Research Article Open Access
Effects of a Paracetamol and Tramadol Fixed-dose Combination on Pain, Asthenia, Cognitive Disorders and Sleep Quality in Fibromyalgia
Ghini M1*, Carpenito G1 and Mascia MT2
1Department in Rheumatology, Modena Local Health Authority, Italy
2Department of Diagnostic, Immuno-Rheumatology Unit, Clinical and Public Health, University of Modena and Reggio Emilia, Italy
- Corresponding Author:
- Ghini M
Modena Local Health Authority
Ospedale di Sassuolo Via Ruini 2 - 41049 Sassuolo (MO), Italy
Tel: 0536846461
Fax: 0536846632
E-mail: m.ghini@ausl.mo.it
Received Date: June 11, 2016; Accepted Date: September 19, 2016; Published Date: September 22, 2016
Citation: Ghini M, Carpenito G, Mascia MT (2016) Effects of a Paracetamol and Tramadol Fixed-dose Combination on Pain, Asthenia, Cognitive Disorders and Sleep Quality in Fibromyalgia. J Pain Relief 5:267. doi:10.4172/2167-0846.1000267
Copyright: © 2016 Ghini M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Objective: Fibromyalgia syndrome (FMS) is a chronic condition characterized by widespread musculoskeletal pain associated with a variety of other symptoms. In order to evaluate the effects of a multimodal analgesia approach to the treatment of FMS, a retrospective observational study was performed. Methods: Sixty-nine consecutive outpatients, seen for the first time and diagnosed with FMS, were identified in our registry between April 2011 and May 2012. All patients were prescribed a fixed combination of tramadol 37.5 mg + paracetamol 325 mg, either alone or combined with other treatments. The patient’s general details and FMSrelated signs and symptoms were recorded. In order to have a more objective evaluation of the severity of pain, we assigned a score (0-4) based on the reaction of the patient to the touch of tender points (Evoked Pain Score: EPS). Results: The study confirmed that the symptoms of FMS take on a number of forms and are treated using various therapeutic approaches; however, patients were still suffering pain at the time of their first visit. Significant reduction in pain and improvement in quality of life were observed at the second visit, with a statistically significant reduction in the VAS (8.1 vs. 5.5; p<0.001), EPS (2.7 vs. 2.0; p<0.001) and FMS-related symptoms. Conclusion: Multimodal analgesia with a tramadolo-paracetamol combination is a valid analgesia option in the treatment of FMS, as it exploits different mechanisms of action on pain and on the associated symptoms, such as asthenia, sleep disturbance, paraesthesia, headache and restless legs.
Keywords
Fibromyalgia syndrome (FMS); Pain; Tramadol; Paracetamol, Combination therapy
Introduction
Fibromyalgia syndrome (FMS) is a chronic condition of diffuse musculoskeletal pain with tenderness in specific points, often associated with asthenia, cognitive and mood disorders, joint stiffness, sleep disorders, paresthesia, restless legs and headache. It is a persistent and potentially debilitating disorder with a severe impact on the quality of life of patients, whose ability to work and perform activities of daily living may be significantly affected [1,2].
The growing interest in FMS in recent years is confirmed by the many published studies regarding various aspects of this disease, including special issues of medical journals [3,4] and books [5]. FMS is a disorder of pain-processing mechanisms; however, factors contributing to the physiopathology of FMS may include genetic influences and environmental triggers, such as exposure to stressors, in addition to abnormal function of the autonomic and neuroendocrine systems [6,7].
Evidence suggests that both the ascending and descending pain pathways operate abnormally, resulting in central amplification of pain signals, which can be compared to the “volume control setting” being turned up too high. Stimuli such as heat and cold, as well as mechanical and ischemic pressure, produce pain responses in patients when applied at levels of intensity that do not evoke pain responses in healthy individuals. Evidence also suggests that FMS involves abnormal levels of serotonin and norepinephrine, which are key neurotransmitters in endogenous pain suppression pathways [8,9]. These factors are also usually associated with disorders that occur concomitantly or overlap with FMS, such as major depressive disorders, irritable bowel disease (IBD), and temporomandibular disorders [10].
Given the unclear aetiology of FMS and the variety of presentations of the disease, it has been established that no one therapy is always effective. As a matter of fact, the treatment of FMS is multidisciplinary, although pain control obviously plays a pivotal role in the management of patients, together with either pharmacological or nonpharmacological measures aimed at improving the other cognitivebehavioral, gastrointestinal (GI) and musculoskeletal symptoms. In particular, the modulatory effects of certain drugs, such as tramadol, on serotoninergic transmission may play an important role in controlling symptoms, such as asthenia, sleep disturbance, paraesthesia, headache, restless legs, etc. [11].
Since no single medication has yet been seen to satisfactorily control all FMS symptoms, multicomponent treatment is required from the outset [12]. The medications employed at the current time include antidepressants, nonsteoroidal anti-inflammatory drugs (NSAID), opioids, tramadol, sedatives, muscle relaxants, analgesics, hypnotic agents and anticonvulsants.
Most of these drugs improve just one or two symptoms, and no drug is capable of overall symptom control. In addition, different classes of drugs with different mechanisms of action are used off-label, including tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs) and sedative hypnotics [13,14]. The best treatment should be identified and combined with patient education and nonpharmacological therapy.
We focused our attention on a fixed combination of tramadol 37.5 mg + paracetamol 325 mg, which could represent a useful approach to the management of FMS because of the combined, synergic analgesic effects of paracetamol on musculoskeletal pain and the serotoninergic activity of tramadol [15] on FMS-related symptoms, such as asthenia, sleep disturbance, paraesthesia, headache and restless legs. In order to evaluate the effects of a fixed combination on FMS, a retrospective observational study was performed.
Materials and Methods
Once the study protocol had been submitted to the local independent ethics committee, we reviewed the registry of our outpatients departments, listing all the patients requiring advice and appointments between April 2011 and May 2012. During this period, a series of 69 consecutive patients diagnosed with FMS by 1990 ACR Cryteria [16] were identified from medical records; they were visited for the first time in our outpatient departments. The patients were invited to our sites for a second visit between July 2011 and August 2012.
Unfortunately, the area around Modena (Italy) was hit by an earthquake in May 2012, and only 40 patients were able to attend the second appointment. Our retrospective observation of patients included a population of 69 patients with fibromyalgia, which provided us with information about the frequency of symptoms reported by patients, and a population of 40 patients, for which data was available on the results achieved with the approach usually adopted by our sites to manage FMS. Patients were treated for 3 months out of total study observation period of approximately 14 months.
The medical records of the selected patients were reviewed paying special attention to the following information: patient’s general details; date of first diagnosis of FMS; concomitant diseases; FMS-related signs and symptoms. The characteristics of pain were assessed at baseline considering the number of painful or tender points and the intensity of pain assessed using a 10 cm visual analogue scale (VAS).
In our clinical practice, we also register a more objective evaluation of pain, assigning a score, that we call “Evoked Pain Score” (EPS) to the intensity of patient’s reaction to the touch of tender points; this score ranges from 0 (no reaction) to 4 (very strong reaction). These measures were usually repeated at the second visit about 3 months later, in line with our standard approach to FMS assessment.
The treatments (active ingredients and dosage) that had been prescribed to the patients prior to the first visit were recorded, together with the treatments prescribed by our sites between the first and second visits. In addition, patients were asked to provide an opinion on the efficacy of the pharmacological treatment prescribed and to report their treatment compliance in terms of the number of medications taken each day; the frequency of patients who reduced the prescribed dosage of their own initiative was also recorded.
The results of the observational study were reported primarily using descriptive analysis. Patients’ general details and medical records at baseline were summarized in frequency tables with central tendency and distribution using the most appropriate parameters for each variable (mean, standard deviation, min-max range).
The changes between the first and second visits in the intensity of pain were analyzed using a Wilcoxon test; p<0.05 were considered statistically significant. Statistical analysis was performed using the software SPSS Statistical Package, version 13.0.
Results
The general details of the 69 patients, who were visited for the first time at our sites, are given in (Table 1). Fibromyalgia was far more common in women (94.2%), however there was no relationship with age, as patients were 19 to 78 years of age, with no age-related cluster; there was no relationship with body weight, although grade 1 (31.9%) or grade 2 (20.3%) overweight patients were more frequently affected than patients of a normal weight (42.0%).
| Sex (M/F; %) | Age (year) | Weight (kg) | Height (cm) |
|---|---|---|---|
| 4-65 (5.8 / 94.2) |
53.6 ± 13.4 (19 – 78) |
69.6 ± 15.6 (39 – 115) |
161.8 ± 6.0 (150 – 180) |
| BMI 26.4 ± 5.1 (16.0 – 40.7) |
Normal (18.5-25): 42.0% Overweight Grade 1 (25-30): 31.9% Overweight Grade 2 (30-40): 20.3% |
||
| Data are indicated as mean ± standard deviation, frequency or min-max range, as appropriate for each variable. | |||
Table 1: General characteristics of the patients at baseline.
FMS was mostly primary (97.1% of patients), and in just two patients the painful syndrome was considered to be secondary to other medical conditions; the most frequently associated conditions were differently located joint arthritis and anxiety-depressive syndrome, which were present in 11 patients (15.9%).
FMS was first diagnosed at the baseline visit in 29 patients (42%), whereas for the other 40 patients (58%) the diagnosis of FMS had been formulated during a previous appointment by their general practitioner or other specialists (Table 2).
| FIBROMYALGIA | |
| primary | 67 (97.1%) |
| secondary | 2 (2.9%) |
| DIAGNOSIS | |
| new diagnosis | 29 (42.0%) |
| previous diagnosis | 40 (58.0%) |
| ASSOCIATED DISEASES | |
| Osteoarthritis (different locations) | 11 (15.9%) |
| Anxiety-depressive syndrome | 11 (15.9%) |
| Others | 13 (18.8%) |
| SYMPTOMS | |
| Asthenia | 6898.6%) |
| Sleep disturbance | 5478.3%) |
| Headache | 4971.0%) |
| Cognitive disturbances | 48 69.6%) |
| Paraesthesia | 48 69.6%) |
| IBS | 45 65.2%) |
| Dizziness | 44 63.8%) |
| Dyspepsia | 40 58.0%) |
| Tachycardia | 40 58.0%) |
| Restless legs | 31 (44.9%) |
| Temporomandibular pain | 31 (44.9%) |
| Anxiety / depressive syndrome | 29 42.0%) |
| Irritable bladder | 28 (40.6%) |
| Vaginismus | 13 (18.8%) |
Table 2: Clinical characteristics of the patients at baseline. Data are indicated in terms of frequency (number and percentage).
The analysis of the symptom pattern showed that the characteristics of FMS vary greatly in form. Asthenia was the symptom most commonly reported by patients (98%), followed by sleep disturbance (78%), headache (71%), cognitive disturbances and paraesthesia (69%), IBD (65%), dizziness (63%), dyspepsia and tachycardia (58%). Other symptoms, such as anxiety/depression, irritable bladder, restless legs, temporomandibular pain and vaginismus, were also present but affected less than 50% of patients (Table 2).
The heterogenic pattern of FMS-related symptoms is reflected by the pharmacological treatments prescribed to patients before they were visited for the first time at our sites.
In about 20% of cases, patients were receiving cyclobenzaprine, a compound that improves both the sleep disturbance and musculoskeletal symptoms; other patients were on treatment with antidepressants, such as amitriptyline, duloxetine and SSRIs (4.3%), or with the muscle relaxant tizanidine (2.8%), or NSAIDs, such as nimesulide (2.8%) (Table 3).
| PRIOR TO VISIT 1 |
Cyclobenzaprine: 14 (20.3%) |
| Amitriptyline hydrochloride: 3 (4.3%) | |
| Nimesulide: 2 (2.8%) | |
| Paracetamol: 2 (2.8%) | |
| Sulfo-adenosyl-L-methionine: 2 (2.8%) | |
| Tizanidine: 2 (2.8%) | |
| Unreported: 31 (44.9%) |
Table 3: Treatments administered for fibromyalgia prior to Visit 1. The frequency of treatment is indicated as the number of patients receiving the drug (percentage between brackets).
In spite of the ongoing treatment, these patients were still suffering from significant painful symptoms. The average number of tender points was 17.6 ± 0.9 (range: 14-18), with a mean VAS of 8.1 ± 1.4 and an EPS of 2.6 ± 0.7. The reaction to evoked pain was “intermediate” and “strong” in 49.3 and 36.2% of patients, respectively, although in nine patients (13%) we registered a “very strong” evoked pain (Table 4). About 45% patients were naïve to any treatment for FMS.
| All population (n = 69) |
Patients attending two visits | ||
|---|---|---|---|
| Visit 1 (baseline) (n = 40) |
Visit 2 (3 months) (n = 40) |
||
| N° tender points | 17.6 ± 0.9 (14–18) |
17.5 ± 1.0 (14 – 18) |
16.4 ± 2.7* (8 – 18) |
| VAS | 8.1 ± 1.4 (4 – 10) |
8.1 ± 1.5 (5 – 10) |
5.5 ± 2.4*** (2 – 10) |
| Evoked Pain Score (EPS) | 2.6 ± 0.7 (1 – 4) |
2.7 ± 0.8 (1 – 4) |
2.0 ± 0.7*** (1 – 3) |
| No reaction (0) | 0 | 0 | 0 |
| Light reaction (1) | 1 (1.4%) | 1 (2.5%) | 9 (22.5%) |
| Intermediate reaction (2) | 34 (49.3%) | 18 (45.0%) | 20 (50.0%) |
| Strong reaction (3) | 25 (36.2%) | 14 (35.0%) | 11 (27.5%) |
| Very strong reaction (4) | 9 (13.0%) | 7 (17.5%) | 0 |
Table 4: Characteristics of pain at baseline and corresponding changes between visit 1 and visit 2.
All patients were given a treatment with a fixed combination tramadol 37.5 mg + paracetamol 325 mg (Patrol®, Alfa Wassermann). Therefore, in 31 patients (44.9%), the tramadol-paracetamol combination was the only therapy they received; in 27 patients (39.1%), the treatment was associated with cyclobenzaprine, and 7 patients (9.8%) received additional treatments for concomitant conditions (Table 5).
In all patients, the recommended dose was 3 tablets daily; however, after at least two weeks of treatment the patients were allowed to increase the dosage up to 4 tablets a day where necessary to control pain, or to reduce the dosage to 2 tablets a day if this dose was still sufficient to control FMS symptoms. 35% of patients reduced the daily dosage to 2 tablets after at least 2 weeks of treatment because it was considered sufficient to control symptoms.
The results achieved with the treatment were assessed only in the 40 patients who were able to attend a second visit at our sites before the earthquake on May 2012 significantly restricted the activities performed by our Hospital. In short, an improvement in pain symptoms was observed in all patients, in terms of a significantly reduced number of tender points from 17.5 ± 1.0 to 16.4 ± 2.7 (p< 0.05).
In a similar way, the VAS score dropped from 8.1 ± 1.5 at Visit 1 to 5.5 ± 2.4 at Visit 2 (p<0.001), whilst the EPS decreased from 2.7 ± 0.8 to 2.0 ± 0.7 (p<0.001). No patient had still a very strong reaction to the touch of tender points and muscles, and most patients (50.0%) had an intermediate reaction (Table 4).
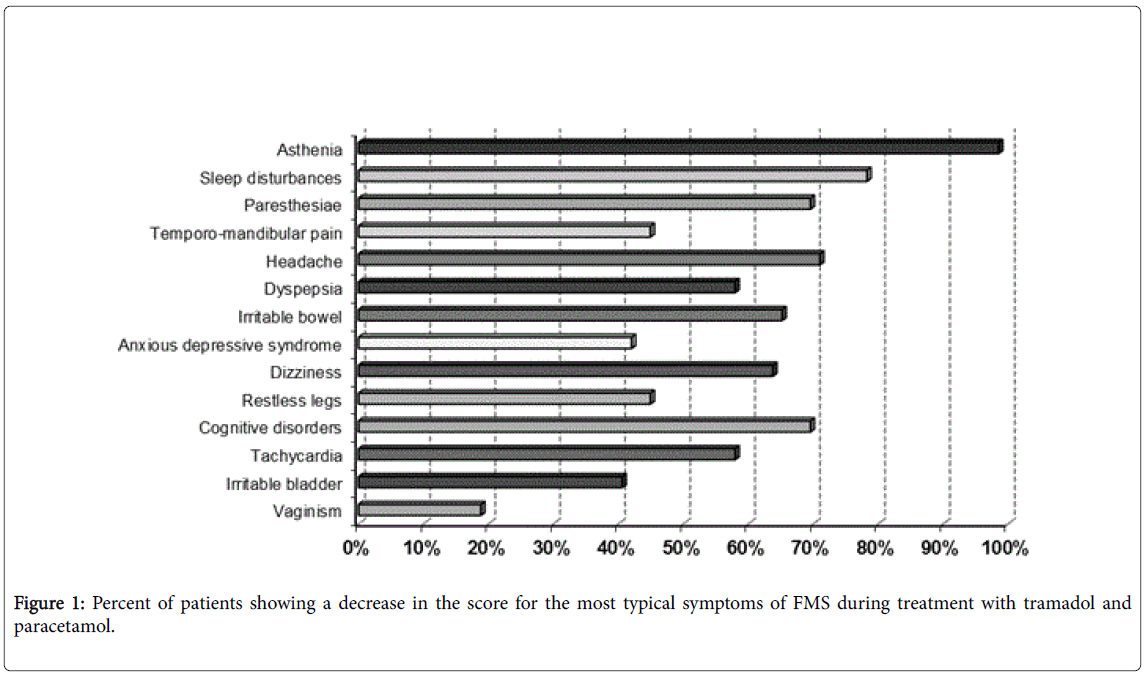
Figure 1 shows the percent of patients reporting an improvement in the most common symptoms of FMS. It is worth mentioning that about 60% of patients reported an improvement in sleep disturbance, followed by about 41 to 45% of patients who reported an improvement in four characteristic, very common symptoms of FMS, i.e. asthenia, paraesthesia, headache and restless legs. These last symptoms are thought to be related to altered serotoninergic neurotransmission within the central nervous system (CNS) and, therefore, they are targeted by serotoninergic compounds, such as tramadol.
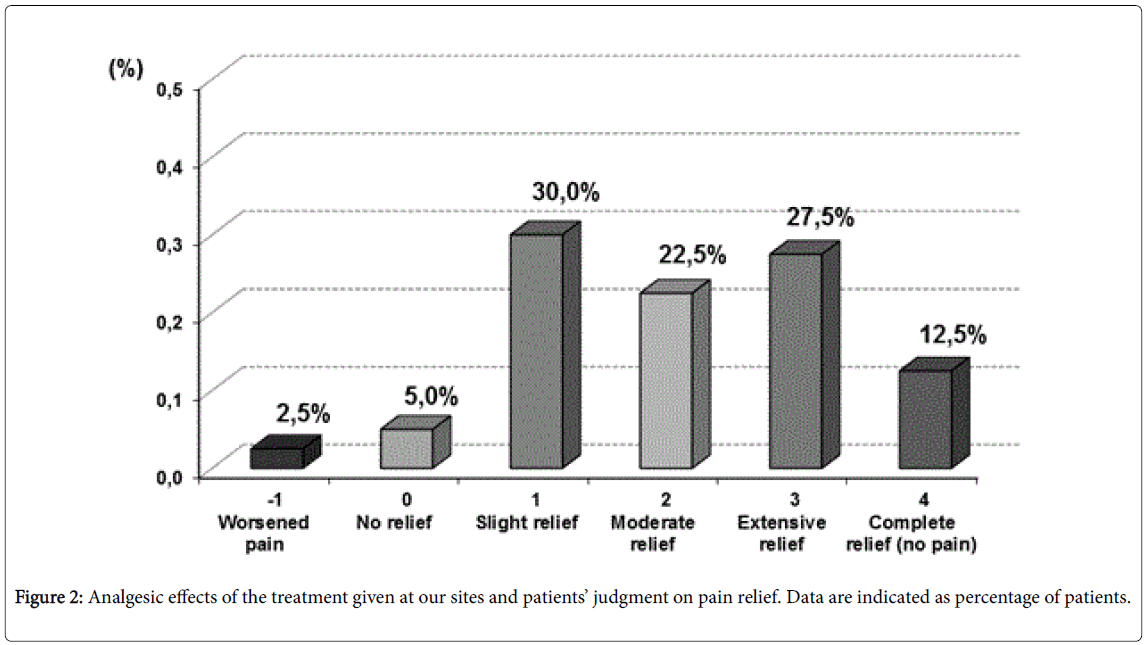
The opinions expressed by physicians and patients on the validity of the analgesic approach followed by our sites in patients with FMS, confirmed the results obtained using the pain indexes. At least 65% of patients reported they had a pain relief from “slight” to “very good”, whereas 35% of patients still claimed that they were suffering from significant pain. On their part, the investigators believed that 62.5% of patients had enjoyed a significant improvement in FMS-related pain, whereas relief was considered to be “slight” in a further 30% of patients and “null” in the remaining subjects (7.5%) (Figure 2).
Side effects were observed in 11 patients and most frequently represented by epigastric pain; severe allergic skin reactions with itchiness; nausea and dizziness; dizziness and cognitive disturbances; dizziness and itchiness; xerostomia; xerostomia and constipation; constipation; general malaise; drowsiness (Table 5).
| TREATMENT COMPLIANCE | |
| As prescribed | 26 (65.0%) |
| Reduced dose | 14 (35.0%) |
| N° TABLETS TAKEN DAILY | |
| 0/1/2/3/4/unreported | 2/1/13/10/14/10 |
| Other Drugs Used During The Study | |
| None | 30 (75.0%) |
| Nimesulide; duloxetine (2); oxycodone; indomethacin/ caffeine/prochlorperazine; NSAIDs; cyclobenzaprine; sertraline (2); herbal remedies. | |
| Side Effects | |
| None | 29 (72.5%) |
| Epigastric pain; severe allergic skin reaction with itchiness; nausea and dizziness; dizziness and cognitive disturbances; dizziness and itchiness; xerostomia; xerostomia and constipation; constipation; general malaise; drowsiness |
Table 5: Treatment compliance and adverse effects observed during the study. The frequency of treatment is indicated as number of patients (percentage between brackets).
Discussion
As FMS is a complex syndrome associated with a wide range of symptoms, treatment should be tailored to suit individual patients, dealing with their particular needs and targeting their most distressing symptoms [7,17]. In addition, non-pharmacological treatment options, including aerobic exercises, physiotherapy, cognitive behavioral therapy, massage and acupuncture can be helpful [18].
We believe, however, that the first aim of treating FMS should be to decrease pain and to increase function by means of a multimodal treatment strategy, which in most cases includes pharmacological intervention. Another important aspect is the concomitant treatment of any possible nociceptive pain caused by a different medical condition, for example, the pain caused by inflamed bursitis or degenerative spondylosis [19].
Non-Oncological Chronic Pain (NOCP) has come to be considered a socially important problem, since it has a severe impact on patient’s life and every year leads to 200 million lost working days in Italy [20].
Based on positive screens for the London Fibromyalgia Epidemiological Study Screening Questionnaire (LFESSQ-4), FMS was estimated to have a prevalence of 4.7% (95% CI: 4.0 to 5.3) in a survey conducted on the general population of five European countries including Italy [21]. This data suggests that about 3,000,000 Italians suffer from FM.
In recent years, the concept of “multimodal analgesia” has been developed for treatment of NOCP in general and FMS in particular. The concept is based on the exploitation of the different mechanisms of action of analgesics, by combining opioids with NSAIDs or paracetamol, in order to boost the resulting analgesic effect compared to the single compounds. Combination therapy could also lead to a reduction in the dose of each single analgesic and an optimization of the risk/benefit ratio and patient compliance; this objective is especially important in older people or in special populations with enhanced intolerance to either NSAIDs or opioids. From this point of view, tramadol is a centrally active analgesic of special interest that is recommended by the EU and American guidelines for the treatment of fibromyalgia [22-24]. By inhibiting the reuptake of norepinephrine and serotonin, it exerts an analgesic effect with minimal activation of the opioid receptors and, therefore, it is not usually considered a “pure” opioid. In particular, tramadol targets certain FMS symptoms, such as asthenia, paraesthesia, headache and restless legs, which are sustained by altered serotoninergic and noradrenergic neurotransmission within the CNS. Tramadol is therefore often used in combination with paracetamol, which works by reducing nitric oxide synthesis and prostaglandin release from the CNS. The fixed tramadol + paracetamol combination was successfully used in the treatment of FMS in a randomized, double-blind, placebo-controlled clinical trial. When the paracetamol 325 mg + tramadol 37.5 mg combination was administered four times a day in 315 patients with moderate-to-severe pain (≥ 40 VAS score), the fixed combination produced a significantly greater reduction in pain than placebo. Statistically significant differences were also observed in the total score and subscales of the Fibromyalgia Impact Questionnaire in favour of the active treatment. It is worth mentioning that the results were obtained by giving a daily dose of tramadol that is about 25% lower than the full dose needed for analgesia, which improved safety, as demonstrated by the absence of any serious adverse events [25].
The advantage of a fixed combination of tramadol+paracetamol in the treatment of FMS seems to be confirmed by our observational experience on 69 patients. Regardless of whether the tramadol +paracetamol combination was added to previous treatments for FMS (about 55% of patients) or started as a first treatment in naïve patients (about 45% of patients), a significant reduction in pain intensity and FMS symptoms was achieved in almost all patients, with an improvement in quality of life that was appreciated by patients. A more objective evaluation of pain, based on an EPS that we assigned to the patients on the basis of their reaction to the touch of tender points, confirmed the results achieved with the validated subjective pain scales. We decided to add this evaluation in order to have a more complete assessment of the patient. In longitudinal studies on FMS, improvements in pain scores are not necessary parallel to changes in evoked pain. This result suggests certain dissociation between the two dimensions of pain.
Most studies analyzing the correlation between psychological processes and pain are in fact based on subjective evaluations, asking directly to the patient information on his painful experience with numerical scales or visual analogue scales, but the data on the correlation between these parameters and evoked pain scores, that describe pain sensitivity to pressure, are lacking [26].
Unlike several compounds that are used (sometimes off-label) in the management of FMS and that act on one or two FMS symptoms (such as tricyclic antidepressants, SSRIs or SNRIs), we noticed that the tramadol-paracetamol combination affected most (if not all) the FMSrelated symptoms. In particular, about half of all patients experienced a reduction in asthenia, headache, paraesthesia and restless legs. It should be noted that these symptoms are not only the most disabling symptoms for patients in their daily activities; they are also the symptoms that most frequently lead to wrong diagnoses and pointless tests or examinations. For instance, in our experience, because they complain of restless legs, FMS patients often undergo electromyography examinations that are completely pointless, or those who complain of headaches are given headache medications that are not effective for FMS-related headaches. All these treatments constitute an unnecessary cost for instrumental exams for the National Health System, and pointlessly expose patients to ineffective drugs.
On the contrary, the combined mechanisms of action of paracetamol and tramadol are not merely analgesic; they may also provide a “ground-based” approach to FMS, as it is important that we remember that FMS is a “syndrome of symptoms” and not a single symptom or a disease sustained by a single pathogenetic mechanism. In addition, the tolerance of a fixed combination of paracetamol and tramadol is now documented by both published clinical trials and multiyear marketing experience [25,27-30].
The ELZA study [22] evaluated the safety of a fixed combination of paracetamol (325 mg) and tramadol (37.5 mg) in a prospective observational survey on 5,495 patients with severe pain; the combination was prescribed as first-line therapy in 37.6% of patients and after treatment failure in the remaining 62.4% of patients. Only 230 (4.2%) patients in the study reported one or more adverse events (AEs); all these adverse events were already known and were expected for this combination. The complementary action of the two active ingredients makes this combination an effective and well-tolerated therapy.
Another observational, multicenter, prospective study (the SALZA study) recruited 2,663 patients aged 65 years or over, who were treated with paracetamol+tramadol either as first-line treatment (30% of patients) or after failed or unsatisfactory efficacy (69.8%), and/or as a result of safety problems (7.8%) with at least one other analgesic. During the one-month study period, 14.7% of patients received an additional rescue analgesic. The study confirmed the efficacy of the paracetamol+tramadol combination with regard to pain intensity, pain relief, patient satisfaction and overall clinical impression evaluated by the patient, regardless of the aetiology of the pain or the duration of the underlying condition. The combination was well-tolerated; a total of 119 patients (4.5%) reported at least one adverse event, all of which were known and expected AEs [29].
The conclusion can be drawn from literature and our experience that “multimodal analgesia” represents a valid approach to patients with fibromyalgia and, in this regard, a combination of tramadol and paracetamol can be a valid analgesic treatment that exploits the different mechanisms of action of the two compounds. It should be remembered that the fixed combination of tramadol+paracetamol was recently suggested as a first-choice treatment in patients with acute exacerbation of pain by the IASP Guidelines on chronic neuropathic pain [30].
Lastly, following the earthquake affecting Modena and neighboring areas in May 2012, we noticed that several patients are reporting a worsening in their FMS and a significant exacerbation of their FMSrelated symptoms. It can be postulated that the post-traumatic stress caused by the earthquake is playing an important role in the outcome of FMS. It would be therefore interesting (and it is our intention) to prospectively evaluate how a dramatic event, such as an earthquake, can affect the progression of FMS in these patients in the months to come.
References
- Arnold LM, Clauw DJ, McCarberg BH, FibroCollaborative (2011) Improving the recognition and diagnosis of fibromyalgia. Mayo ClinProc86: 457-464.
- Williams DA, Schilling S (2009) Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am 35: 339-357.
- Sarzi-puttini P, Buskila D, Mease P (2015) “Fibromyalgia and related syndromes”. Clinical and Experimental Rheumatology 33: 1-88.
- Sarzi-Puttini P, Atzeni F, Perrot S (2012) La Fibromialgia. ReumatismoNumeroMonografico 64: 4.
- Blotman F, Branco J (2007) “Fibromyalgia, Daily aches and pain” Clinical and Experimental Rheumatology 3: 1
- Dell'Osso L,Carmassi C, Consoli G, Conversano C, Ramacciotti C, et al. (2011) Lifetime post-traumatic stress symptoms are related to the health-related quality of life and severity of pain/fatigue in patients with fibromyalgia. ClinExpRheumatol 29:S73-S78
- Cohen H, Neumann L, Glazer Y, Ebstein R, Buskila D (2009) “The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158) met and fibromyalgia” ClinExpRheumatol27: S51-S56.
- Carli G, Suman AL, Biasi G, Marcolongo R (2002) Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain 100: 259-269.
- Nielsen LA, Henriksson KG (2007) Pathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): the role of central and peripheral sensitization and pain disinhibition. Best Pract Res ClinRheumatol 21: 465-480.
- Bradley LA (2009) Pathophysiology of fibromyalgia. Am J Med 122 (Suppl 12): 1-13.
- Clauw DJ (2014) Fibromyalgia: a clinical review. JAMA 311: 1547-1555.
- Carbonell-Baeza A, Aparicio VA, Chillón P, Femia P, Delgado-Fernandez M, et al. (2011) Effectiveness of multidisciplinary therapy on symptomatology and quality of life in women with fibromyalgia. ClinExpRheumatol 29: S97-103.
- Giacomelli C, Sernissi F, Sarzi-Puttini P, Di Franco M, Atzeni F, et al. (2013) Fibromyalgia: a critical digest of the recent literature. ClinExpRheumatol 31: S153-157.
- Malemud CJ (2009) Focus on pain mechanisms and pharmacotherapy in the treatment of fibromyalgia syndrome. ClinExpRheumatol 27: S86-91.
- Gong YH, Yu XR, Liu HL, Yang N, Zuo PP, et al. (2011)antinociceptive effects of combination of tramadol and acetaminophen on painful diabetic neuropathy in streptozotocin-induced diabetic rats. ActaAnaesthesiologicaTaiwanica49:16-20.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160-172.
- Smith HS, Harris R, Clauw D (2011) Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 14: E217-245.
- Chong YY, Ng BY (2009) Clinical aspects and management of fibromyalgia syndrome. Ann Acad Med Singapore 38: 967-973.
- Buskila D (2009) Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res Ther 11: 242.
- Di Franco M, Iannuccelli C, Bazzichi L, Atzeni F, Consensi A, et al. (2011) Misdiagnosis in fibromyalgia: a multicentre study. ClinExpRheumatol 29: S104-108.
- Branco JC, Bannwarth B, Failde I, AbelloCarbonell J, Blotman F, et al. (2010) Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 39: 448-453.
- American Pain Society (2005) Guideline for the Management of Fibromyalgia Syndrome Pain in Adults and Children. Clinical Practice Guidelines Series 4
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62:600-610.
- Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, et al. (2008) EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 67: 536-541.
- Bennett RM,Kamin M, Karim R, Rosenthal N (2003) Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med114:537-545.
- Peñacoba Puente C, Velasco Furlong L2, Écija Gallardo C2, Cigarán Méndez M2, Bedmar Cruz D3, et al. (2015) Self-efficacy and affect as mediators between pain dimensions and emotional symptoms and functional limitation in women with fibromyalgia. Pain ManagNurs 16: 60-68.
- Pergolizzi JV, van de Laar M, Langford R, Mellinghoff HU, Merchante IM, et al. (2012) Tramadol/paracetamol fixed-dose combination in the treatment of moderate to severe pain. J Pain Res 5: 327-346.
- SerrieA, Ganry H, Creuzé A, Schatz B (2011) Epidemiological data, efficacy and safety on a fixed combination of paracetamol (325 mg) and tramadol (37.5 mg) in the treatment of moderate to severe pain, in general practice (ELZA survery; Efficacité et ToLerance de ZAldiar®. J ApplTher Res 8:3-14.
- Mejjad O, Serrie A, Ganry H (2011) Epidemiological data, efficacy and safety of a paracetamol-tramadol fixed combination in the treatment of moderate-to-severe pain. SALZA: a post-marketing study in general practice. Curr Med Res Opin 27: 1013-1020.
- IASP (2010) International Association for the Study of Pain: Pharmacological management of neuropatic pain. IASP 18:1-8.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 14007
- [From(publication date):
September-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13011
- PDF downloads : 996