover the plant Fe requirements. On the other hand, another possible explanation could be that pea plants prevent organic acids decrease from the tissue pool, thus increasing the availability of these compounds for translocation through the xylem. | |
| Taken together, the observed changes, mainly the increase in PEPC activity and organic acid concentrations of Pisum roots, may contribute to the iron deficiency stress response. In fact, increased PEPC activity would sustain the carbon replenishment in the tricarboxylic acid cycle, the enhanced synthesis of malic, citric and amino acids [8-24]. Furthermore, since iron deficiency can induce the accumulation of other metals such as Mn, Zn and Cu [25], organic acids could be involved in metal binding to avoid oxidative damage due to other catalytic ions [26]. | |
| Conclusion | |
| In summary, we conclude that Douce cultivar showed several responses characteristics of Strategy I-efficient plants under iron deficiency conditions, such as enhanced root PEPC activity and malic, citric concentrations, in addition to some enzyme activities involved in the organic acid metabolism. These physiological responses could be partly related to tolerance in biochemical and molecular studies, would be helpful to gain more knowledge concerning the regulation of intracellular ion transport, when plants are under Fe deficiency conditions. | |
| Acknowledgements | |
| This work was funded by the Tunisian Ministry of Higher Education, Research and Technology (LR02CB02). Thanks are also to the anonymous reviewers for additional valuable comments. | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
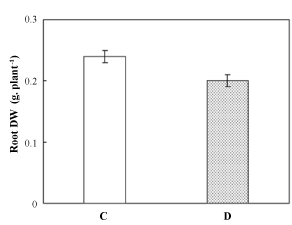 |
| Figure 1 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 16805
- [From(publication date):
May-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11900
- PDF downloads : 4905
