Research Article Open Access
Effectiveness of a Specific Care Plan in Alzheimers Disease in the Oldest Old
Sandrine Sourdet1*, Sophie Guyonnet1,2,3, Maria E. Soto1,2,3, Sandrine Andrieu2,3,4, Christelle Cantet2,3, Bruno Vellas1,2,3 and Fati Nourhashemi1,2,3
1Gérontopôle, Hôpital La Grave-Casselardit, 170 avenue de Casselardit, 31059 Toulouse Cedex 9, France
2Inserm Unit 1027, Toulouse, France
3University of Toulouse III, 37 Allées Jules Guesde, 31073 Toulouse Cedex, France
4Department of Epidemiology and Public Health, Toulouse University Hospital, 37 Allées Jules Guesde, 31073 Toulouse Cedex, France
- Corresponding Author:
- Sandrine Sourdet
Service de Médecine Interne et de Gérontologie Clinique
Pavillon Junod, 170 avenue de Casselardit Hôpital La Grave-Casselardit
TSA 40031, 31059 Toulouse Cedex 9, France
Tel : (33) 5 61 77 79 29
E-mail: sourdet.s@chu-toulouse.fr
Received date: September 01, 2015; Accepted date: October 12, 2015; Published date: October 19, 2015
Citation: Sourdet S, Guyonnet S, Soto ME, Andrieu S, Cantet C, et al. (2015) Effectiveness of a Specific Care Plan in Alzheimer’s Disease in the Oldest Old. J Alzheimers Dis Parkinsonism 5:194. doi: 10.4172/2161-0460.1000194
Copyright: © 2015 Sourdet S, wt al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: To investigate the effectiveness of a multicomponent specific care and assistance plan, in reducing the rate of functional decline in oldest-old patients (age > 85 years) with Alzheimer’s disease (AD), compared with usual care. Patients and methods: This is a post-hoc analysis of a cluster randomized trial (the PLASA study), assessing the impact of a specific care plan in AD patients in 50 memory clinics in France. Two hundred community-dwelling mild to moderate AD patients aged 85 or more, were analyzed: 97 patients were enrolled in the intervention group and 103 in the control group (usual care). Patients and their caregivers in the intervention group had a twice-yearly follow-up, with a comprehensive standardized and global assessment. If any complication was identified during the assessment, standardized management protocols were proposed to guide the intervention of the physician, along with an information and training of the caregiver. The primary outcome was measured by change on the Alzheimer’s disease Cooperative Study-Activities of Daily Living (ADCS-ADL) scale at 24 months, and analyzed on an intentionto treat-basis, using a mixed model. Results: Of the 200 participants randomized and analyzed, 89 completed the study: 36 (37.11 %) in the intervention group and 53 (51.46 %) in the control group. The participants’ mean age were respectively 88.1 years (SD 3.1) and 87.8 (SD 2.3) in the intervention and control group. Intervention showed no effectiveness in reducing the rate of functional decline at two years. Indeed, the decline in the ADCS-ADL score was -12.8 (SE=4.0) in the intervention group, and -9.0 (SE=3.0) in the control group (p=0.46). Conclusion: A comprehensive specific health care plan did not reduce functional decline rate in oldest-old patients with mild to moderate AD, followed-up in memory clinics. More research is needed to identify actions that will lessen functional decline in this high-risk population.
Keywords
Functional decline; Alzheimer’s disease; Specific care plan; Oldest-old
Abbreviations
AD: Alzheimer’s Disease; SD: Standard Deviation; MMSE: Mini-Mental State Examination; ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; NPI: Neuropsychiatric Inventory; ADCS-ADL: Alzheimer’s Disease Cooperative Study- Activities of Daily Living
Introduction
The ageing population is rapidly growing and the oldest-old group (aged > 85 years) represents the fastest growing age group in western countries. From 16 million people in OECD (Organization for Economic Co-Operation and Development) countries in 2000, their number is projected to increase fourfold to 70 million by 2050, and will represent 5% of the total population [1].
The soaring number of the oldest old population will lead to significant social and economic challenges. With prevalence estimates of dementia up to 40% in this age group [2], there will be more people with dementia aged > 85 in the middle of the century than currently at all ages. Alzheimer’s disease (AD) is the most common cause of dementia worldwide [3], and probably one of the most common in the oldest-old population [4,5]. AD is associated with cognitive but also functional decline, which increases the risk of institutionalization and mortality, and lowers quality of life [6].
Several authoritative groups have published consensus guidelines for the care of patients with AD, and have suggested regular follow-up [7–9] aiming to delay disability. We recently published a cluster randomized trial which assessed the effectiveness of a comprehensive specific care plan in decreasing the rate of functional decline in communitydwelling patients with mild to moderate AD, compared with usual care in memory clinics [10]. We supported the idea that a regular and standardized follow-up in AD (including cognitive, functional, nutritional, psychological, behavioural and social assessment), with comprehensive guideline-based intervention in each field, may decrease the progression of disability. We did not conclude on a positive effect of this specific assessment and management on functional decline, in a study population with an average age of 80.2 years.
However, patients at particular risk of decline, such as oldest-old patients (aged > 85 years), may be more likely to benefit from such an intervention. The prevalence of disability is very high in this population [11], owing to numerous comorbidities and medications [12,13], depression [14], or decrease in physical activity [15]. These patients experience a high number of hospital admissions and readmissions, in particular linked to dementia [16], with an increased risk of hospitalization-associated disability [17]. Furthermore, in oldest-old AD patients, progression of dependency seems to be more rapid than in the younger elderly, even for a comparable cognitive decline [18]. We hypothesize that a standardized specific care and assistance plan may be effective in this vulnerable population.
The aim of this study was to evaluate the effectiveness of a comprehensive specific care plan in decreasing the rate of functional decline in community-dwelling patients with mild to moderate AD, aged 85 years or more, compared with usual care in memory clinics.
Patients and Methods
Design and participants
The PLASA study (Plan de Soin et d’Aide dans la maladie d’Alzheimer, or specific care and assistance plan for Alzheimer’s disease) is a two-year cluster randomized trial, comparing an intervention group receiving a comprehensive specific care plan for AD, with a control group receiving usual care. The main purpose is to assess whether or not a specific care and assistance plan can decrease the rate of functional decline in this population. The study took place in 50 memory clinics in France (University and General Hospitals). To minimize the potential of contamination across groups, memory clinics were the unit of randomization and participants were the unit of analysis. After randomization, 26 memory clinics made up the intervention group and 24 memory clinics made up the control group. Patients were recruited between June 2003 and July 2005. Details regarding the study, intervention and randomization are published elsewhere [10].
The study protocol was approved by the Institutional Ethics Review Committee. Written informed consent was obtained from all patients before beginning protocol-specific procedures.
To be eligible for the PLASA study, patients had to meet the following inclusion criteria: age >65 years, diagnosed with probable or possible AD according to the NINCDS-ADRDA criteria [19], with a Mini-Mental State Examination (MMSE) score between 12 and 26, not bedridden or in a wheelchair, living at home with a clearly identified informal caregiver, and not participating in any other research program. In this sub-analysis, we selected only patients aged 85 years or more.
Intervention: Alzheimer’s disease specific care and assistance plan
The plan was developed by a multidisciplinary working group comprising neurologists, geriatricians, psychiatrists, and general practitioners. It was based on data from scientific literature, on personal experience of the working group members, and incorporated opinions of representatives from the medical and social sectors, of patients’ families, and of care team members. We established the necessity of a comprehensive, standardized, twice-yearly assessment in several fields: caregiver information and support, social and health resources, supervision of drugs (including AD treatment), nutritional status, gait disorders and walking capacities, psychological and behavioural symptoms, cognitive decline, dependency, risks and legal protection, appropriateness of the environment and admission to an institution, advanced stages of the disease, and communication with other professionals. Patients in the intervention group were assessed every six months at the memory clinic, along with their caregivers. Additional appointments were fixed if considered necessary. The patients underwent physical, neuropsychological, geriatric and social assessment, using standardized tools. Progression of cognitive decline was measured with the MMSE [20]. Functional capacities were measured with the Activities Of Daily Living (ADL) scale [21], and Instrumental Activities Of Daily Living (IADL) scale [22]. Nutritional status was determined with the mini-nutritional assessment (MNA) [23]. Behavioural disturbances were evaluated with the Neuropsychiatric Inventory (NPI) [24]. Gait disorders were assessed with the one-leg balance test. The Zarit Burden interview was used to evaluate the strain on caregivers [25]. Caregivers and patients were both asked about living arrangements, chronic conditions, and current medication. Cardiovascular risk factors (diabetes mellitus, past or current smoking, hypertension, dyslipidemia) and co-morbidities (peripheral atherosclerosis, stroke, chronic obstructive pulmonary disease [asthma, chronic bronchitis, pulmonary emphysema], painful osteoarthritis and cancer) were also recorded.
Furthermore, in each field, we developed standardized management protocols that were initiated depending on the results of the comprehensive assessment (full details of these protocols are available at http://cm2r.enamax.net/onra/images/stories/ fiches_ plasa. pdf). Written and digital (CD-ROM) documents containing the details of the assessment, its analysis, and possible pharmacological or nonpharmacological management, was provided to the health professionals and in particular to the physician. Written support material was also offered to the caregivers and patients’ relatives, to improve their knowledge and understanding of the disease, and to offer solutions to specific problems.
Control - Usual care
Patients in the control-usual care arm were monitored according to the center’s established practice. We nevertheless decided on a regular annual visit in the control group, in order to reduce the number of patients lost during follow-up. Physicians in control memory clinics did not have access to the intervention.
Outcome measures
The efficacy of the specific care and assistance plan was assessed on the basis of loss of independence in the activities of daily living, using the ADCS-ADL (Alzheimer’s Disease Cooperative Study-Activities Of Daily Living) scale [26]. The questionnaire was submitted to all patients at baseline, one year and two years, during the scheduled visits in the centers.
Statistical analysis
We conducted a post-hoc analysis restricted to patients 85 years of age or more. An intention-to-treat analysis was used, including all randomized patients. The primary efficacy endpoint was change from baseline in the ADCS-ADL score at two years. We used two models to compare the rate of functional decline in the intervention group and the control group. The primary model of analysis was an imputation method. As the ADCS-ADL score was calculated by adding item responses, missing values were observed for certain item responses and total scores. The imputation procedure replaced each missing score with a reweighed score, derived from a rule of three sums from non-missing items, if five or fewer items were missing. If more than five items were missing, the score was considered as missing. Analysis was then performed using a mixed model. The secondary model of analysis was a mixed model that did not impute data in case of missing outcomes. Statistical analyses were carried out using SAS software version 9.1.
Results
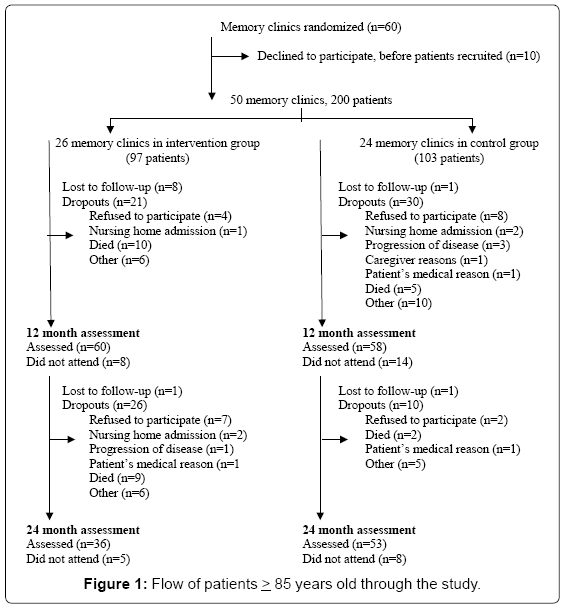
The study cohort consisted of 200 patients aged 85 years or more: 97 in the intervention group and 103 in the control group (Figure 1). The patients’ baseline characteristics were similar (Table 1). The patients’ mean age was 88.1 years (SD 3.1) in the intervention group and 87.8 years in the control group (SD 2.3). The majority of participants was female and was receiving acetylcholinesterase inhibitors at baseline. According to the mean MMSE score (18.9 (SD 3.7) in the intervention group and 19.1 (SD 3.9) in the control group patients were at a mild to moderate stage of dementia. Forty-two per cent of the entire cohort lived alone, and in 27.5% of cases caregivers were the spouse, and in 59.5% were children.
| Characteristics | Intervention group (n=97) |
Control group (n=103) |
|---|---|---|
| Patients | ||
| Mean age, years (SD) | 88.1 (3.1) | 87.2 (2.3) |
| Women (n, %) | 63 (64.95) | 72 (69.9) |
| Centre of recruitment: University hospital (n,%) | 43 (44.33) | 58 (56.31) |
| Mean MMSE score (0-30) (SD) | 18.9 (3.7) | 19.1 (4.0) |
| Use of cholinesterase inhibitors or memantine at baseline (n, %) | 75 (77.3) 93 (95.9) |
82 (79.6) 99 (96.1) |
| Number of non-dementia-related drugs (n, %) ≤ 3 >3 |
38 (39.2) 59 (60.8) |
51 (49.5) 52 (50.5) |
| Mean number of chronic diseases (SD) | 2.2 (1.4) | 2.2 (1.2) |
| Living arrangements (n, %) Living alone Living with spouse Other |
41 (42.3) 29 (29.9) 27 (27.8) |
43 (41.8) 37 (35.9) 23 (22.3) |
| Caregivers | ||
| Mean age (years) (SD) | 64.9 (11.9) | 66.0 (12.7) |
| Women (n, %) | 70 (72.2) | 71 (68.9) |
| Living with patient (n, %) | 37 (38.5) | 45 (44.1) |
| Relationship to patient (n, %) Spouse Child Other relationship |
24 (24.8) 59 (60.8) 14 (14.4) |
31 (30.1) 60 (58.3) 12 (11.7) |
AD: Alzheimer’s disease; SD: Standard deviation; MMSE: Mini-Mental State Examination
Table 1: Baseline characteristics of patients with AD and their caregivers randomized to a specific care plan for the management of AD or to usual care (control). Values are numbers (percentages) unless stated otherwise.
A total of 37.1% (n=36) of patients in the intervention group and 51.5% (n=53) in the control group completed the two-year follow-up visit (Figure 1). During the study, 19.6% of patients in the intervention group and 6.8% in the control group died. Furthermore, 11.3% of subjects in the intervention group refused to participate, versus 9.7% in the control group. There were no differences between the baseline characteristics of patients who completed and of patients who dropped out of the study.
Disease progression and main outcome measures
The baseline ADCS-ADL total scores were comparable in the intervention and control groups.
Both statistical models failed to show a beneficial effect of the intervention on the primary outcome (difference in change over the two years of follow-up in total score for ADCS-ADL). In the first model, with no imputation value, the two-year decrease in ADCS-ADL score was -12.8 (SE=4.0 in the intervention group and -9.0 (SE=3.0) in the control group (p=0.46). After imputation by the rule of three sum for missing data, the two-year decrease in the ADCS-ADL score was -11.0 (SD=2.3) in the intervention group and -9.7 (SD=1.9) in the control group (p=0.65) (Table 2).
| ADCS –ADL score (0-78) | Crude change or estimate |
P values |
|||
|---|---|---|---|---|---|
| Variables | Baseline | 12 months | 24 months | ||
| Analysis with no imputation for missing data a |
|||||
| Intervention group | 50.6 (2.1) | 44.2 (2.5) | 37.8 (4.1) | - 12.8 (4.0) | |
| Control group | 49.3 (1.9) | 44.8 (2.0) | 40.3 (2.9) | -9.0 (3.0) | 0.46 |
| Analysis using imputation by rule of three for missing data | |||||
| Intervention group | 48.2 (1.9 ) | 42.7 (2.0) | 37.1 (2.7) | - 11.1 (2.31) | |
| Control group | 44.4 (1.8 ) | 39.6 (1.9) | 34.7 (2.4) | - 9.7 (1.9) | 0.65 |
a Only patients with scores. The ADCS-ADL scale is a caregiver-rated questionnaire of 23 items assessing functional capacities, with a score ranging from 0 to 78; the highest score represents full functioning with no impairment.
ADSC-ADL: Alzheimer’s Disease Cooperative Study-activities of daily living
Table 2: Mean scores on alzheimer’s disease cooperative study-activities of daily living (ADSC-ADL) scale at baseline and at 12 and 24 months. Values are means (standard error) unless stated otherwise.
Discussion
In this national multicentre study, 200 oldest-old AD patients were recruited. They lived in both rural and urban environments, and despite their follow-up in memory clinics, their characteristics are probably similar to those of AD patients treated in other clinical settings, as only specialists (neurologists, psychiatrists and geriatricians) can initiate specific treatment for dementia in France. However, certain characteristics may differ from the general population or other studies, and may limit the generalization or the comparison of our results. First, we reported a baseline ADCS-ADL score of 50.6 (2.1) and 49.3 (1.9) respectively in the intervention and control groups. In a recent study investigating the tolerability of the rivastigmine patch in mild to moderate AD patients (mean MMSE = 19.6 (SD=4.3)), the baseline ADCS-ADL was similar to our study (50.3 (SD=20.1)), but in a younger sample of patients (mean age=74 (SD=7.7)). In another study exploring the benefit of a switch from donepezil to galantamine, in patients with mild to moderate AD, baseline ADCS-ADL mean score was 57.2 (SE=1.5), in a population with a mean age of 74.6 years (SE=0.8). We may have recruited older subjects with a relatively high level of functioning, based on these results.
Our study focused on oldest-old AD patients living at home with a clearly identified caregiver. The results of this study did not show any positive results of a comprehensive and specific care plan on delaying disability. Th ere was no difference in the two-year rate of functional decline, measured by the ADCS-ADL scale in the intervention group, compared with the usual-care control group, in mild to moderate AD patients, aged 85 years or more.
To the best of our knowledge, this is the first study evaluating the effectiveness of a specific care plan for AD in an oldest-old population in memory clinics. Despite the fact that the oldest old represent the largest and most expanding part of the dementia population [27], few studies have investigated the management of patients with AD amongst the oldest old. This age group is usually excluded from drug trials because of their comorbidities. Moreover, even non-pharmacological studies are often limited in such an aged population due to the difficulties with enrollment and follow-up that this population group presents.
We reported a two-year decrease of -12.8 (SE=4.0) and -9.0 (SE=3.0) in the ADCS-ADL score, respectively in the intervention and control group. As it is commonly used in clinical trials, no study has explored decline in the ADCS-ADL score in oldest-old patients, restricting comparisons with other oldest age cohorts. In fact, only one study has investigated decline in the functional score of an AD population aged 85 and over, using the Katz ADL score [18].
We hypothesized that a specific and more intensive care plan could be effective because of the particular characteristics of the population, named very elderly patients, with a high number of comorbidities, a high risk of hospitalization, and a more rapid functional decline [18]. Our intervention is centered on assessment and management of complications linked to AD. But oldest-old patients have several chronic diseases that contribute to the burden of disability [12]. In our study, the mean number of chronic diseases besides AD is 2.2 (SD=1.4 and SD=1.2 respectively in the intervention and control group) which is similar to other studies [14]. Even if the twice-yearly consultation includes a geriatric evaluation, it doesn’t include specific assessment and treatment of each medical condition. Dementia is a strong predictor of functional decline in the oldest-old population, but several chronic conditions like congestive heart failure or history of depression contribute as well [14].
Furthermore, information and training of caregivers are an important part of the study’s intervention. The demographic characteristics of the caregivers in our study are quite different from the general population of caregivers for AD patients, as only 25% of caregivers are spouses and 59.5% are children [28]. The advanced age of patients probably explains these results, as we can expect a greater number of widowed subjects. Younger caregivers, such as children, may be less committed to care, as they probably have other responsibilities, jobs or family commitments [29]. Furthermore, more than 40% of patients were living alone, which is a higher percentage than what is usually observed in longitudinal studies [28,30,31]. The fact that patients live alone means that the caregiver has limited opportunity and time to get involved in patient’s home care. The lesser involvement of children could reduce the impact of the intervention.
Other hypotheses may also be put forward to account for the lack of effect of the intervention. A major limitation in our study, and one that is inherent to studies of the oldest old, is the high attrition rate, due in particular to death and noncompliance. Dementia is associated with shorter survival. The probability of dying at the oldest ages is very high, and so consequently is the death rate. Moreover, due to the diversity and complexity of the intervention, adherence to the intervention program was difficult to assess. Finally, a lack of power in this post-hoc analysis may probably contribute to the in significance of our results.
Conclusion
The oldest old are the fastest growing age group in western countries, and therefore disability and neurodegenerative diseases, such as AD, represent a huge financial burden, that is likely to continue to increase in the coming decades. In this cluster randomized trial assessing the effect of a specific care plan on functional decline in very old AD patients living in the community, there was no evidence that a structured, twice-yearly evaluation and management had a beneficial effect. Specific investigations are required to improve treatment of AD and prevent disability in this part of the population. Dementia and disability have a major impact on public health burden and planning, confirming the need for future research in the oldest-old population.
This study was supported by a grant from the French Ministry of Health (PHRC 02-006-01). The sponsor had no role in the study.
We thank the investigators from the following hospitals for help with data collection for the PLASA study: Albi General Hospital: Alain Quinçon; Ales General Hospital: Liliane Peju; Anger University Hospital: Gilles Berrut; Annecy General Hospital: Francoise Picot; Bar Le Duc General Hospital: Gabrielle De Guio; Bordeaux University Hospital: Muriel Rainfray; Brest University Hospital: Armelle Gentric; Carcassonne General Hospital:Christian Tannier; Carvin General Hospital: Nathalie Taillez; Chambéry General Hospital: Françoise Declippeleir, Claude Hohn; Champcueil General Hospital: Marie Françoise Maugourd; Dieppe General Hospital: Thierry Pesque; Elbeuf General Hospital: Thibault Simon; Grasse General Hospital: Jacques Ribiere; Grenoble University Hospital: Alain Franco; Lannemezan General Hospital: Serge Bordes; Lavaur General Hospital:Françoise De Pemille; Le Havre General Hospital: Isabelle Landrin; Lens General Hospital: Olivier Senechal; Lille University Hospital: Jean Roche, Florence Pasquier; Lyon University Hospital: Marc Bonnefoy; Marseille University Hospital: Bernard Michel; Montpellier University Hospital: Claude Jeandel, Jacques Touchon; Nice General Hospital: Jean Yves Giordana; Nice University Hospital: Patrice Brocker, Philippe Robert, Olivier Guerin; Nimes General Hospital: Denise Strubel; Niort General Hospital: Jean Albert Chaumier; Paris General Hospital: Bernard Durand-Gasselin; Paris General Hospital: Michel Samson; Paris University Hospital: Joel Belmin, Sylvie Legrain, Anne Sophie Rigaud, Laurent Teillet, Marc Verny, Sylvie Pariel; Pau General Hospital: François de la Fournière; Plaisir General Hospital: Olivier Drunat; Reims University Hospital: François Blanchard; Rennes University Hospital: Pierre Jouanny; Roubaix General Hospital: Pierre Forzy; Rouen General Hospital: Moynot; Rouen University Hospital: Philippe Chassagne, Didier Hannequin, Frédérique Dugny, Caroline Levasseur; Saint Dizier General Hospital: Anne Aubertin; Sezanne General Hospital: Elisabeth Quignard; Valenciennes General Hospital: Pascale Leurs; Vannes General Hospital: Christian Le Provost; Villejuif General Hospital: Dorin Feteanu, Christophe Trivalle; Wasquehal General Hospital: Frigard. We also thank M C Cazes for administrative support and Julie Léger for her statistical help. The CD-ROM was produced with financial support from the French Ministry of Health.
Funding
This study was supported by a grant from the French Ministry of Health (PHRC 02-006-01). The sponsor had no role in the study.
References
- Lafortune G, Balestat G (2007) Trends in severe disability among elderly people: Assessing the evidence in 12 OECD countries and the future implications. OECD Health Working Papers, n° 26, OECD Publishing.
- Gardner RC, Valcour V, Yaffe K (2013) Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimer’s Research and Therapy5: 27.
- Fratiglioni L, De Ronchi D, Agüero-Torres H (1999) Worldwide prevalence and incidence of dementia. Drugs and Aging15: 365–375.
- Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH (2010) Dementia incidence continues to increase with age in the oldest old: the 90+ study. Annals of Neurology67: 114–121.
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, et al. (2002) Dementia and Alzheimer disease incidence: A prospective cohort study. Archives of Neurology59: 1737–1746.
- Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P (2004) Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health and Quality of Life Outcomes2: 52.
- Waldemar G, Phung KTT, Burns A, Georges J, Hansen FR, et al. (2007) Access to diagnostic evaluation and treatment for dementia in Europe. International Journal of Geriatric Psychiatry22: 47–54.
- Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, et al. (2006) Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease14: 561–572.
- Nourhashémi F, Olde Rikkert MG, Burns A, Winblad B, Frisoni GB, et al. (2010) Follow-up for Alzheimer patients: European Alzheimer Disease Consortium position paper. The Journal of Nutrition, Health and Aging14: 121–130.
- Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Giraudeau B, Cantet C, et al.(2010) Effectiveness of a specific care plan in patients with Alzheimer’s disease: cluster randomised trial (PLASA study). BMJ (Clinical Research Ed)340: c2466.
- Berlau DJ, Corrada MM, Kawas C (2009) The prevalence of disability in the oldest-old is high and continues to increase with age: Findings from The 90+ Study. International Journal of Geriatric Psychiatry24: 1217–1225.
- Klijs B, Nusselder WJ, Looman CW, Mackenbach JP (2011) Contribution of chronic disease to the burden of disability. PloS One6: e25325.
- Nogueira SL, Ribeiro RCL, Rosado LEFPL, Franceschini SCC, Ribeiro AQ et al. (2010) Determinant factors of functional status among the oldest old. Revista Brasileira De Fisioterapia (São Carlos (São Paulo, Brazil))14: 322–329.
- Berlau DJ, Corrada MM, Peltz CB, Kawas CH (2012) Disability in the oldest-old: Incidence and risk factors in the 90+ study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry20: 159–168.
- Baert V, Gorus E, Mets T, Geerts C, Bautmans I (2011) Motivators and barriers for physical activity in the oldest old: a systematic review. Ageing Research Reviews10: 464–474.
- Goebeler S, Jylhä M, Hervonen A (2004) Use of hospitals at age 90. A population-based study. Archives of Gerontology and Geriatrics39: 93–102.
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, et al. (2003) Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. Journal of the American Geriatrics Society51: 451–458.
- Nourhashémi F, Gillette-Guyonnet S, Rolland Y, Cantet C, Hein C, et al. (2009) Alzheimer’s disease progression in the oldest old compared to younger elderly patient: data from the REAL.FR study. International Journal of Geriatric Psychiatry24: 149–155.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology34: 939–944.
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research12: 189–198.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. Jama185: 914–919.
- Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist9: 179–186.
- Guigoz Y, Vellas B, Garry PJ (1996) Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutrition Reviews54: S59–65.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, et al. (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology44: 2308–2314.
- Zarit SH, Todd PA, Zarit JM (1986) Subjective burden of husbands and wives as caregivers: A longitudinal study. The Gerontologist26: 260–266.
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, et al. (1997) An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders11: S33–39.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W et al. (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association9: 63–75
- Reynish E, Cortes F, Andrieu S, Cantet C, Olde Rikkert M, et al. (2007) The ICTUS Study: A Prospective longitudinal observational study of 1,380 AD patients in Europe. Study design and baseline characteristics of the cohort. Neuroepidemiology29: 29–38.
- Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R (1998) Predictors of institutionalization for people with dementia living at home with a carer. International Journal of Geriatric Psychiatry13: 682–690.
- Hoe J, Katona C, Orrell M, Livingston G (2007) Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: The LASER-AD study. International Journal of Geriatric Psychiatry, 22: 1031–1036.
- Nourhashemi F, Amouyal-Barkate K, Gillett e-Guyonnet S, Cantet C, Vellas B and REAL.FR Group (2005) Living alone with Alzheimer’s disease: cross-sectional and longitudinal analysis in the REAL.FR Study. The Journal of Nutrition, Health and Aging9: 117–120.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14157
- [From(publication date):
December-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9642
- PDF downloads : 4515