Effects of Cucurbitacins E, D and I on the Gene Expressions of Apoptotic, Autophagic and AKT-Mtor Pathways in SW480 Colorectal Cancer Cells
Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 /
Abstract
Backgorund: Many studies have reported the anticancer effects of cucurbitacins. However, related molecular events need to be described. The current study aims at evaluating the impact of cucurbitacins D, E, and I on death and survival pthways in colorectal cancer (CRC).
Methods: Cell viability was determined by MTT assay in both SW-480 and HT-29 (only cucurbitacin-E). Total RNA was extracted, and the expression of mRNA was quantified by real-time RT-PCR analysis.
Results: The MTT results for SW-480 revealed that cucurbitacins E and I had almost similar potency in different doses, but cucurbitacin-D only had up to almost 50% lethality as a function of dose. Cucurbitacin-E reached IC50 in a very lower dosage at 6 micromolar in HT-29. The expression levels of BAX mRNA were significantly decreased or remained almost unaltered (HT-29) and that of BCL-2 mRNA witnessed a considerable increase (p˂0.05), which is a non-canonical paradigm. What is more, while the expression levels of p53 and AIF mRNA was increased in all treatments of cucurbitacins in SW-480, they were suppressed in HT-29 cells treated with cucurbitacin-E. Caspase-3 expression increased in both colon cell lines. According to expression patern, only cucurbitacin-I had the possibility of suppressing the AKT-mTOR pathway. While autophagy genes were increased in cucurbitacins, cucurbitacin-I decreased the ATG5 expression level.
Conclusion: We noticed that cucurbitacins have the potential to reveal more about both non-canonical interactions of death pathway and BAX/BAk independent apoptosis. These results indicate that cucurbitacins might contribute to BAX/BCL-2-independent cell death in CRC cells.
Keywords
Colorectal cancer; Cucurbitacin; Apoptosis; Autophagy; Gene expression; BAX/BCL-2-independent cell death; AKT-mTOR pathway; Non-canonical death pathway
Introduction
Colorectal cancer is the third most common cancer and the fourth most frequent cause of cancer-related mortality worldwide [1]. Colon carcinogenesis is the result of a gradual transformation of colonic epithelial cells, which accumulates genetic as well as epigenetic changes that both increase their growth and alter their phenotypes [2]. Cucurbitacins are a class of highly oxidized tetracyclic triterpenoids and the various degrees of substitution and saturation allows cucurbitacins to have a variety of chemical compounds; however, they have several common characteristics like presenting a double bond between C-5—C-6, and many of them showing a double bond at C-1 (E and I) and/or C-23 (E, D and I). Cucurbitacin E represents C-25 acetoxyl while cucurbitacins D and I have C-25 hydroxyl. Natural and semi-synthetic cucurbitacins have demonstrated promising anticancer activities ranging from anti-proliferation, cell cycle arrest to induction of apoptosis [3,4].
Some cucurbitacins have been shown to have cytotoxic effects [5,6]. The most critical mechanisms relating to the apoptotic effects of cucurbitacins are their ability to change transcriptional activities (nuclear factors or genes), and also their capability of activating or inhibiting pro- or anti-apoptotic proteins [7,8].
Studies have demonstrated that both classical and non-classical apoptosis pathways exist. Previous findings have indicated that under specific conditions, anti-apoptotic BCL2 family members can be cleaved and thereby converted into pro-apoptotic molecules directly facilitating cytochrome c release [9-11]. What is more, mitochondriamediated apoptosis can be activated in the absence of BAX/BAK.
This situation is significant because in several tumors, resistance to chemotherapy is due to the downregulation of BAX and BAK [12] that reflect a substantial clinical challenge. As such, it is essential to identify novel apoptosis inducers that bypass BAX/BAK.
Some exterior factors were reported that forced cells a non-classical death pathway [13-16]. For instance, Mullauer, F. B., et al. reported that BAX/BAK double-deficient mouse embryonic fibroblasts displayed the release of cytochrome c, caspase activation, DNA fragmentation, and PARP cleavage upon betulinic acid treatment. This result designates that BetA does not induce a classical mitochondrial pathway to apoptosis [15].
It seems counter-intuitive, but several studies have shown that high levels of anti-apoptotic factors correlated with better prognosis in specific cancers. It has shown that a high level of expression of antiapoptotic BCL‑2 is associated with favorable results in some human cancers. Notably, the increase in anti-apoptotic BCL‑2 does not necessarily lead to a decline in apoptotic sensitivity, and indeed the opposite can hold true. In contrast, a high level of expression of proapoptotic BAX can correlate with poor results. Studies demonstrated that high levels of BAX can be associated with decreased survival and increased risk of relapse in all kinds of cancers [17-22]. As we described above, findings challenge the dogma and suggest this viewpoint that anti/pro-apoptotic factors can be served in an unusual direction to inhibit cancer.
The mutant TP53 is in approximately 50% of human cancers. The modified TP53 loses its tumor-suppressive function and obtain new oncogenic activities whether through transcriptional effects on various genes or by protein-protein interactions. Thus, it turns into an active antithetical protein having its own “social network” of interacting proteins and transcriptional targets which endows it with a gain of function (GOF) activities. Tumor cells gain resistance to cell death and become chemoresistance by recruiting the mutant TP53 interacting with proteins such as caspase 3, P300, P73, VDR, etc. [23,24]. SW480 and HT-29 are primary colorectal adenocarcinoma cell-lines with mutant TP53 [25], from which can benefit binding caspase-3 and inhibits its activation.
Autophagy is an intracellular degradation system that delivers cytoplasmic constituents to the lysosome [26]. Autophagy is activated in response to multiple stresses during cancer progressions, such as nutrient starvation, the unfolded protein response (ER stress), and hypoxia; besides, it is observed upon treatment of cancers with a broad spectrum of cytotoxic and targeted chemotherapeutic agents [27].
The mammalian kinase target of rapamycin (mTOR) is a primary regulator of the autophagic process and is regulated by starvation, growth factors, and cellular stressors [28]. Upstream of mTOR the AKT/PTEN pathway modulates mTOR activity. The interplay between the AKT/PTEN/mTOR pathway and the autophagic process is complex, and disruption of the molecular effectors of the negative feedback loop of the AKT/PTEN/mTOR pathway may unbalance the effects towards cell death with several outcomes [29].
In the extensive number of oncological researches, the isolation and purification of biologically active compounds from plants have been increased due to the discovery of potent antitumor drugs with high biodiversity and minimum side effects [30]. We, therefore, set out to compare the cytotoxic effects of the cucurbitacins E, D and I through measuring their IC50 and also by evaluating the expression of some prominent genes in death (apoptotic and autophagic) and survival (Akt/mTOR) pathways to infer (if feasible) the cause of the differences in cytotoxicity effects of these three types of cucurbitacins. The candidate genes were BCL-2, BAX, p53, Aif and caspase-3 for apoptosis pathway, and LC3, Beclin and ATG5 for autophagy as well as Akt, mTOR and PTEN for survival in the signaling pathway.
Materials and Methods
Reagents
Fetal bovine serum (FBS) and RPMI 1640 were bought from Gibco (Denmark). RPMI 1640 supplemented with 2 g/l NaHCO3, 30 mg/l L-glutamine, 100 U/ml penicillin and 10 μg/ml streptomycin (Gibco, Invitrogen, Carlsbad, CA) in pH 7.2 was prepared and after sterilization by 0.2 μm filter kept at 4°C before use. Cucurbitacins D, E, and I were obtained from the stock of our previous purification study [30]. The methanolic extract of E. elaterium fruits was fractionated to petroleum ether, chloroform, and ethyl acetate fractions. The chloroform fraction was chosen for further purification with column chromatography. Finally, cucurbitacins D, E, and I were isolated by column chromatography and identified by NMR spectroscopy. MTT and propidium iodide were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Cell culture and drug treatment
Human colorectal carcinoma cell lines, SW-480 and HT-29 were obtained from the Iranian Biological Resources Center’s Cell Bank (Tehran, Iran). The cells were maintained as monolayers in RPMI medium containing 10% heat inactivated FBS and kept in a fully humidified atmosphere at 37°C with 5% CO2 in the air. The cells (1 × 106) were seeded for 12 h and then treated with various concentrations (0- 60 μM) of cucurbitacins, which were dissolved in RPMI medium. After 24 h cells were detached by trypsinization, pooled by centrifugation, and washed twice with sterile PBS.
MTT assay
Survival evaluation of SW-480 cells was performed by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells (2 × 104 cells/well) were seeded into a 96-well plate (Nunclon, Denmark) in the absence and presence of different concentrations of the drug (0-60 μM). 10 μl of MTT reagent (5 mg/ml in H2O) was added, and the plates were incubated at 37°C with 5% CO2 in the air for four hours in the dark. The violet formazan crystals were dissolved entirely in 150 μl DMSO, and the absorbance measured at 570 nm using a microplate reader (Model Power Wave XS2, Bio Tek, USA). The percentage of surviving cells was calculated from the ratio of the absorbance between treated and untreated cells. The proliferation of the cells is presented as an average (±SD) of at least three independent experiments.
RNA purification, reverse transcription, and real-time RT PCR amplification
After 24 hours of drug treatment, total RNA was isolated from SW-480 cells using TRIzol (Invitrogen) as recommended by the manufacturer and cDNA synthesized using an M-MuLV reverse transcriptase kit (Vivantis, Malaysia) at 42°C for 60 min, followed by 85°C for 5 min. Duplicate PCR reactions were performed using the real-time PCR kit (Jena Bioscience, Germany) according to the manufacturer’s instructions, using GAPDH as normalizers for genes. The cycling program was as a pre-incubation at 95°C for 5 min followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 secs and 72°C for 30 secs which was followed by a final extension step of 72°C, 5 min. Thermal amplification was carried out on a Rotor-Gene 6000 instrument (Corbett, Sydney, Australia). All reactions were run in triplicate, and the expression of the genes was analyzed based on the cycle threshold (Ct) and relative expression levels were determined as 2- [ΔΔC (t)]. The sequences of primers are listed in Table 1.
Statistical analysis
All data were expressed as means ± SD. The student t-test was used to determine statistical significance at p˂0.05. SPSS 22.0 and GraphPad Prism 7 software was used for the statistical analyses.
Results
Cytotoxic effect of Cucurbitacins D, E and I on SW-480 and HT-29 cells
The potential effect of cucurbitacins on the survival rate of colorectal cancer cells was analyzed with MTT assay. Cells in the absence of cucurbitacins D, E, I show more than 90% viability (e.g., 98%, 96% and 97% viability for the absence of cucurbitacins D, E and I respectively). Upon addition of cucurbitacins D, E and I, the cell viability was reduced to 78%, 61% and 62% at 10 μM of cucurbitacins until it reaches 61%, 20% and 25% at 50 μM, respectively. There was a significant decrease (p˂0.01) in the cell viability after cucurbitacins E and I exposure, especially at high concentrations (≥80 μg/ml). The behavior of cucurbitacins E and I are similar, and at approximately 20 μM of cucurbitacins E and I cells reach to IC50; however, cucurbitacins D can only reduce the viability of the cells by 60 % (Figure 1).
Cytotoxic effect of Cucurbitacin-E on HT-29 cells
Cells in the absence of cucurbitacins-E showed more than 90% viability. Cell viability was reduced to 50% at 6 μM, and it reached to 60%, at about 50 μM. There was not a significant decrease in the cell viability at higher concentrations (≥30 μg/ml) (Figure 1).
Figure 1: Effect of cucurbitacins on the viability of SW-480 cells analyzed by MTT assay after exposure to various concentrations of cucurbitacins. The viability of cells was significantly decreased in a dose-dependent manner. The cells at a density of 106 cells/ml were cultured with various concentrations of cucurbitacins. Data are presented as means ± SD of three independent experiments. *p˂0.05 and **p˂0.01 versus control.
Effect of Cucurbitacins on BCL2, BAX, p53, Caspase-3 and AIF mRNA expression in SW-480 cells
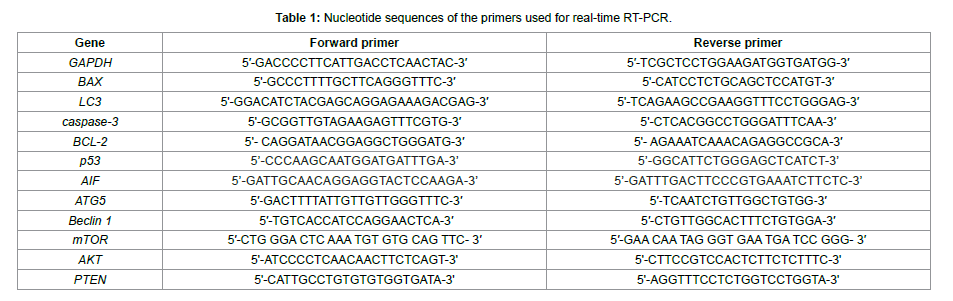
Upon addition of E and I cucurbitacins, p53 mRNA level was increased, but the effect of cucurbitacin D on p53 mRNA expression levels was significantly (p< 0.001) more than that of cucurbitacins E and D with the approximately 7-fold increase in mRNA level. The expression of AIF mRNA in SW-480 cells was increased after exposure to all types of cucurbitacins (Figure 2). The caspase-3 mRNA level of SW-480 cells was significantly increased (p˂0.001) by cucurbitacin treatments in a concentration-dependent manner. Interestingly, the level of BAX mRNA was significantly decreased in SW-480 cells. By contrast, the expression of BCL-2 mRNA was significantly increased (Figure 3).
Figure 2: Effect of cucurbitacin-E on the viability of HT-29 cells analyzed by MTT assay after exposure to various concentrations. The viability of cells was significantly decreased in a dose-dependent manner. The cells at a density of 106 cells/ml were cultured with various concentrations of cucurbitacins. Data are presented as means ± SD of three independent experiments. *p˂0.05 and **p˂0.01 versus control.
Figure 3: The relative expression level of BCL-2, BAX, p53, AIF, and Casp- 3 mRNA was examined by real-time RT-PCR. Upon addition of cucurbitacins E, D and I, p53, Bcl-2, caspase-3, and AIF mRNA level was increased, and the level of BAX mRNA was significantly decreased. GAPDH was chosen as an internal control. The expression of the genes was analyzed based on the cycle threshold (Ct), and relative expression levels were determined as 2-[ΔΔCt]. Data are presented as means ± SD of three independent experiments * indicates significant difference from control; *p˂0.05, **p˂0.01, ***p˂0.001
Effect of Cucurbitacin-E on BCL2, BAX, p53, Caspase-3 and AIF mRNA expression in HT-29 cells
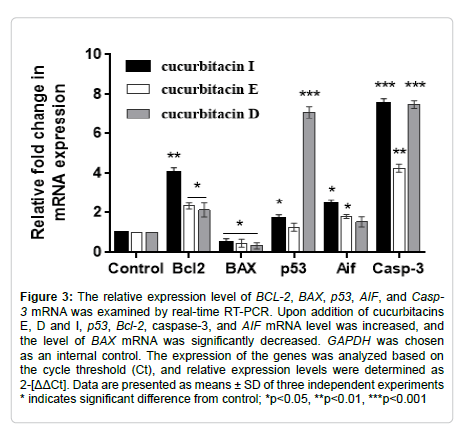
We evaluated just apoptotic gene expressions for only HT-29 cells treated with cucurbitacin-E to have a comparative apoptotic gene analysis in a different primary cell line. Upon addition of cucurbitacins E, p53 mRNA was downregulated. The expression of AIF mRNA was also decreased after exposure to cucurbitacin-E (Figure 4). The expression of caspase-3 was considerably increased (p˂0.05), while that of BAX significantly dropped. Conversely, BCL-2 mRNA was significantly increased (Figure 4).
Effect of Cucurbitacins on ATG5, LC3 and Beclin-1 mRNA expression in SW-480 cells
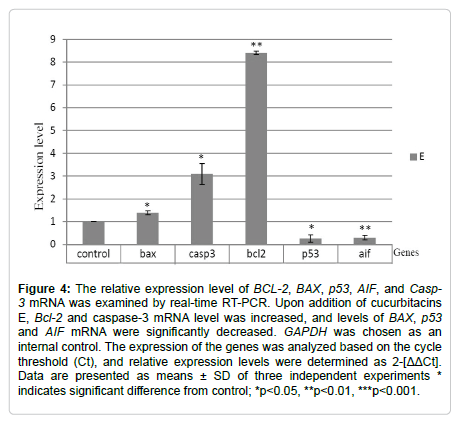
After the treatment of the SW-480 cells with cucurbitacins, the expression of LC3 and Beclin-1 mRNA was increased. Upon addition of cucurbitacin D and E, the expression of ATG5 mRNA increased, but the effect of cucurbitacin I on ATG5 mRNA expression was different with an approximately 3-fold decrease in mRNA levels (Figure 5).
Figure 4: The relative expression level of BCL-2, BAX, p53, AIF, and Casp- 3 mRNA was examined by real-time RT-PCR. Upon addition of cucurbitacins E, Bcl-2 and caspase-3 mRNA level was increased, and levels of BAX, p53 and AIF mRNA were significantly decreased. GAPDH was chosen as an internal control. The expression of the genes was analyzed based on the cycle threshold (Ct), and relative expression levels were determined as 2-[ΔΔCt]. Data are presented as means ± SD of three independent experiments * indicates significant difference from control; *p˂0.05, **p˂0.01, ***p˂0.001.
Figure 5: The relative expression level of ATG5, LC3, and Beclin-1 mRNA was examined by real-time RT-PCR. After treatment of the SW-480 cells with all types of cucurbitacins, the expression of LC3 and Beclin-1 mRNA was increased. Upon the addition of cucurbitacins, the expression of ATG5, LC3, and Beclin-1 mRNA was increased, but the effect of cucurbitacin I on ATG5 mRNA expression was so different with an approximately 3-fold decrease in mRNA levels. GAPDH was chosen as an internal control. The expression of the genes was analyzed based on the cycle threshold (Ct), and relative expression levels were determined as 2-[ΔΔCt]. Data are presented as means ± SD of three independent experiments * indicates significant difference from control; *p˂0.05, **p˂0.01, ***p˂0.001.
Effect of Cucurbitacins on AKT, mTOR and PTEN mRNA expression in SW-480 cells
After treatment of cells with cucurbitacins D and E, the mRNA expression of AKT and mTOR was increased, and the expression of PTEN mRNA was decreased. However, the expression pattern of AKT and PTEN was different after the treatment of SW-480 cells with cucurbitacin I. Upon addition of cucurbitacin I, the expression of AKT and PTEN was increased and the expression of mTOR mRNA was decreased (Figure 6).
Figure 6: The relative expression level of AKT, mTOR and PTEN mRNA was examined by Real-time RT-PCR After treatment of cells with cucurbitacins D and E, the mRNA expression of AKT and mTOR was increased, and the expression of PTEN mRNA was decreased. However, after the addition of cucurbitacin I, the expression of AKT and PTEN was increased, and the expression of mTOR mRNA was decreased. GAPDH was chosen as an internal control. The expression of the genes was analyzed based on the cycle threshold (Ct), and relative expression levels were determined as 2-[ΔΔCt]. Data are presented as means ± SD of three independent experiments * indicates significant difference from control; *p˂0.05, **p˂0.01, ***p˂0.001
Discussion
MTT results demonstrated that the IC50 for cucurbitacins E, I and D in SW-480 were 20, 20 and 40 μM respectively. Cucurbitacins E and I had almost similar cytotoxicity effect as a function of increasing their doses and also were more fatal to SW-480 CRC cells than cucurbitacin D. Unlikely, with increasing the dosage of cucurbitacin D, lethality went only up to 50% compared to 70% (cucurbitacin E) and 65% (cucurbitacin I). One reason why cucurbitacin D was less potent, would be the increase in the expression of AKT (1.9-fold) and mTOR (2.6 fold), and fall in the expression of PTEN (0.8 fold) which are in favor of the survival pathway.
Wild-type TP53 plays a significant role in suppressing tumorigenesis by inducing genomic stability, cell cycle arrest, or apoptosis while mutant TP53 resists to cell death and apoptosis through binding and interacting with various proteins like caspase-3 [23,24]. P53 is mutant in SW-480 and the expression level of P53 (7 fold) in the cucurbitacin D treated cells was much more than E (expression levels was similar to control) and I (1.6 fold) treated cells, so this could be another explanation why cucurbitacin-D was less potent. Thus, it implicates that although cucurbitacin D treated cells induced caspase-3 gene expression 5 fold compared to control, this treated cells became resistant to cucurbitacin D through suppressing of caspase-3 by mutant p53. To study the cytotoxicity effects of the cucurbitacins and the influence of p53 in a different scenario, we exposed another colorectal primary cancer cell line HT-29 to cucurbitacin E, which similarly has mutant p53. we noticed that although the expression levels of caspase-3 in HT-29 was lower than that of SW-480 under the influence of cucurbitacin E, it reached to IC50 in a lower dosage (6 μM), and interestingly, evaluation of the gene expression of p53 indicated that it was nearly suppressed with the 0.25 of expression level compared to non-treated HT-29 cells. Thus, the HT-29 cell line might miss the function of mutant p53 as a survival factor. Assessing the AKT-mTOR survival pathway demonstrated that cucurbitacin-I almost suppressed the expression of mTOR (just under 0.5), and AKT was expressed nearly in a similar quantity of control (1.28), while PTEN was upregulated 2.5-fold which overall is against the survival pathway. In cucurbitacin E, there was not any considerable tendency in favor or against of survival pathway compared to control.
Apoptosis pathway examination in SW-480 showed an unusual gene expression model. It was with the characteristics of upregulating BCL-2 and downregulating BAX, while caspase-3 and AIF were upregulated. As we explained earlier, the overexpression of BCL-2 and downregulation or suppression of BAX can be against cancer cell survival (9-22). The result for HT-29 cell line treated with cucurbitacin-E is in agreement with this idea, since even though the BCL-2 expression status was higher (8 fold), the IC50 was at a lower dosage (about 6 μM), and surprisingly the expression levels of caspase-3, on the other hand, was lower (3 fold) compared to the results of all three types of cucurbitacins in the SW-480 cell line. Our previous flow cytometry results demonstrated that purified cucurbitacins D, E and I induced apoptotic cell death in the human gastric cancer cell line (AGS). However, they showed a negligible effect on the BAX mRNA level [31].
The autophagy gene expressions showed that cucurbitacin E and D treated cells in SW-480 upregulated the expression levels of LC-3, Becline-1 and ATG5 which is in favor of autophagic cell death. On the other hand, ATG5 was suppressed in cucurbitacin-I treated cells, and therefore autophagic cell death seemed unlikely to happen. This might be one reason that although caspase-3 expression was the highest in treatment with cucurbitacin-I, and conversely cucurbitacin-E had the lowest expression levels of caspase-3 (just over 4), there were no considerable differences in their MTT results.
AIF is a mitochondrial protein, which can participate in caspaseindependent apoptosis. AIF gene is a transcriptional target of p53 [32,33]. The effect of cucurbitacin-E on the HT-29 cell line caused the suppression of p53 expression, and thus the AIF gene had no expression. Conversely, the p53 gene was expressed in all treatments of SW-480, which was followed by the expression of AIF. These results illustrate that SW-480 capable of recruiting AIF to respond to caspaseindependent apoptosis while HT-29 was not able to make it.
Conclusion
A better understanding of the mechanisms of BAX/BCL-2- independent cell death is crucial because various tumor cell lines have been shown to resist classical mitochondrial death pathways, as they lacking BAX or p53, or harboring mutations of these proteins which fail to respond to chemotherapeutic drugs and death ligands. Agents that overcome drug resistance in this type of cancer are of particular interest in drug development and cancer therapy. What is more, these results and other findings challenge the viewpoint that a pro- or antiapoptotic factor serves solely to inhibit or promote cancer, arguing instead that the factors in the apoptosis pathway have a dark side that can actually be served in their opposite direction. Thus, fundamental research on this unusual and specific network of interactions could be promising in clinical settings. Cucurbitacins seem to have the potential to understand more about unusual interactions of apoptotic factors in cellular pathways and could also be more investigated for BAX/BAK independent apoptosis.
Acknowledgment
This study was supported by a grant from the University of Tehran dedicated to the master’s dissertation of Mohammad Reza Sheikhi. We thank Dr. Naser Jafargholizadeh for helping with technical editing.
Declaration of interest
This work was a thesis subject confirmed by the University of Tehran. The author(s) declare that there is no conflict of interest. The authors alone are responsible for the content of the paper.
References
1. Haggar, F. A., & Boushey, R. P. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg., 2009; 22(4): 191.
2. Vaiopoulos, A. G., Athanasoula, K. C., & Papavassiliou, A. G. Epigenetic modifications in colorectal cancer: molecular insights and therapeutic challenges. Biochim Biophys Acta., 2014; 1842(7): 971-980.
3. Chung, S. O., Kim, Y. J., & Park, S. U. An updated review of cucurbitacins and their biological and pharmacological activities. Excli J., 2015; 14: 562-566.
4. Perez Gutierrez, R. M. Review of Cucurbita pepo (pumpkin) its phytochemistry and pharmacology. Med chem., 2016; 6(1): 012-021.
5. Witkowski, A., Woynarowska, B., & Konopa, J. Inhibition of the biosynthesis of deoxyribonucleic acid, ribonucleic acid and protein in HeLa S3 cells by cucurbitacins, glucocorticoid-like cytotoxic triterpenes. Biochem Pharmacol., 1984; 33(7): 995-1004.
6. Duncan, M. D., & Duncan, K. L. Cucurbitacin E targets proliferating endothelia. J Surg Res., 1997; 69(1): 55-60.
7. Alsayari, A., Halaweish, F., & Gurusamy, N. The role of cucurbitacins in combating cancers: A mechanistic review. Pharmacogn Rev., 2018; 12(24): 157.
8. L Rios, J., Andújar, I., M Escandell, J., M Giner, R., & C Recio, M. Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds. Curr Pharm Des., 2012; 18(12): 1663-1676.
9. Grimm, S., & Brdiczka, D. The permeability transition pore in cell death. Apoptosis., 2007; 12(5): 841-855.
10. Jonas, E. A., Hickman, J. A., Chachar, M., Polster, B. M., Brandt, T. A., Fannjiang, Y. et al. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci., 2004; 101(37): 13590-13595.
11. Kirsch, D. G., Doseff, A., Chau, B. N., Lim, D. S., de Souza-Pinto, N. C., Hansford, R., et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem., 1999; 274(30): 21155-21161.
12. Westphal, D., Dewson, G., Czabotar, P. E., & Kluck, R. M. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta, 2011; 1813(4): 521-531.
13. Urban, C., Rheme, C., Maerz, S., Berg, B., Pick, R., Nitschke, R., et al. Apoptosis induced by Semliki Forest virus is RNA replication dependent and mediated via Bak. Cell Death Dis., 2008; 15(9): 1396-1407.
14. Murphy, A. M., Sheahan, B. J., & Atkins, G. J. Induction of apoptosis in BCL‐2‐expressing rat prostate cancer cells using the Semliki Forest virus vector. Int J Cancer., 2001; 94(4): 572-578.
15. Mullauer, F. B., Kessler, J. H., & Medema, J. P. Betulinic acid induces cytochrome c release and apoptosis in a Bax/Bakindependent, permeability transition pore dependent fashion. Apoptosis., 2009; 14(2): 191-202.
16. Mizuta, T., Shimizu, S., Matsuoka, Y., Nakagawa, T., & Tsujimoto, Y. A Bax/Bak-independent mechanism of cytochrome c release. J Biol Chem., 2007; 282(22): 16623-16630.
17. Ichim, G., & Tait, S. W. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer., 2016; 16(8): 539.
18. Shinoura, N., Yoshida, Y., Nishimura, M., Muramatsu, Y., Asai, A., Kirino, T., et al. Expression level of Bcl-2 determines anti-or proapoptotic function. Cancer Res., 1999; 59(16): 4119-4128.
19. Kaparou, M., Choumerianou, D., Perdikogianni, C., Martimianaki, G., Kalmanti, M.,Stiakaki, E. Enhanced levels of the apoptotic BAX/BCL-2 ratio in children with acute lymphoblastic leukemia and high-risk features. Genet Mol Biol., 2013; 36(1): 7-11.
20. Hogarth, L. A., & Hall, A. G. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood., 1999; 93(8): 2671-2678.
21. Köhler, T., Schill, C., Deininger, M. W., Krahl, R., Borchert, S., Hasenclever, D., et al. High Bad and Bax mRNA expression correlate with negative outcome in acute myeloid leukemia (AML). Leukemia., 2002; 16(1): 22-29.
22. Bairey, O., Zimra, Y., Shaklai, M., Okon, E., & Rabizadeh, E. Bcl-2, Bcl-X, Bax, and Bak expression in short-and long-lived patients with diffuse large B-cell lymphomas. Clin Cancer Res., 1999; 5(10): 2860-2866.
23. Stein, Y., Rotter, V., & Aloni-Grinstein, R. Gain-of-function mutant p53: All the roads lead to tumorigenesis. Int J Mol Sci., 2019; 20(24): 6197.
24. Frank, A. K., Pietsch, E. C., Dumont, P., Tao, J., & Murphy, M. E. Wild-type and mutant p53 proteins interact with mitochondrial caspase-3. Cancer Biol Ther., 2011; 11(8): 740-745.
25. Ahmed, D., Eide, P. W., Eilertsen, I. A., Danielsen, S. A., Eknaes, M., Hektoen, M., et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis, 2013; 2(9): e71-e71.
26. Mizushima, N. Autophagy: process and function. Genes Dev., 2007; 21(22): 2861-2873.
27. Chen, N., & Debnath, J. Autophagy and tumorigenesis. FEBS let., 2010; 584(7): 1427-1435.
28. Kim, Y. C., & Guan, K. L. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest., 2015; 125(1): 25-32.
29. Tapia, O., Riquelme, I., Leal, P., Sandoval, A., Aedo, S., Weber, H., et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Archiv, 2014; 465(1): 25-33.
30. Dias, D. A., Urban, S., & Roessner, U. A historical overview of natural products in drug discovery. Metabolites, 2012; 2(2): 303-336.
31. Jafargholizadeh, N., Zargar, S. J., & Aftabi, Y. The cucurbitacins D, E, and I from Ecballium elaterium (L.) upregulate the LC3 gene and induce cell-cycle arrest in human gastric cancer cell line AGS. Iran J Basic Med Sci., 2018; 21(3): 253.
32. Stambolsky, P., Weisz, L., Shats, I., Klein, Y., Goldfinger, N., Oren, M., et al. Regulation of AIF expression by p53. Cell Death Differ., 2006; 13(12): 2140-2149.
33. Daugas, E., Susin, S. A., Zamzami, N., Ferri, K. F., Irinopoulou, T., Larochette, N., et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J., 2000; 14(5): 729-739.
Citation: Sheikhi MR, Zargar SJ, Zia A (2021) Effects of Cucurbitacins E, D and I on the Gene Expressions of Apoptotic, Autophagic and AKT-Mtor Pathways in SW480 Colorectal Cancer Cells. Cell Mol Biol 67: 164.
Copyright: © 2021 Sheikhi MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1811
- [From(publication date): 0-2021 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 1022
- PDF downloads: 789