Research Article Open Access
Effectiveness of Nanomaterial Copper Cold Spray Surfaces on Inactivation of Influenza A Virus
Sundberg K*, Champagne V, McNally B, Helfritch D and Sisson RMaterials Science and Engineering, Worcester Polytechnic Institute, Worcester, MA, USA
- Corresponding Author:
- Kristin Sundberg
Materials Science and Engineering, Worcester Polytechnic Institute
Worcester, MA 01609, USA
Tel: 978-844-4511
E-mail: kristinsundberg7@ yahoo.com
Received date:: September 10, 2015; Accepted date:: October 14, 2015; Published date:: October 21, 2015
Citation: Sundberg K, Champagne V, McNally B, Helfritch D, Sisson R (2015) Effectiveness of Nanomaterial Copper Cold Spray Surfaces on Inactivation of Influenza A Virus. J Biotechnol Biomater 5:205. doi:10.4172/2155-952X.1000205
Copyright: © 2015 Sundberg K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Bacterial and viral contamination of touch surfaces allows for transmission of pathogens leading to increased risk of infection. Previous work has demonstrated the antimicrobial properties of copper for contact-killing of microbes for use in hospitals. Less research exists on copper as an antiviral surface and on the effects of nanomaterial copper surfaces in the contact-killing of viruses. Nano agglomerate and conventional copper powder feedstock is used in the cold spray process to form copper coatings on aluminum substrates. The nano and conventional copper surfaces formed are tested for antiviral contact-killing of influenza A virus. After a two hour exposure to the surfaces, the surviving influenza A virus was assayed and the results compared. The differences in the powder feedstock used to produce the test surfaces were examined in order to explain the mechanism that caused the observed differences in influenza A virus killing efficiency. Results showed that the nano copper surface was antiviral, but less effective than a study on antimicrobial killing of MRSA on copper surfaces. The nano copper surface was more effective at percent reduction of influenza A virus than that of conventional copper. It was determined that the work hardening caused by the cold spray process in combination with the high number of grain boundaries results in a copper microstructure that enhances ionic diffusion. Copper ion diffusion is the principle mechanism for microbial and viral destruction on copper surfaces. Testing determined significant microbiologic differences between nano- and conventional Cu surfaces and demonstrates the importance of nano copper surfaces as an antiviral agent. The nano agglomerate powder shows superior antiviral effectiveness to that of conventional Cu due to an increase in grain boundaries at the nano level. Further research is needed to determine the effects of nano and conventional copper surface roughness on the contact-killing rate of viruses versus microbes on both a micro and nano-scale.
Keywords
Cold spray; Influenza A virus; Nanomaterial; Copper; Antiviral
Background
The epidemiology of nosocomial pathogens is a cause of concern in hospitals due to the possibility of increased risk of infection [1,2]. Influenza A, a viral pathogen, is one of the leading causes of infection and mortality of young children and the elderly (>65) [3]. During the 2012 and 2013 influenza seasons, the percentage of deaths attributed to pneumonia and influenza in the United States peaked at 9.9%, exceeding the epidemic threshold for 13 consecutive weeks [4].
It is indicated by the Center for Disease Control and Prevention (CDC), that the three main mechanisms of influenza virus transmission are the spread of infectious droplets through: airborne, person to person, or surface contact [5]. Research shows, influenza that is distributed by airborne nuclei can contaminate objects in the environment, leading to easy transfer at the hand-surface contact interface [6-8]. The spread of bacteria and viruses via hospital surfaces, including medical equipment, work stations, and patient rooms is well documented [2,6,9,10]. The addition of self-disinfecting surfaces in conjunction with required cleaning practices further prevents the spread of infectious diseases [11].
Research exists supporting the role of copper surfaces as an antimicrobial material [12-15]. A correlation is shown between the increase in copper content and the increased contact-killing of E. coli, Listeria monocytogenes, and Methicillin-Resistant Staphylococcus aureus [12]. Two hypothesized mechanisms for contact killing of microbes is the interaction of copper ions with the cellular membrane causing an influx of ions in the intracellular matrix, and copper induced DNA fragmentation, both resulting in cell death [16]. Testing of S. aureus and E. coli on nano Cu-incorporated diamond-like carbon films shows post-incubation TEM images of cytoplasm leakage from cell membranes and data from Transmission electron microscopy- Energy dispersive X-ray spectroscopy (TEM-EDS) indicating copper ion presence in the membranes of both microbes. This shows the primary contact-killing mechanism to be membrane deformation by copper ions [17].
Research also exists supporting the role of antimicrobial copper surfaces in the contact-killing of influenza A virus [18]. It is shown that Cu(II) ions added in solution to influenza A virus result in morphological abnormalities [19]. Similar studies with copper ion solutions show the increased sensitivity of enveloped viruses to Cu(II) ions as compared to non-enveloped viruses, suggesting that the virus’s lipid membrane becomes overwhelmed by intracellular copper, similar to the microbial kill mechanism [20].
The Copper Development Association (CDA) currently lists more than 450 wrought and cast copper alloys under the unified numbering system (UNS) that are registered with the U.S. Environmental Protection Agency (EPA) as antimicrobial [21]. All EPA approved surfaces contain a copper content of at least 60%. EPA-approved laboratory studies show that copper kills 99.9% of disease-causing bacteria including, Staphylococcus aureus, Enterobacter aerogenes, Escherichia coli O157:H7, Pseudomonas aeruginosa, Vancomycin-resistant Enterococcus faecalis (VRE) and MRSA, within 2 hours of contact. All EPA registered materials can be used for frequently touched surfaces, such as hospitals, for control and inactivation of microbes [22]. While there are specific guidelines for testing the efficacy of antimicrobial surfaces against microbes, there are no EPA approved protocols for testing their efficacy against viruses.
Increasing the kill rate of influenza A virus is important, as the survival of infectious influenza on fomites can last up to 72 hours [6]. Research shows that processing of copper material does affect the ability of copper to inactivate microbes [23]. Copper surface coatings can be created using various metal spray techniques including plasma, arc, and cold spray processes. During the cold spray process, a heated carrier gas brings copper powder to supersonic velocities, causing them to plastically deform and adhere to a metal substrate, forming a thin solid copper coating. A schematic of the cold spray process is shown in Figure 1. Powder particles are fed into a converging-diverging nozzle filled with a stream of heated helium or nitrogen gas and then blasted at the substrate, forming a mechanical bond with the surface upon impact. In general, the cold spray process operates at a temperature between 100-500°C and at a velocity range of 600-1000 m/s, resulting in <1% porosity and <1% of oxides.
Champagne et al. [23] indicates that of the plasma, arc, and cold spray processes, the later has the higher velocity impact of copper particles, resulting in increased material hardness and higher kill rate of Staphylococcus aureus. The aim of this research is to increase copper material hardness through the use of nano copper cold spray particles, which will create a harder copper coating that will show a higher kill rate towards Influenza A Virus.
Test Procedure
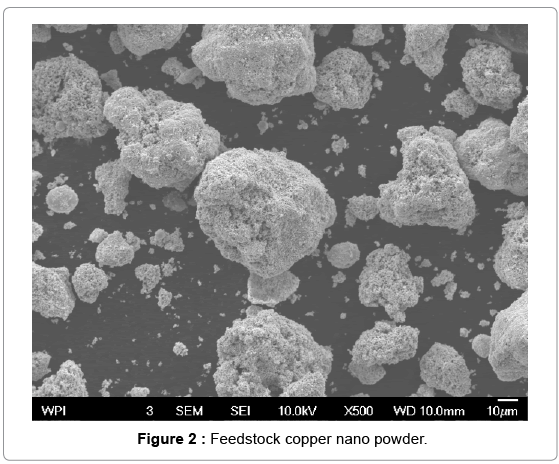
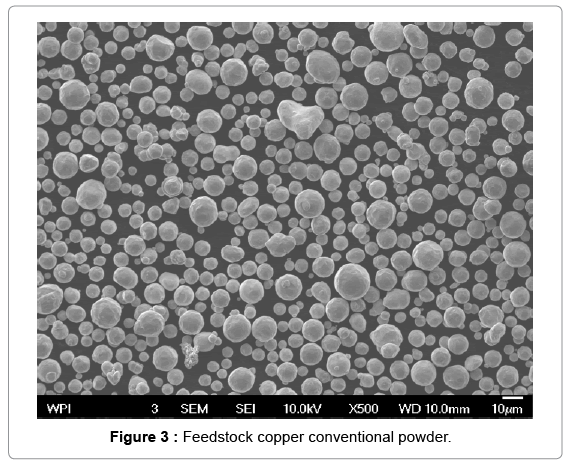
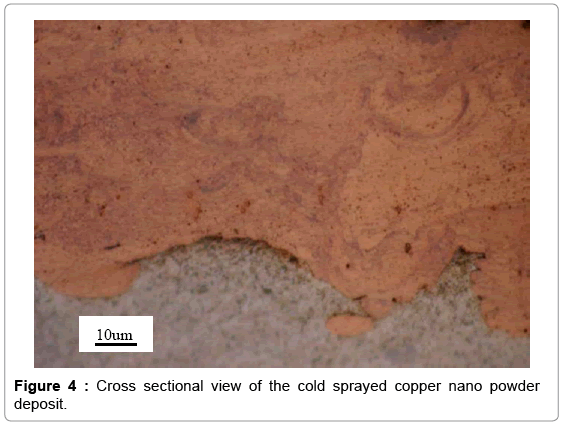
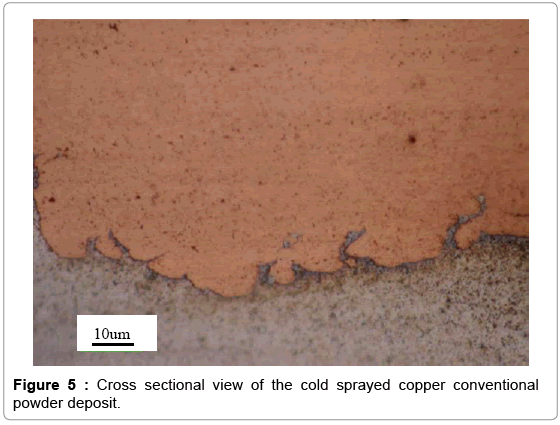
The cold spray surface coating technique was used to produce copper coatings approximately 0.05 mm thick on aluminum substrates. The nano and conventional copper powder feedstock used for cold sprays is shown in Figures 2 and 3. The particle size distribution for the nano copper (Eltron) and conventional copper (Praxair Cu-159) is an average of 25 microns and a range of -31/+5 microns, respectively [24,25]. To confirm powder particle size as reported by the powder producer, a , particle size distribution analysis was performed on the Horiba LA- 910 Laser Scattering Particle Size Distribution Analyzer, in a 10 g/L solution of Sodium Hexametaphosphate in DI Water. The nano and conventional powder are both pure copper. The conventional powder is produced by gas atomization. For the nano powder, nanosized particles were dispersed in a solvent with a pure copper binder precursor and spray dried under controlled atmosphere to form perfectly spheroidized micron sized particles (~ 20 um in diameter) by Eltron Research and Development Inc. [24,26] Copper nanoparticles offer many benefits over convention copper powders in terms of strength, hardness, wear and reactivity but cannot be used in the cold spray process due to insufficient mass. During the cold spray process, particles less than 5 microns in diameter can have insufficient momentum to penetrate the “bow shock” region next to the substrate [27]. Eltron utilizes spray drying to achieve strong, nanoparticle agglomerates with tight control of particle shape, size, distribution, and porosity with an ultra-fine grained (UFG) inorganic metallic binder that can match the nanoparticles in composition and that have sufficient mass and momentum to be used as a feedstock for the cold spray process [26]. Cross sections of the nano and conventional copper coupons produced are shown in Figures 4 and 5. Both deposits formed dense coatings with low porosity. Unlike the conventional Cu coating, the nano-structured deposit has areas of nano-Cu agglomerates surrounded by conventional Cu, which serves as a binder, as indicated by the darker colored regions of Figure 4. The clear difference in microstructure suggests a change in biological activity from conventional to nano copper surfaces.
The copper coated coupons were inoculated with influenza A virus, held at room temperature for two hours, and then survivors were resuspended and cultured. There are no EPA approved test procedures for copper alloy surfaces as a sanitizer of viruses.
The EPA antimicrobial procedure, “Test Method for Efficacy of Copper Alloy Surfaces as a Sanitizer”, was used with some changes [27]. The full details of the modified procedure are given below.
Carrier surfaces and preparation
Carrier surfaces and preparation: The surfaces of the nano and conventional copper coupons were the test carriers. The surfaces of the stainless steel squares were the control carriers. The carriers were sterilized with ethanol and allowed to dry prior to use in the test. Post sterilization, carriers were placed into Petri dishes.
Inoculation of carriers: A 100 μL aliquot of influenza A virus, ATCC VR-544, strain Hong Kong was inoculated into the test and control carriers. The inoculum was spread within 1/8 inch of the carrier edge. The virus was then air-dried at room temperature (20ºC) in a relative humidity of 23.5% until visibly dry (30 minutes). This temperature and humidity was selected to maximize virus survival while drying. Exposure time began at the 30 minute drying mark.
Neutralization of subculture: Following the two hour exposure time, a 1.00 μL aliquot of neutralizing test medium was inoculated onto the carriers and the carriers were scraped with a sterile cell scraper. The test medium is comprised of minimum essential medium (MEM) and 100 μg/mL penicillin, 10 μg/mL gentamicin, 2.5 μg/mL amphotericin B, 2 μg/mL TPCK-Trypsin, 0.2% bovine serum albumin (BSA), and 25 mM HEPES. Post scraping, 10-fold serial dilutions were performed in the test medium. An 100μL aliquot from each dilution was inoculated into the indicator cell cultures in quadruplicate. Indicator cell cultures were MDCK (canine kidney) cells.
Incubation and observation: The cell cultures were incubated at 36-38ºC in 5-7% CO2 for seven days. Following incubation, for each test the average TCID50 was calculated. The average log and percent reduction in viral titer of each test substance were calculated using the corresponding average TCID50 of the control carriers (stainless steel).
Results and Discussion
The ability to increase the contact-killing rate of influenza A virus on copper coatings is suggested to relate to the increase in Cu ion diffusion through the virus’s lipid membrane [20]. Champagne et al. shows that diffusion of copper ions from conventional copper surfaces is increased by copper surface hardness, resulting in increased microbial destruction [23]. Surface hardness of copper coatings can be increased further by using nano copper powder as opposed to the conventional powder typically used, as detailed in the below research results.
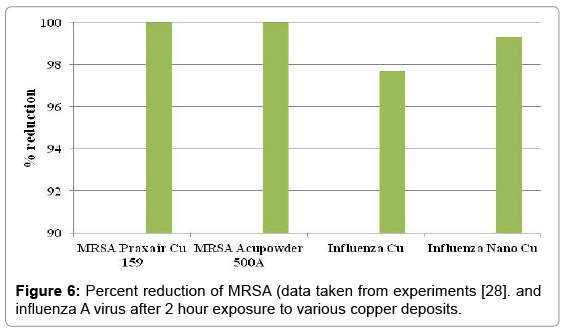
The percent reduction of influenza A virus after two hours on the surface of nano and conventional copper cold spray surfaces is shown in Figure 6. Also included in Figure 6 is data provided by the Army Research Laboratories on testing of copper cold spray surfaces for percent reduction of MRSA [28]. The results show that nano copper and conventional copper have a 99.3% and 97.7% reduction in influenza A virus, respectively. The use of copper as an antimicrobial material shows >99.9% reduction of MRSA after 2 hours. Copper is antiviral, but less effective at contact-killing of viruses than microbes.
The contact-killing of viruses by nano copper surfaces is more efficient than that of conventional copper. This difference in antiviral effectiveness between nano and conventional copper requires an examination of how the powder feedstock for the cold spray deposition process affects the nature of a copper coating.
The cold spray process operates at temperatures between 100- 500ºC and velocities between 600-1000 m/s. Due to the unique cold spray process conditions, two specific properties can be attributed to the copper cold spray coating. The first, high velocity impact of the powder particles creates a low porosity coating. Generally, cold spray coatings result in <1% porosity and <1% of oxides. Second, by blasting the powder particles at a high velocity and relatively low temperature, work hardening is induced. Work hardening results in high material dislocation density cause by the plastic deformation of particles’ as they hit the substrate and neighboring particles. Champagne et al. has shown that work hardening by cold spray increases dislocation density within copper [29]. By increasing the number of particles that collide with each other, the work hardening increases. This in turn will increase dislocation density and ultimately material hardness.
Particle size distribution analysis shows that the average particle size for nano and conventional copper powder was 37.8 microns and 10.4 microns, respectively. While the cold spray nano powder feedstock is larger, it contains many nano sized particles on the scale of 60-80 nm (Sky Spring Nanomaterials) [26]. Post agglomeration, the micron sized nano powder is heat treated using a proprietary process developed by Eltron to form micron sized particles with a nano grained structure [26]. The increase in nano grained boundaries in the nano powder cold spray feedstock allows for increased dislocation density post-processing. The relationship between dislocation density and diffusivity is Dp ∝ ρ [30]. The diffusion of copper ions is increased with the hardness increase produced by nano grain boundaries, which enhances copper ion flow for viral destruction.
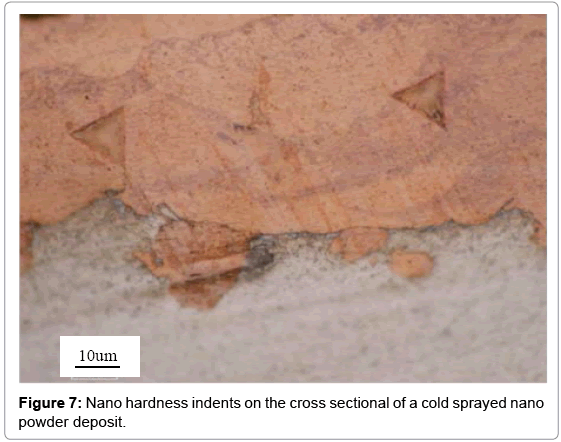
As illustrated in Figure 7, nano hardness testing was completed, with the resulting values for the nano copper and conventional copper surfaces as 2.44 and 1.45, respectively. The relationship between hardness and dislocation density is H2 ∝ ρ [31]. It follows that the nano copper surface has higher nano hardness, meaning a higher dislocation density from work hardening. Due to the reduced size of nano copper powder in comparison to the conventional powder, there are a greater number of particles present in the nano copper powder when sprayed. This means that there are a greater number of grain boundaries too. The more grain boundaries present upon impact with the substrate, the greater the work hardening resulting in higher dislocation density.
Dislocations at grain boundaries of metals increase ion diffusion, also known as pipe diffusion [32]. The increase in dislocation on the nano copper coating led to higher ion diffusivity reflected in a greater reduction in influenza A virus than conventional copper surfaces.
Conclusion
The effectiveness of copper as antimicrobial for contact-killing on touch surfaces has been well documented [12-15]. Less research exists on the effectiveness of copper as an antiviral agent and on the effects of nano grained copper surfaces on contact-killing rate of viruses. The significant differences between nano and conventional copper surfaces produced by cold spray technique, as shown here, demonstrate the importance of the copper powder processing and of the resulting deposition structure. The nano copper surface showed antiviral effectiveness caused by high dislocation density imparted to the sprayed particles by a combination of cold spray velocity and pre-processing heat treatment of nano particle agglomerates. The high dislocation density, measured by an increase in material hardness, allowed for higher copper ion diffusivity.
Cold spray technology is currently in use to produce metal copper coatings for hospital applications. Touch surfaces such as the copper coated hospital tray in Figure 8; show the current use of copper as an antimicrobial surface. Data collected on virus contact-killing properties of copper supports the potential future use of copper in hospitals and other industries for antiviral applications.
Both the nano and conventional copper for influenza A virus reduction have lower % reduction values than that of the copper surfaces used for contact-killing of MRSA. Surface roughness is documented to effect microbe retention [33]. Roughness of the copper surface could be a contributing factor to the higher contact-killing levels of influenza A virus by nano copper. Microbes, like MRSA, range in size from 0.5- 1.0 μm in diameter, whereas viruses like influenza A, are on a 80-120 nm scale [34,35]. The size difference may affect the ability of microbes and viruses to form reservoirs of survivors in microscopic pockets and crevices on copper surfaces. Further analysis of surface metrology on a micron and nano scale is needed to determine the effect of conventional and nano copper surface structures on the contact-killing of influenza A virus.
Acknowledgements
The sanitizer testing conducted against influenza A virus and reported in this paper was carried out under contract by ATS Labs at Eagan MN. The experiments were performed by Shanen Conway.
References
- Page K, Wilson M, Parkin I (2009) Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Mater Chem 19: 3819-3831.
- Hota B (2004) Contamination, Disinfection, and Cross-Colonization: Are Hospital Surfaces Reservoirs for Nosocomial Infection? Clinical Infectious Diseases 39:1182-1189.
- Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montaño LF, et al. (2009) Influenza control in the 21st century: Optimizing protection of older adults. Vaccine 27: 5043-5053.
- Centers for Disease Control and Prevention (CDC) (2013) Influenza activity--United States, 2012-13 season and composition of the 2013-14 influenza vaccine. MMWR Morb Mortal Wkly Rep 62: 473-479.
- CDC (2005) Infection control measures for preventing and controlling influenza transmission in long-term care facilities.
- Boone SA, Gerba CP (2007) Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 73: 1687-1696.
- Goldmann DA (2000) Transmission of viral respiratory infections in the home. Pediatr Infect Dis J 19: S97-102.
- Weber TP, Stilianakis NI (2008) Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J Infect 57: 361-373.
- Neely AN, Maley MP (2000) Survival of enterococci and staphylococci on hospital fabrics and plastic. J ClinMicrobiol 38: 724-726.
- Bergen LK, Meyer M, Høg M, Rubenhagen B, Andersen LP (2009) Spread of bacteria on surfaces when cleaning with microfibre cloths. J Hosp Infect 71: 132-137.
- Tamimi AH, Carlino S, Gerba CP (2014) Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am J Infect Control 42: 1178-1181.
- Michels H, Wilks S, Noyce J, Keevil C (2005) Copper Alloys for Human Infectious Disease Control. Proceedings of the Materials Science and Technology Conference, Pittsburgh, PA Copper for the 21st Century Symposium.
- Copper Development Association (2007) Copper Clinical Trial: Investigating the antimicrobial properties of copper alloys in healthcare facilities.
- Noyce J, Michels H, Keevil C (2006) Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. Journal of Hospital Infection 63: 289-297.
- Weaver L, Michels HT, Keevil CW (2008) Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J Hosp Infect 68: 145-151.
- Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77: 1541-1547.
- Lee F, Wang D, Chen L, Kung C, Wu Y, et al. (2013) Antibacterial nanostructured composite films for biomedical applications: microstructural characteristics, biocompatibility, and antibacterial mechanisms. Biofouling 29: 295-305.
- Noyce JO, Michels H, Keevil CW (2007) Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73: 2748-2750.
- Horie M, Ogawa H, Yoshida Y, Yamada K, Hara A, et al. (2008) Inactivation and morphological changes of avian influenza virus by copper ions. Arch Virol 153: 1467-1472.
- Sagripanti JL, Routson LB, Lytle CD (1993) Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol 59: 4374-4376.
- Copper Development Association (2015) Properties of Wrought and Cast Copper Alloys.
- Copper Development Association. The Copper Advantage: A Guide to Working with Copper and Copper Alloys.
- Champagne VK, Helfritch DJ (2013) A demonstration of the antimicrobial effectiveness of various copper surfaces. J BiolEng 7: 8.
- Praxair: Surface technologies. Powder Solutions, product brochure.
- Test method for efficacy of copper alloy surfaces as a sanitizer.
- Rolfe S (2014) email conversation and Phase I SBIR Final Report, “Advanced Nanostructured Powders for Cold Spray Applications”, Eltron Research and Development Inc., 4600 Nautilus Court South Boulder, CO 80301-3241.
- Liu J (2006) Cold spray formation of thin metal coatings. US Patent Application US 2006/0090593.
- Eltron: Research and development. Copper Nanograined Agglomerates.
- Helfritch D (2014) Email conversation.
- Mittemeijer E (2010) Fundamentals of Materials Science. Springer, Verlag, Berlin, Heidelberg.
- Mehrer H (2007) Diffusion in solids: Fundamentals, methods, materials, diffusion-controlled processes. Springer, Berlin, Heidelberg, New York.
- Champagne V, Helfritch D, Trexler M (2007) Some Material Characteristics of Cold-Sprayed Structures. Res Lett Mater Sci.
- Center for Disease Control Prevention. Electron Micrograph of Methicillin-Resistant Staphylococcusaureus (MRSA).
- Hart E (1957) On the role of dislocations in bulk diffusion. ActaMetall 5: 597.
- Rapid reference to influenza. The influenza virus: structure and replication. Elsevier Ltd.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15574
- [From(publication date):
December-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14371
- PDF downloads : 1203