Effects of Carrying Out a Low-Intensity Isometric Contraction of the Proximal Thigh Muscles on the Electromyographic Activity of the Leg Muscles when Simulating an Ankle Sprain
Received: 21-Nov-2017 / Accepted Date: 06-Dec-2017 / Published Date: 10-Jan-2018 DOI: 10.4172/2329-910X.1000251
Abstract
Background: The patterns of activation of the leg musculature can be altered after suffering from a sprained ankle, which can contribute negatively to the biomechanics of the leg. Ankle sprains can generate changes in muscle electromyographic activity in both the thigh and ankle regions, for which physiotherapy is the most common treatment. This study aimed to determine the effect of a low-intensity isometric contraction of the proximal thigh muscles (gluteus maximus (Gmax), gluteus medius (Gmed), tensor fasciae latae (TFL) on the electromyographic response of the tibialis anterior (TA), peroneus longus (PL), lateral gastrocnemius (LG), and soleus muscles (SL) in a sudden ankle supination.
Methods: Fifteen healthy volunteers were subjected to 2 series of three trials on a sudden ankle supination platform (50°). This involved a multiple analysis of variance per trial and muscle for the dependent variables and an analysis of variance in the measures repeated under study for the different time windows evaluated, according to the intended factors and muscle studied.
Results: No significant differences were observed in the variables concerning the time of activation, the moment of maximum EMG registration or registered peak value.
Conclusion: Inducing a low-intensity isometric contraction of the abductor leg muscles did not generate changes in the muscular values studied.
Keywords: Ankle injuries; Hip; Electromyography; Postural control
Introduction
Ankle sprains are one of the injuries with the greatest impact on the lower extremities, particularly amongst athletes [1–3]. The scope of this injury affects lower extremities globally and usually there is an alteration of the sensory motor system [4,5]. Likewise, several other alterations were observed both locally in the ankle [2,6] and distally in the point of the injury given that weakness of the abductor musculature in the lower extremities was also observed [7–9]. The latter suggests that ankle injuries may have an effect on the entire chain of leg muscles [7,8,10] due to a possible alteration of the central sensory motor system [4,5].
This could explain why several authors have reported a delay in the activation of the peroneus muscles and thus an alteration in the sequence of muscular contraction in the kinetic chain in situations of sudden supination of the ankle [10–13].
On the other hand, it is believed that a key element in the control of postural changes is the level of electromyographic (EMG) activity of the various muscles involved in counteracting alterations in balance. An increase in stiffness may lead to an improvement in the spindle output and this increased sensitivity in the spindle muscle may improve the overall stability of the individual due to an increase in motor control and in the execution of motor response [14–18].
At this point we should mention two moments described in the EMG motor control, namely the Anticipatory Postural Adjustments (APA), muscular activity of the Feed forward type that takes place between approximately -500 ms prior to the action and the initiation of the action, and the adjustments in postural control (Control Postural Adjustments (CPA)) up to +400 ms after the action [19–21]. On taking all these factors into consideration, it is possible to believe that as a result people who have suffered a previous sprain may suffer various alterations in the lower limb and in postural control [4,8,7,12,13].
Since it seems that spindle output may be modified through training [18], a question arises. In order to answer this unknown topic, assuming that it is possible to train selectively a muscle contraction, and focusing the attention in the activation pattern of the lower limb chain, the objective of this study was to examine the effect of a lowintensity isometric contraction (<25% MVIC) of the proximal thigh muscle (GMax, GMed and TFL) on the electromyographic (EMG) response of the TA, PL, lateral gastrocnemius LG and the SL in a sudden ankle supination situation. The hypothesis of this study was that an anticipated contraction of the thigh musculature would increase the electromyographic activity of the shank, and this contraction could generate changes in the contraction pattern, therefore creating a preventive strategy for an ankle sprain.
Methodology
Ethical approval was obtained from the Scientific Research Ethics Committee of the Catalan Sports Council (ID number: 03_2013_CEICGC; March 25, 2013) and all participants signed an informed consent. Fifteen students from the Fundació Universitària del Bages (Universitat Autònoma de Barcelona- Spain) were recruited to participate in the study (10 women, 5 men; mean and standard deviation: age 22 ± 2.0; weight 61.8 kg ± 7.9; height 169.9 cm ± 7.1; foot measurement 25.4 cm ± 2.7). The sample size was calculated from the work of Vaes et al. [22] according to which, 30 subjects should have been recruited (15 per group) to obtain a statistical power of 80% and an error α of 0.05. The inclusion criteria were the following: physically active individuals, with no history of pathology or surgical intervention in the lower extremities (ever), without visual or vestibular alterations. The leg under study was the one that the participants would use to kick a penalty.
Instruments
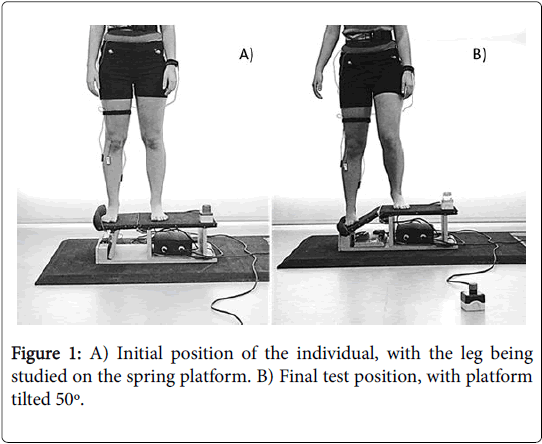
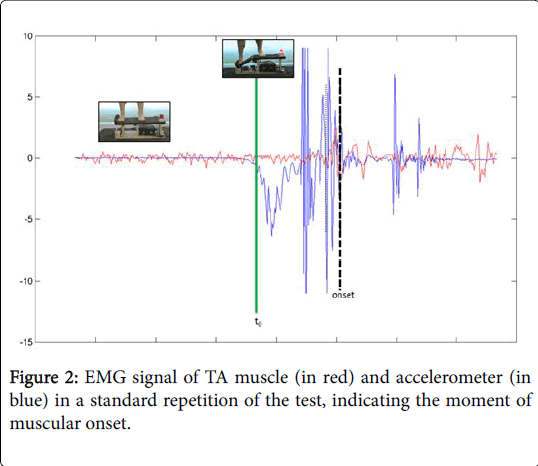
A tilting platform (up to 50°) with a spring mechanism was designed and built according to models previously used [23–25]. The EMG activity was recorded by surface electrodes SX230 with a signal amplifying system (Biometrics Ltd, Gwent, Wales) connected to a Biometrics© Datalogger. All the different data was captured at a frequency of 1000 Hz. and stored in the Datalogger itself. In order to know the exact moment that the spring platform dropped (t0), an accelerometer of the type ACL300 (Biometrics Ltd, Gwent, Wales) affixed to the spring platform and connected to the Biometrics© Datalogger was used.
EMG procedures
All data collection for each subject was carried out in a single session. After preparing the skin of the subject, the electrodes were placed on the abductor muscles of the leg (GMax, GMed, and TFL) and on the distal muscles (TA, PL, LG, and SL), in accordance with the instructions of Hermens et al. [26]. The reference electrode was placed on the ulnar styloid of the right arm. Three recordings of five seconds each were performed to determine the GMax, GMed, and TFL (based on Daniels instructions [27]) maximum voluntary isometric contraction (MVIC) value, calculating the mean of the three trials.
Intervention Protocol
The participants had to stand on the platform with their arms by their sides and placing as much weight as possible on the leg being studied, looking at a fixed point on the wall 3 meters in front of them. Each individual performed two series (control situation-situation A, active situation-situation B) of three attempts, with a five-minute rest in between each attempt and another 30-minute rest in between the two series. The order in which the participants developed each situation (A-B or B-A) was randomized throwing a coin. The opening mechanism of the platform was activated in a random and unexpected manner (Figure 1). During the situation B the participants were required to induce an isometric contraction of the GMax, GMed and TFL muscles at the threshold of 25% of the raw signal of the MVIC, given that at this threshold value of activation there cannot be associated any fatigue component neither any structural dysfunction in the muscle [28]. The order to the participants was to generate an abduction torque conducting an action like bringing the greater trochanter toward the iliac crest. An auditory feedback was provided to achieve this assignment. The electromyographic variables studied are detailed in Table 1 and Figure 2. In all cases, the variables studied were the average of the EMG recorded value and the root mean square (RMS). The EMG data was processed and analysed offline by an investigator blinded to the group allocation, and using a program developed by Matlab R2011a (The Mathworks, Inc©, Massachusetts, USA). The variables tonset, tpeak, and EMGpeak were processed based on the original corrected signal, without smoothing. The data obtained in the analysis of the different time windows went through a 20 ms RMS filter and then corrected through a frequency range of 20-350 Hz.
| EMG variables | Description |
|---|---|
| Baseline | the average of the EMG signal from -1000ms to -750ms (in relation to t0) |
| t0 | the moment when the recording of the accelerometer activity exceeded by 2 standard deviations (SD) the average recorded when at rest |
| tonset | the moment when the original EMG signal exceeded the base signal by two SD and remained above this threshold for 4ms |
| EMGpeak | the highest EMG recorded value after t0 |
| tpeak | the time that passed between t0 and EMGpeak |
| time window -3 | from -500 ms to -350ms (in relation to t0) |
| time window -2 | from -350ms to -200 ms (in relation to t0) |
| time window -1 | from -200ms to -50ms (in relation to t0) |
| time window +1 | from 0 to +400ms (in relation to t0) |
Table 1: Detailed description of the electromyographic variables studied.
Data analysis
In these repeated measures design, the normal distribution of the data was evaluated using the Mauchly's sphericity test criterion (W=0338, p<0.005), taking the result into account to correct the freedom degrees in the statistical values analysed. A multiple variance analysis was carried out so as to detect the influence of the independent variables: situation (A/B) and muscle (TA, PL, GL, SL) on the dependent variables: tonset, tpeak, and EMGpeak.
A variance analysis for repeated measures (ANOVA) was used to study the average EMG variable recorded and the RMS of the time windows described (-3, -2, -1, +1), considering the situation factors (A/B) and the muscles studied (TA, PL, GL, SL). The size of the time windows -1/+1 effect was analysed in accordance with Cohen’s d. The analysis was performed using the SPSS program, version 21 (SPSS Inc., Chicago, IL, USA).
Results
Analysis of the variables tonset, tpeak, EMGpeak
As regards the trial (A: without instructions/B: with voluntary activation of muscular activity), there was no statistically significant influence for time dependent variables. The averages for the time to onset were for trial A/B: TA: 13.874 ms ± 25.493/31.208 ms ± 40.42; PL: 28.653 ms ± 27.55/22.667 ms ± 21.234; LG: 31.889 ms ± 30.961/43.583 ms ± 41.457; SL: 32.014 ms ± 42.264/26.333 ms ± 27.231). As regards the time to peak, the averaged values were for A / B trials: TA: 108.417 ms ± 56.37 / 121.625 ms ± 46.757; PL: 124.055 ms ± 45.717/104.542 ms ± 48.321; LG: 91.999 ms ± 53.866 / 106.375 ms ± 58.955; SL: 105.833 ms ± 63.973/108.583 ms ± 55.386. Regarding the peak at EMG, the values were (A/B): TA: 0.334 mV ± 0.46/0.507 mV ± 0.619; PL: 0.258 mV ± 0.274/0.26 mV ± 0.212; LG: 0.321 mV ± 0.563/0.495 mV ± 0.818; SL: 0.231 mV ± 0.166/0.312 mV ± 0.314. Table 2 shows more statistical details on this data.
| Variable | F | p | ɲ2 | 1-β |
|---|---|---|---|---|
| tonset | 0.416 | 0.521 | 0.005 | 0.098 |
| tpeak | 0.6 | 0.807 | 0.001 | 0.057 |
| EMGpeak | 1.215 | 0.273 | 0.014 | 0.193 |
Table 2: Statistical results according to situation A (without action) and B (with muscular activation), for variables tonset, tpeak, EMGpeak.
Analysis of the variables according to the time window studied
Average EMG: No statistically significant differences were found between the different situations (A and B) (F=1.571; p= 0.197; η2=0.018; 1-β=0.412), nor among the comparisons of the different muscles (F=1.047; p=0.403; η2=0.034; 1-β=0.518) (Table 3).
| Muscle | Average -3 | Average -2 | Average -1 | Average +1 | ||||||||||||
| A | B | A | B | A | B | A | B | |||||||||
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| TA | 0.008 | 0.019 | 0.021 | 0.046 | 0.008 | 0.012 | 0.013 | 0.023 | 0.012 | 0.012 | 0.041 | 0.069 | 0.049 | 0.048 | 0.05 | 0.03 |
| PL | 0.006 | 0.012 | 0.017 | 0.021 | 0.008 | 0.012 | 0.016 | 0.01 | 0.022 | 0.039 | 0.03 | 0.041 | 0.036 | 0.022 | 0.04 | 0.02 |
| LG | 0.02 | 0.057 | 0.058 | 0.147 | 0.024 | 0.069 | 0.055 | 0.141 | 0.024 | 0.049 | 0.069 | 0.169 | 0.048 | 0.089 | 0.08 | 0.17 |
| SL | 0.009 | 0.008 | 0.019 | 0.016 | 0.01 | 0.004 | 0.015 | 0.011 | 0.012 | 0.013 | 0.023 | 0.019 | 0.029 | 0.02 | 0.03 | 0.01 |
Table 3: Summary of the tests descriptive results for the average variable of EMG (mV) for time windows -3, -2, -1, +1.
RMS: No statistically significant differences were found between the different situations (A and B) (F=0.803; p=0.493; η2=0.009; 1-β=0.223) nor among the comparisons of the different muscles (F=1.673; p=0.096; η2=0.054; 1- β=0.765) (Table 4).
| Muscle | RMS -3 | RMS -2 | RMS -1 | RMS +1 | ||||||||||||
| A | B | A | B | A | B | A | B | |||||||||
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| TA | 0.011 | 0.024 | 0.025 | 0.055 | 0.01 | 0.015 | 0.015 | 0.025 | 0.013 | 0.014 | 0.046 | 0.078 | 0.068 | 0.058 | 0.074 | 0.04 |
| PL | 0.006 | 0.012 | 0.018 | 0.023 | 0.008 | 0.012 | 0.018 | 0.013 | 0.028 | 0.053 | 0.032 | 0.045 | 0.049 | 0.03 | 0.044 | 0.02 |
| LG | 0.033 | 0.094 | 0.074 | 0.187 | 0.037 | 0.108 | 0.067 | 0.178 | 0.036 | 0.082 | 0.078 | 0.189 | 0.063 | 0.116 | 0.105 | 0.21 |
| SL | 0.013 | 0.01 | 0.022 | 0.02 | 0.013 | 0.008 | 0.018 | 0.011 | 0.014 | 0.019 | 0.026 | 0.021 | 0.036 | 0.022 | 0.048 | 0.02 |
| 0.011 | 0.024 | 0.025 | 0.055 | 0.01 | 0.015 | 0.015 | 0.025 | 0.013 | 0.014 | 0.046 | 0.078 | 0.068 | 0.058 | 0.074 | 0.04 | |
Table 4: Summary of the tests descriptive results for the variable RMS of EMG (mV) for time windows -3, -2, -1, +1.
Effect Size: Calculated according to the difference between two averages by means of Cohen’s d; it was observed that the effect size for the developments of situation A and for time windows ± 1, was very large for muscles TA (1.057) and SL (1.008) and moderate for muscles PL (0.442) and GL (0.334). As for situation B, the effect size of the response obtained in the comparison of time windows ± 1, was small for muscles PL (0.194) and GL (0.082), moderate for muscle TA (0.248), and large for muscle SL (0.692) (Table 5).
| Muscle | Situation A | Situation B | |||||
| window -1 | window +1 | ES | window -1 | window +1 | ES | ||
| TA | Average | 0.012 | 0.049 | 1.057 | 0.041 | 0.054 | 0.248 |
| Typical deviation | 0.012 | 0.048 | 0.069 | 0.027 | |||
| PL | Average | 0.022 | 0.036 | 0.442 | 0.03 | 0.036 | 0.194 |
| Typical deviation | 0.039 | 0.022 | 0.041 | 0.015 | |||
| GL | Average | 0.024 | 0.048 | 0.334 | 0.069 | 0.083 | 0.082 |
| Typical deviation | 0.049 | 0.089 | 0.169 | 0.173 | |||
| SL | Average | 0.012 | 0.029 | 1.008 | 0.023 | 0.034 | 0.692 |
| Typical deviation | 0.013 | 0.02 | 0.019 | 0.012 | |||
Table 5: EMG recordings (mV) of time windows -1 and +1 and their relation with increase, according to Cohen´s d.
This was interpreted according to Cohen [29]: d<=0.20 (small), 0.20>d<=0.50 (moderate), 0.50>d<=0.80 (large), and Rosenthal [30], who added 0.80>d<=1.30 (very large).
Discussion and implications
The results of this study did not show significant differences in EMG activity for variables tonset, tpeak and EMGpeak when the foot was subjected to sudden supination in either of the two situations described. The intervention was not significant either for the values of the EMG variables for muscles TA, PL, GL, and SL in the recordings of the different time windows. Nevertheless, even though there were no statistically significant differences, a different behaviour was observed in the pattern of muscular activity during the trials carried out in situation B, which focused on muscular activity in the proximal thigh muscles.
The muscle spindle, under the control of the Central Nervous System (CNS), is primarily responsible for the regulation of activities that occur to control any imbalances and maintain a stable position [18,31]. Various predetermined motor programs, which are activated following a string of established muscular actions [32], intervene in this control. Various authors have speculated that greater basal stiffness could contribute positively to active joint rigidity, thus generating greater muscle spindle sensitivity output and thereby, creating greater initial stability to facilitate the task of maintaining balance [15,16,18,33]. Similarly, Deniskina et al. [34] observed in their study an important relationship between muscle tone in the trunk and hip, and postural control in movements generated on a frontal plane, as is the case of an ankle sprain. For these reasons, in the present study we decided to focus on the increased initial level of voluntary stiffness, with the intention of modifying the levels of muscular electrical activity and improving the stability of the ankle in a situation of sudden inversion due to the above mentioned mechanical implications in the lower limb after an ankle sprain.
In this sense, Vaes et al. [22] did not observe significant differences in the times of peroneal latency nor in other electromyographic variables analysed, when comparing a group of healthy people with another group made up of people with chronic ankle instability, in a test done with a spring platform with a 50° inclination drop. However, they did observe differences in the time spent on the first deceleration of the fall of the foot, which was greater for the group with a history of prior injuries and thus, some biomechanical alterations were detected in these participants.
In this regard, both Tsao and Hodges [35] and Küng et al. [36] associated the ability to learn new strategies and generate changes in learned motor patterns with CNS plasticity whether in the preparation for a gesture during Feedforward [35] or in the ability to increase the number of motor units recruited at once [36], thus obtaining more sensory information and greater ability to generate force in the motor response.
The tool used in this study was designed according to previous studies. Vaes et al. [25] used and evaluated this type of device for the study involving healthy individuals (with a tilt of up to 50°), obtaining an ICC=0.90. Similarly, Eechaute et al. [23] and Benesch et al. [11] verified the reliability of this tool for the study of t-onset for the peroneal musculature, obtaining an ICC=0.83 (using a 50° tilt) [23] and a Spearman’s rho of p>0.99 (with a tilt of 30°) [11].
In a similar way to our study, Dias et al. [37] used the test with a spring platform to evaluate the results of a 4-week-long specific training oriented towards improvement in the peroneal activation times in healthy individuals, again seeking a possible strategy for preventing ankle sprains, but they did not find any significant differences among the groups evaluated. On the other hand, both Grüneberg et al. [38] and Lofvenberg et al. [24] detected significant differences in the observation of peroneal onset when comparing healthy individuals to subjects with a history of prior injuries, in inversion tests on platforms at 25° and 30°, respectively.
Another aspect analysed in this study was the level of EMG activity in the ankle musculature during the moments prior to and the ones immediately after a change in balance. Santos et al. [19] and Krishnan et al. [20] presented the existing relationship between APA activity, which occurs approximately 200 m before tonset [20,21] and CPA activity, which is generated immediately after and up to the first 400 m after t0. In this sense, there were no significant differences between the different situations (A and B) in the overall analysis. However, in the individual analysis of the muscles, some changes in the activation patterns were detected. When observing the EMG behaviour trend of the 4 time windows in this study, it is worth mentioning that the EMG activity in situation B always tended to be higher for each muscle studied.
It is also interesting to note that the muscular activity observed during time window -1 was greater in situation B, as it was also the case for window +1. In this particular comparison (window ± 1) it can be observed that according to the effect size shown by the difference in both situations, the increase in muscular activity is much greater in situation A, thus corroborating that the events that occur during the instants prior to an articular homeostasis alteration determine the muscular behavior that will occur after the perturbation.
This could indicate that the APA activity increased in this study after a prior isometric contraction of the thigh musculature; this fact could provide a better balance control strategy when facing possible alterations in balance [19,20]. These studies by Santos et al. [19] and Krishnan et al. [20] coincided in describing muscle sensory receptors in a higher state of alert when a movement can be predicted, since APA activity was observed to a greater extent than CPA activity. On the other hand, if the perturbation is unexpected, which is what happens in an ankle sprain, the APA is virtually non-existent in contrast with the CPA, which is much more intense in order to correct postural imbalances [19–21].
It is also interesting to observe the change registered in the behavior of EMG activity in the musculature observed in window +1, where the role of each muscle changed due to a modification in the intensity of muscular activation. Thus, during situation A, TA was the muscle that showed greater electrical activity, followed by GL, PL, and SL. On the other hand, in situation B, the levels of muscular activity in this time window were, from greater to lesser: GL and TA for both variables, followed by PL and SL for the average EMG, and SL and finally PL for the RMS. It is important to keep in mind that these changes in the pattern of activation can also be influenced by both the deceleration of the muscle itself during the fall and by changes in the muscle torque [39].
Although traditionally the actions of the invertor and evertor musculature of the foot have been given importance when it comes to the control of joints, especially when a sudden inversion activity takes place [12,40], nowadays there is sufficient evidence for us to consider the need to include a hip musculature training using the protocols to prevent an ankle injury. An example of these would be the references that link the effect on the hip musculature that follows an ankle injury and the global alteration of the sensory motor system [4,8,10,18,41]. The results of this study contribute to reinforce this idea since they show that the proximal muscle activation in the reaction chain of the lower extremity could interfere in the activation pattern of the ankle muscles, which makes them a possible preventive strategy for the type of injuries that these perturbations could cause. Future studies using muscular training with low-intensity muscle contractions in the hip area are necessary to evaluate more extensively their effect on activation patterns. Tsao and Hodges [35] found that it is possible to selectively train a muscle at low intensity so as to generate changes in its activation during the Feed forward of a motor action, and that these changes could be maintained over the long-term.
This study has three limitations. Firstly, it is necessary to assume that, in the different situations that we evaluated, there were no significant changes in the torque of the different joints involved in the movement given that this could lead to different levels of activation. We must also consider the fact that this study was carried out on a selection of healthy individuals and that the same study should therefore be carried out on individuals who have previously suffered ankle sprain injuries and thus, determine their exact impact. Lastly, the small sample size may contribute in the fact of not finding significance in the variables studied in the test.
Conclusions
The results of this study suggest that no significant differences were observed in the level of EMG activation of the muscles TA, PL, LG, and SL when performing a low-intensity (<25% MVIC) isometric contraction of the proximal thigh musculature (GMax, GMed and TFL) prior to the realization of an ankle sprain, even though no significant changes were detected in the activation patterns of the shank muscles.
References
- Fong DT, Chan YY, Mok KM, Yung PSH, Chan KM (2009) Understanding acute ankle ligamentous sprain injury in sports. Sport Med Arthrosc Rehabil Ther Technol 1: 1-14.
- McKay GD, Goldie PA, Payne WR, Oakes BW (2001) Ankle injuries in basketball: Injury rate and risk factors. Br J Sports Med 35: 103-108.
- Waterman BR, Belmont PJ, Cameron KL, Deberardino TM, Owens BD (2010) Epidemiology of ankle sprain at the united states military academy. Am J Sports Med 38: 797-803.
- Brown CN, Mynark R (2007) Balance deficits in recreational athletes with chronic ankle instability. J Athl Train 42: 367-373.
- Hass CJ, Bishop MD, Doidge D, Wikstrom EA (2010) Chronic ankle instability alters central organization of movement. Am J Sports Med 38: 829-834.
- Ross ES, Guskiewicz KM, Bing YU (2005) Single-leg jump-landing stabilization times in subjects with functionally unstable ankles. J Athl Train 40: 298-304.
- Beckman SM, Buchanan TS (1995) Ankle inversion injury and hypermobility: Effect on hip and ankle muscle electromyography onset latency. Arch Phys Med Rehabil 76: 1138-1143.
- Friel K, McLean N, Myers C, Caceres M (2006) Ipsilateral hip abductor weakness after inversion ankle sprain. J Athl Train 41: 74-78.
- Webster KA, Gribble PA (2013) A comparison of electromyography of gluteus medius and maximus in subjects with and without chronic ankle instability during two functional exercises. Phys Ther Sport 14: 17-22.
- Lee SP, Powers CM (2014) Individuals with diminished hip abductor muscle strength exhibit altered ankle biomechanics and neuromuscular activation during unipedal balance tasks. Gait Posture 39: 933-938.
- Benesch S, Pütz W, Rosenbaum D, Becker H (2000) Reliability of peroneal reaction time measurements. Clin Biomech 15: 21-28.
- Mcvey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD (2005) Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int 26: 1055-1061.
- Menacho MD, Pereira HM, Oliveira BIR DE, Chagas LMPM, Toyohara MT, et al. (2010) The peroneus reaction time during sudden inversion test: Systematic review. J Electromyogr Kinesiol 20: 559-565.
- Wilkerson GB, Nitz AJ (1994) Dynamic ankle stability: Mechanical and neuromuscular interrelationships. J Sport Rehabil 3: 43-57.
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) Stiffness control of balance in quiet standing. J Neurophysiol  80: 1211-1221.
- Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D (2003) Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. J Neurophysiol 90: 3774-3782.
- Suzuki Y, Nomura T, Casadio M, Morasso P (2012) Intermittent control with ankle, hip, and mixed strategies during quiet standing: A theoretical proposal based on a double inverted pendulum model. J Theor Biol 310: 55-79.
- Ashton Miller JA, Wojtys EM, Huston LJ, Fry-Welch D (2001) Can proprioception really be improved by exercises? Knee Surgery, Sport Traumatol Arthrosc 9: 128-136.
- Santos MJ, Kanekar N, Aruin AS (2010) The role of anticipatory postural adjustments in compensatory control of posture: Electromyographic analysis. J Electromyogr Kinesiol 20: 388-397.
- Krishnan V, Latash ML, Aruin AS (2012) Early and late components of feed forward postural adjustments to predictable perturbations. Clin Neurophysiol 123: 1016-1026.
- Vedula S, Kearney R, Wagner R, Stapley PJ (2010) Decoupling of stretch reflex and background muscle activity during anticipatory postural adjustments in humans. Exp Brain Res 205: 205-213.
- Vaes P, Duquet W, Van Gheluwe B (2002) Peroneal reaction times and eversion motor response in healthy and unstable ankles. J Athl Train 37: 475-480.
- Eechaute C, Vaes P, Duquet W, Van Gheluwe B (2007) Test-retest reliability of sudden ankle inversion measurements in subjects with healthy ankle joints. J Athl Train 42: 60-65.
- Lofvenberg R, Karrholm J, Sundelin G, Ahlgren O (1995) Prolonged reaction time in patients with chronic lateral instability of the ankle. Am J Sports Med 23: 414-417.
- Vaes P, van Gheluwe B, Duquet W (2001) Control of acceleration during sudden unstable ankle supination in people. J Orthop Sport Phys Ther31: 741-752.
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361-374.
- Hislop HJ, Montgomery JD,Worthingham (2003) Técnicas de Balance Muscular Elsevier.
- De Luca CJ (1997) The use of surface electromyography in biomechanics. J Appl Biomech 13: 135-163.
- Cohen J (1988) Satatistical Power analysisi for the behavioral sciences. 2nd edn. Lawrence Erlbaum Associates.
- Rosenthal JA (1996) Qualitative descriptors of strength of association and effect size. J Soc Serv Res 21: 37-59.
- Hertel J (2008) Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin J Sport Med 27: 353-370.
- Hiller CE, Nightingale EJ, Lin CWC, Coughlan GF, Caulfield B, et al. (2010) Characteristics of people with recurrent ankle sprains: A systemic review with meta analysis. Br J Sports Med 45: 660-672.
- Paillard T (2012) Effects of general and local fatigue on postural control: A review. Neurosci Biobehav Rev 36: 162-176.
- Deniskina I, Levik Y, Gurfinkel V (2001) Relative roles of the ankle and hip muscles in human postural control in the frontal plane during standing. Hum Physiol 27: 317-321.
- Tsao H, Hodges PW (2008) Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol 18: 559-567.
- Küng UM, Horlings CG, Honegger F, Allum JH (2010) The effect of voluntary lateral trunk bending on balance recovery following multi-directional stance perturbations. Exp Brain Res 202: 851-865.
- Dias A, Pezarat-Correia P, Esteves J, Fernandes O (2011) The influence of a balance training program on the electromyographic latency of the ankle musculature in subjects with no history of ankle injury. Phys Ther Sport 12: 87-92.
- Grüneberg C, Nieuwenhuijzen PH, Duysens J (2003) Reflex responses in the lower leg following landing impact on an inverting and non-inverting platform. J Physiol 550: 985-993.
- Lee AJ, Lin WH (2008) Twelve-week biomechanical ankle platform system training on postural stability and ankle proprioception in subjects with unilateral functional ankle instability. Clin Biomech 23: 1065-1072.
- Munnn J, Sullivan SJ, Schneiders AG (2010) Evidence of sensorimotor deficits in functional ankle instability: A systematic review with meta-analysis. J Sci Med Sport 13: 2-12.
- Franettovich SMM, Honeywill C, Window N, Crossley KKM, Creaby MW (2014) Neuromotor control of gluteal muscles in runners with achilles tendinopathy. Med Sci Sports Exerc 46: 594-599.
Citation: Borao O, Planas A, Susin A, Corbi F (2018) Effects of Carrying Out a Low-Intensity Isometric Contraction of the Proximal Thigh Muscles on the Electromyographic Activity of the Leg Muscles when Simulating an Ankle Sprain. Clin Res Foot Ankle 6:251. DOI: 10.4172/2329-910X.1000251
Copyright: © 2017 Borao O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9902
- [From(publication date): 0-2018 - Nov 01, 2025]
- Breakdown by view type
- HTML page views: 8735
- PDF downloads: 1167