Research Article Open Access
Effects of Cleome gynandra Linn: Leaf Extract on Ovarian Folliculogenesis of Albino Mice
Jupitara Deka* and J C KalitaDepartment of Zoology, Gauhati University, Guwahati-781014, Assam, India
- Corresponding Author:
- Jupitara Deka
Department of Zoology, Gauhati University, Guwahati-781014, Assam, India
E-mail: jdjupitara1@gmail.com
Received Date: October 14, 2016; Accepted Date: October 26, 2016; Published Date:November 02, 2016
Citation: Deka J, Kalita JC (2016) Effects of Cleome gynandra Linn: Leaf Extract on Ovarian Folliculogenesis of Albino Mice. J Tradi Med Clin Natur 5:196.
Copyright: © 2016 Deka J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
It is well known that the plant kingdom contains numerous bioactive substances affecting the regulation of reproduction. Cleome gynandra plant extracts contain phytoestrogenic compounds. These compounds act as agonist or antagonist estrogen receptors, thus affecting the steroid hormones level. In traditional medicine, Ceome gynandra is used by lactating females for enhancement of milk and as a housing drug. The aim of this study was to investigate the effects of methanolic extract of C. gynandra leaves on the folliculogenesis of female albino mice. The effect of C. gynandra methanolic leaf extracts on folliculogenesis was studied in sixteen (N=16) sexually matured female albino mice with regular oestrus cycle. Mice were randomly divided into four (4) groups of four (n=4) mice per group. The experimental groups were treated as follows: Group I treated with 250 mg/kg, Group II with 500 mg/kg, Group III with 0.01 mg/kg 17β estradiol and Group IV with 1% Tween 80 (control). Follicular growth and changes were studied through standard histological protocols. For each ovary, every 12th and 20th section was examined for counting primordial, primary, secondary, graafian and atretic follicles, respectively to obtain an overall view of the follicular populations per ovary. In this experiment, the dose of 500 mg/kg BW/day showed a significant (p<0.05) decrease in primordial, primary, secondary and Graafian follicle compared to that of normal control mice. Significant increase in the number of atretic follicles was recorded in dose of 500 mg/kg BW/day compared to normal control mice. The dose of 250 mg/kg BW/day showed a similar decrease in primordial, primary, secondary and Graafian follicle compared to that of control mice (p<0.05). 17β Estradiol treated group showed a statistically significant (p<0.05) decrease in number of primordial, primary, secondary and Graafian follicle compared to that of normal control mice.
Keywords
Cleome gynandra, Folliculogenesis, Phytoestrogen, Nutraceuticals
Introduction
The relationship between female fertility and ovarian follicle development is well recognized [1]. Studies in mice [2] and rats [3,4] suggest that differential follicle counts may provide a sensitive means of estimating the extent of ovarian toxicity in females exposed to xenobiotics. As reported in a preliminary study [5] a three stage classification system based on follicle diameter and structure [6] as adapted by Mattison, et al. [7] appears to provide a quantifiable screening procedure for use in subchronic toxicity bioassays.
In 1989, DeFelice hypothesised the occurrence of biological interventions not related to pharmacological methods and wrote about ‘‘nutraceutical’’ products, i.e., ‘‘a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease’’ [8,9]. The original hypothesis was that these foods can protect the human body from adverse events because of the beneficial effects of some phytochemicals. Several studies have reported the validity of this idea in clinical practice [10-12]. Certain synthetic or natural compounds present in the environment mimic, enhance or inhibit endogenous hormones. These compounds are called as environmental estrogens [13]. These chemicals have been a source of concern because of their possible health threats to human being in particular. These are also called as xenoestrogens which cause change in cellular function of animals’ body by binding with estrogen receptor sites [14,15]. These chemicals present in the environment interfere in the biosynthetic pathway of the endogenous hormones or modifying hormone metabolism thereby which an animal maintain a normal homeostatic system with which it responds to its surrounding environment.
Estrogens inhibit mouse oocyte nest breakdown and follicle assembly [16]. Estrogenic action reduces follicle assembly leading to fewer primary and subsequent developing follicles. Thus, the study of follicular populations provides important information about the function of the ovary, in particular the relationship between folliculogenesis and also environmental factors having estrogenic property that regulate it [17].
Plant kingdom contains numerous bioactive substances affecting the regulation of reproduction in animals and humans. Cleome gynandra plant extracts contain numerous bioactive compounds. There are reports that these compounds act as agonist or antagonist of estrogen receptors, thus affecting the steroid hormones level. In traditional medicine, C. gynandra was used by lactating females for enhancement of milk and as a housing drug. The aim of the present study was to investigate the effects of methanolic extract of C. gynandra leaves on the ovarian folliculogenesis in albino mice (Figure 1).
Materials and Methods
Collection of plant materials: Fresh leaves of C. gynandra were collected from different parts of Kamrup district. They in fresh condition were washed under running tap water and then again with distilled water. The plant material was air dried in the shade for 5 days and then homogenized to fine powder and stored in airtight bottles with proper labeling.
Preparation of extract: Powdered plant materials were collected and weighed carefully. A 50 g of the plant material was weighed and soaked in 300 mL of methanol. The mixture was kept in shaker for 48 h and filtered. The filtrate was kept in a rotary evaporator in low temperature under reduced pressure till dryness. Extract thus obtained was examined chemically and screened for phytochemical screening. The extract was kept in a refrigerator when not in use.
Treatment procedure and route of administration: Two doses of 250 mg/kg BW and 500 mg/kg BW of plant extracts, respectively were used which corresponds to a 1/12th and 1/6th, respectively of the highest tested dose (3000 mg/kg BW). 1% v/v Tween-80 (P8074, CAS 9005-65-6) which is a polyethylene sorbitol ester was used to prepare the extract suspension of the test plant extract. To prepare the 17β-Estradiol stock solution, the same was dissolved in Ethanol (analytical grade) and it was diluted with normal saline to prepare the desired dose of working solution. Animals were exposed to the test compounds through standard gastric gavages feeding syringe (Feedy-I, FG-05). The doses were administered at 24 h of interval for a period of 21 days.
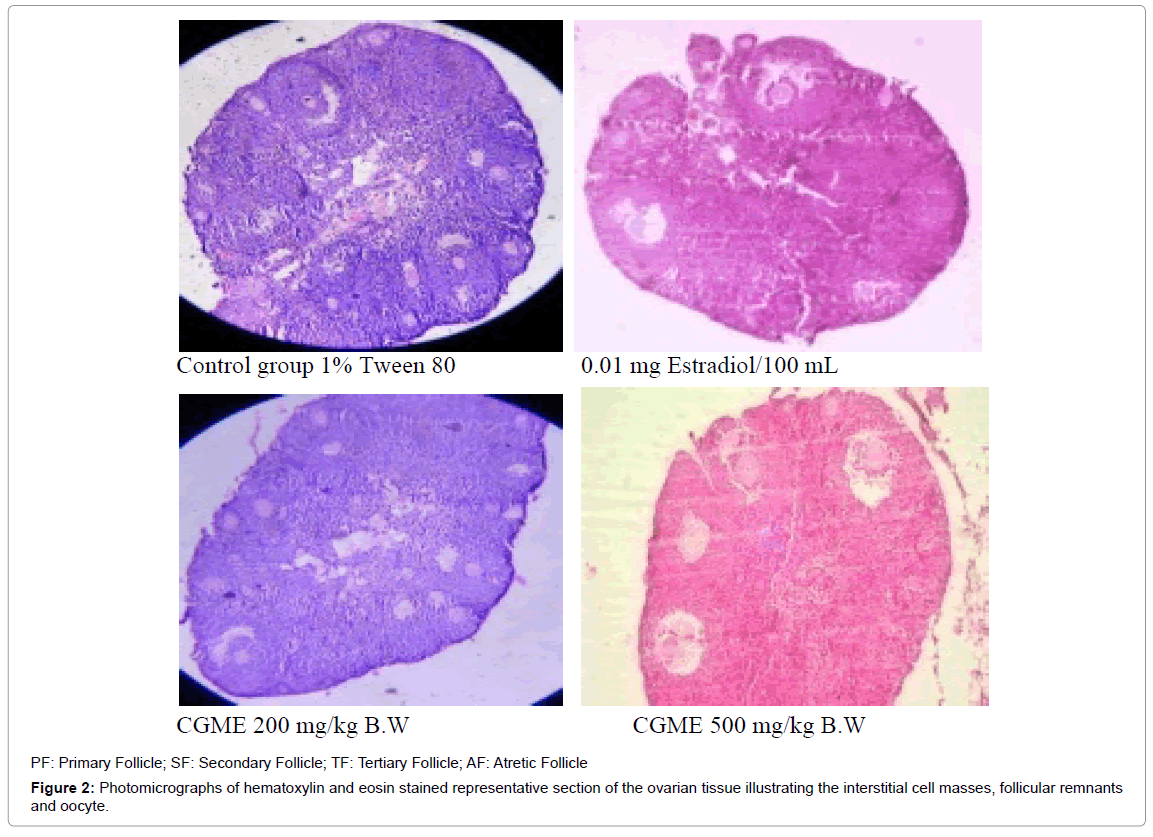
Morphological classification of follicles: Ovaries of five animals of each group were taken for the follicular studies. Ovaries were selected based on the stage and comparability of the weight with respective control ovaries. The ovaries were fixed in Bouin’s fluid, embedded in paraffin and sectioned at 6 μm thickness. The sections were separated for every 10th section and stained with hematoxylin and eosin. Sections of the ovary were examined under a light microscope and the general histologic appearance of the ovary was assessed. All serial sections of the ovary were counted for various stages of development of follicles as described by Moawad, et al., [18,19]. Follicles and atretic follicles were classified according to the method described by Swartz and Mall and Bucci [20,21].
Quantification of follicles: To determine the total population of different types of follicles per ovary the method used by Pedersen and Peters and Butcher and Kirkpatric-Keller were followed [22]. At an average, about 200 serial sections were obtained and for each ovary, every 12th and 20th section was examined for counting smaller (primordial, primary and secondary) and larger (graafian and atretic) follicles, respectively to obtain an overall view of the follicular populations per ovary (Myers et al., [23].
Statistics: Statistical analyses for all the data of animal experimentations were performed using MS Office Excel 2007. The results were expressed as mean ± Standard Error (SE) of mean. The means in both negative as well as positive control versus treated animals were analyzed for significant by Student’s independent t-test distribution. A value of p<0.05 was considered statistically significant for all the tests.
Results
The results of the ovarian follicular counting on treatment with CGME were showed in the Table 1. In this experiment, the dose of 500 mg/kg BW/day showed a significant (p<0.0001) decrease in primordial follicle (892.45 ± 5.97), primary follicle (252.34 ± 4.67), secondary follicle (152 ± 9.78) and Graffian follicle (11.89 ± 2.55) compared to that of normal control mice. Significant increase in the number of atretic follicles (48.12 ± 4.67) was also recorded in dose of 500 mg/kg BW/day compared to normal control mice (18.06 ± 3.27). The dose of 250 mg/kg body weight/day showed a similar decrease in primordial follicle (928.43 ± 4.11), primary follicle (268.51 ± 5.39), secondary follicle (159.72 ± 2.17) and Graffian follicle (16.44 ± 5.13) compared to that of olive oil control mice (p<0.0001). 17β Estradiol treated group showed a statistically significant (p<0.0001) decrease in number of primordial follicle (816.75 ± 44.47), primary follicle (179.42 ± 11.56), secondary follicle (142.51 ± 6.33) and Graffian follicle (12.34 ± 7.21) compared to that of normal control mice (Figure 2).
| Treatment group | Primordial follicle | Primary follicle | Secondary follicle | Graffian follicle | Atretic follicle |
|---|---|---|---|---|---|
| Control, 1% Tween 80 | 983.62 ± 10.52 | 286.37 ± 6.48 | 169.26 ± 8.32 | 19.43 ± 4.86 | 18.06 ± 3.27 |
| Estradiol treated, 0.01mg/100 mL | 816.75 ± 44.47*** | 179.42 ± 11.56*** | 142.51 ± 6.33** | 12.34 ± 7.21** | 52.81 ± 1.57*** |
| Low dose, 250mg/kg BW | 928.43 ± 4.11 | 268.51 ± 5.39 | 159.72 ± 2.17 | 16.44 ± 5.13 | 39.68 ± 2.41*** |
| High dose, 500mg/kg BW | 892.45 ± 5.97*** | 252.34 ± 4.67** | 152.53 ± 9.78 | 13.89 ± 2.55** | 48.12 ± 4.6*** |
Table 1: Effect of CGME on the ovarian follicular population after 21 days of treatment.
Discussion
In this experiment, the dose of 500 mg/kg BW/day showed a significant (p<0.05) decrease in primordial follicle (892.45 ± 5.97), primary follicle (252.34 ± 4.67), secondary follicle (152 ± 9.78) and Graafian follicle (11.89 ± 2.55) compared to that of normal control mice. Significant increase in number of atretic follicle (48.12 ± 4.67) was also recorded in dose of 500 mg/kg BW/day compared to normal control mice (18.06 ± 3.27). The dose of 250 mg/kg body weight/ day showed a similar decrease in primordial follicle (928.4 ± 4.11), primary follicle (268.51 ± 5.39), secondary follicle (159.72 ± 2.17) and Graafian follicle (16.44 ± 5.13) compared to that of tween 80 control mice (p<0.05). 17β Estradiol treated group showed a statistically significant (p<0.05) decrease in number of primordial follicle (816.75 ± 44.4), primary follicle (179.42 ± 11.56), secondary follicle (142.51 ± 6.33) and Graafian follicle (12.34 ± 7.21) compared to that of normal control mice. The results showed a clear vision of the effect of CGME on the ovarian folliculogenesis of the albino mice. It is evident that the higher dose of CGME has much reductive activity on it than those of lower dose. The increasing number of atretic follicles due to the higher dose reveals that there are certain phyto-compounds in CGME which affect normal folliculogenesis. These compounds must have a definite estrogenic property which affects the steroid hormone level.
The experimental results of the present investigation showed a reducing effect upon the follicular development in all the experimental groups of female mice with respect to control upon treated with C. gynandra leaf extract (methanolic) for a period of consecutive 21 days. The number of primordial, primary, secondary and graafian follicles decreased significantly in both the CGME treated groups along with the Estradiol treated group, whereas degenerative nature was seen clearly resulting in the increase in the number of atretic follicles. Another study reported that methanolic extract of Rumex steudelii has potential to disrupt ovarian folliculogenesis when administered orally for 30 consecutive days by inhibiting further development of the recruited ovarian follicles (Solomon, [24]. Many other investigations reported the disrupting effect of various plant extracts on ovarian folliculogenesis. The number of primordial follicle reduced in the ovaries of gerbils when treated with Cannabis extract at 2.5 mg/day for 60 days [25]. There was a total loss of primordial follicles in the ovaries of rats treated with an aqueous extract of dried seed powder of Sapindus trifoliatus at dose level of 50, 100 and 150 mg/100kg BW for consecutive 30 days (Singh and Singh, [26] resembling the effect of hexane extract of Ferula jaeschkeana in guinea pigs (Pathak et al., [27]. Another study revealed the reducing effect of nicotine on the number of graafian follicles at a dose level of 0.3 mg/kg for 15 days (Patil et al., [28]. A study by Roop et al., [29], reported the significant reduction at 6 mg polar fraction of Azadirachta extract treatment. This significant reduction in the number of healthy follicles in all the experimental groups leads to the assumption of disruption of the process of follicle selection due to atresia (Guraya), [30,36-39]. These changes in the oocyte growth and maturation have been influenced by gonadotrophins and steroids along with maturation promoting and growth factors (Guraya, Driancourt and Thuel), [31-35] suggesting the possible reason of the inhibitory effect of these plant extracts on the folliculogenesis in female [40-42].
Conclusion
Lots of researches are going on the phytochemicals of C. gynandra L. and its application in different aspects of human welfare. My aim was to investigate the estrogenic property of C. gynandra L. on ovarian folliculogenesis of the albino mice.
References
- Takizawa K, Mattison DR (1983) Female reproduction. Am J Ind Med 4: 17-30.
- Weitzman GA, Miller MM, London SN, Mattison DR (1992) Morphometric assessment of the murine ovarian toxicity of 7,12-dimethylbenz(a)anthracene. Reprod Toxicol 6: 137-141.
- Toaff ME, Abramovici A, Sporn J, Liban E (1979) Selective oocyte degeneration and impaired fertility in rats treated with the aliphatic monoterpene, citral. J Reprod Fertil 55: 347-352.
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB (1994) Destruction of preantral follicles in adult rats by 4-vinyl-cyclohexene diepoxide. Reprod Toxicol 8: 509-514.
- Anderson LD, Hirshfield AN (1992) An overview of follicular development in the ovary: from embryo to the fertilized ovum in vitro. Md Med J41: 614-620
- Heindel JJ, Thomford PJ, Mattison DR (1989) Histological assessment of ovarian follicle number in mice as a screen for ovarian toxicity. In: Hirshfield AN(ed.) Growth Factors and the Ovary. Plenum, New York
- Pedersen RA, Peters H (1968) Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 83: 555-557.
- Mattison DR, Nightingale MS (1982) Oocyte destruction by polycyclic aromatic hydrocarbons is not linked to the inducibility of ovarian aryl hydrocarbon (benzo(a)pyrene) hydroxylase activity in (DBA/2N X C57BL/6N) F1 X DBA/2N backcross mice. Pediatr Pharmacol 2: 11-21.
- DeFelice SL (1995) The nutraceutical revolution: Its impact on food industry R&D. Trend Food Sci Technol 6: 59-61.
- Kalra EK (2003) Nutraceutical–Definition and introduction. AAPS PharmSci 5: 27-28.
- Estruch R., Ros E, Salas-Salvado J (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368: 1279-1290.
- Massaro M, Scoditti E, Carluccio MA (2010) Nutraceuticals and prevention of atherosclerosis: Focus on omega-3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc Ther 28: e13-e19.
- Scicchitanoa P, Cameli M, Maiello M, Modesti PA, Muiesan ML, et al. (2014) Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods6: 11-32.
- Odum J, Ashby J, Sumpter JP (1998) Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol153: 12-19
- Kummerer K (2001) Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks (3rd edn.) Berlin: Springer publisher.
- Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76: 122-159.
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME (2007) Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinol 148: 3580-3590.
- Raymond Whish S, Loretta PM, O Neal T, Martinez A, Sellers MA, et al. (2007) Drinking water with uranium below the U.S. EPA Water Standard causes estrogen receptor dependent responses in female mice. EnvHealth Perspect 115: 1711-1716.
- Moawad AH, Rakoff AE, Kramer SA (1965) Histologic study of the effects of lowdosage irradiation of rabbit ovaries. Fertil Steril 16: 370-381.
- Bolon B, Bucci IJ, Warbritlon AR, Chen JJ, Mattison DR, et al. (1997) Differential follicle counts as a screen for chemically-induced ovarian toxicity in mice: results from continuous breeding bioassays. Fundam Appl Toxicol39: 1-10.
- Swartz WJ, Mall GM (1989) Chloredecone induced follicular toxicity in mouse ovaries. Reprod Toxicol 3: 203-206.
- Bucci TJ, Bolon B, Warbritton AR, Chen JJ. Heindel JJ (1997) Influence of sampling on the reproducibility of ovarian follicle counts in mouce toxicity studies. Reprod Toxicol11: 689-696.
- Butcher RL, Kirkpatri Keller D (1984) Pattern of follicular growth during the four-day oestrus cycle in the rat. Biol Reprod31: 280-286.
- Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB (2004) Methods for quantifying follicular numbers within the mouse ovary. Reprod 127: 569-580.
- Solomon T, Largesse Z, Mekbeb A, Eyasu M, Asfaw (2010) Effect of Rumex steudelii methanolic root extract on ovarian folliculogenesis and uterine histology in female albino rats. Afr Health Sci 10: 353-361
- Dixit VP, Arya M, Lohiya NK (1976) Mechanism of action of chronically administered Cannabis extract on the female genital tract of gerbils Meriones hurrianae. Indian J Physiol Pharmacol 20: 38-41.
- Singh SP, Singh K (1994) Effect of Sapindus trifoliatus seed on the fertility of female albino rats. Cell Signalling and Ova Implantation (Abstracts). The International Symposium on Cell Signalling andOva-Implantation. All India Institute of Medical Science pp: 21-23.
- Pathak S, Jonathan S, Prakash AO (1995) Timely administration of extract of Ferula jaeschkeana causes luteolysis in the ovary of cyclic guinea pig. Indian J Physiol Pharmacol39: 395-399.
- Patil SR, Ravindra Patil SR, Londonkar R, Patil SB (1998) Nicotine induced ovarian and uterine changes in albino mice. Indian J Physiol Pharmacol42: 503-508.
- Roop JK, Dhaliwal PK, GurayaSS (2005) Extracts of Azadirachta indica and Meliaazedarach seeds inhibit folliculogenesisin albino rats. Braz J Med Biol Res 38: 943-947.
- Guraya SS (1997) Ovarian Biology in Buffaloes and Cattle. Directorate of Information and Publications of Agriculture. Indian Council of Agricultural Research.
- Guraya SS (2000) Comparative Cellular and Molecular Biology of Ovary in Mammals. Fundamental and Applied Aspects. Oxford &IBH Publishing Co. Pvt. Ltd.
- Chen YT, Mattison DR, Feigenbaum L, Fukui H, Schulman JD (1981) Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science214: 1145-1147.
- Halpin DM, Jones A, Fink G, Charlton HM (1986) Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary-ovarian axis. JReprod Fertility77: 287-296.
- Haque A, Kalita JC, Deka DD, Baruah BK (2010) Effect of effluent water downstream to the Nagaon Paper Mill, Assam on ovarian follicular population of immature female C3h mice. The Bioscan2: 529-535.
- Maheshwar A, Bhattacharya S (2007)Ovarian ageing and fertility-review. CurrentWomen’s Health Reviews3: 63-67
- Mattison DR, Thomford PJ (1989) The mechanisms of action of reproductive toxicants. ToxicolPathol17: 364-376.
- Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, et al. (1989) The effect of smoking on oogenesis, fertilization and implantation. SeminReprodEndocrinol7: 291-304.
- Mattison DR, Shiromizu K, Nightingale MS (1983) Oocyte destruction by polycyclic aromatic hydrocarbons. Am J Ind Med 4: 191-202.
- Osman P (1985) Rate and course of atresia during follicular development in the adult cyclic rat. JReprod. Fertil73: 261-270.
- Tilly JL (2003)Ovarian follicle counts – not as simple as 1, 2, 3. Reprod Biol. Endocrinol1: 11
- U.S.Environmental Protection Agency (1998) Health Effects Test Guidelines, OPPTS 870.3800, Reproduction and Fertility Effects. EPA 712-C-98-239. U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Washington, DC.
- U.S.Food and Drug Administration, Redbook (2000) Toxicologial Principles for the Safety Assessment of Food Ingredients. IV.C.9.a. Guidelines for Reproductive Studies. U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 12941
- [From(publication date):
November-2016 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 11920
- PDF downloads : 1021