Research Article Open Access
Effectiveness of a Compound Supplement Containing Piperines, Theanine, Creatine, α-Lipoic Acid, and Proteoglycan for Low Back Pain: A Double-Blind Placebo-Controlled Parallel Comparison Study
| Chie Tarumizu1*, Tomohiro Ohno1, Sachiko Handa2, Sayuri Matsuoka1 and Hajime Orimo3 | |
| 1FANCL Research Institute, FANCL Corporation, 12-13 Kamishinano, Totsuka-ku, Yokohama, Kanagawa-244-0806, Japan | |
| 2FANCL Health Science Corporation, 89-1 Yamashita-cho, Naka-ku, Yokohama, Kanagawa-231-8528, Japan | |
| 3Kenkoin Medical Corporation foundation, 6-7-4 Ginza, Chuo-ku, Tokyo-104-0061, Japan | |
| Corresponding Author : | Chie Tarumizu FANCL Research Institute FANCL Corporation-12-13 Kamishinano Totsuka-ku, Yokohama, Kanagawa-244-0806, Japan Tel: +81-45-820-3443 Fax: +81-45-820-3526 E-mail: chie_0904@fancl.co.jp |
| Received June 10, 2015; Accepted July 16, 2015; Published July 20, 2015 | |
| Citation: Tarumizu C, Ohno T, Handa S, Matsuoka S, Orimo H (2015) Effectiveness of a Compound Supplement Containing Piperines, Theanine, Creatine, α-Lipoic Acid, and Proteoglycan for Low Back Pain: A Double-Blind Placebo-Controlled Parallel Comparison Study J Pain Relief 4:190. doi:10.4172/21670846.1000190 | |
| Copyright: © 2015 Tarumizu C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Objective: Low back pain is one of the most common subjective symptoms presenting in Japan, but has no established curative therapy. Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed for low back pain, and are generally effective in alleviating pain; however, such drugs are frequently associated with adverse effects such as gastrointestinal symptoms. The present study thus investigated the effectiveness of a compound supplement containing piperines, theanine, creatine, α -lipoic acid, and proteoglycan for managing the subjective symptoms of low back pain.
Methods: We conducted a double-blind, placebo-controlled, parallel-comparison study in 79 adult men and women with chronic low back pain. Subjects took either placebo or the investigational compound supplement containing long pepper extract powder at a dose of 150 mg (45 μ g as piperines), theanine at 50 mg, creatine at 250 mg, α -lipoic acid at 3 mg, and salmon nasal cartilage extract powder at 50 mg (10 mg as proteoglycan) or placebo for 8 weeks. Subjective symptoms were assessed immediately before starting to take the supplement or placebo (baseline), 4 weeks after the start (Week 4), and 8 weeks after the start (Week 8).
Results: Japan Low Back Pain Evaluation Questionnaire (JLEQ) scores were significantly lowered at Week 4 and Week 8 in the supplement group compared to those measured at baseline, and they were significantly lower at Week 8 compared to those of the placebo group (P=0.022). Approximately 30% of the JLEQ score of low back pain in the supplement group showed significantly improved compared to the placebo group. No clinically significant adverse events were reported during the study period.
Conclusions: The compound supplement tested herein, provided some relief for subjects with low back pain, and therefore could be useful in managing low back pain, and safe alternative or complement to NSAID therapy.
| Keywords |
| Low back pain; Supplement; JLEQ (Japan Low Back Pain Evaluation Questionnaire) |
| Introduction |
| Various pathologies and diseases underlie the onset and progression of low back pain, which is one of the most common subjective symptoms reported in Japan. According to the 2010 Ministry of Health, Labour and Welfare (MHLW) Comprehensive Survey of Living Conditions, low back pain ranked first among the health issues complained about by men (89.1 men per 1,000) and second among those affecting women (117.6 women per 1,000) [1]. Low back pain is defined mainly by such elements as location of the pain, duration since onset, and cause. In general, low back pain is defined as a pain localized between the palpable inferior end of rib and the gluteal fold, and is classified based on duration into acute low back pain (less than 4 weeks from onset), sub-acute low back pain (from 4 weeks to less than 3 months from onset), and chronic low back pain (3 months or longer from onset) [2,3]. With regard to classification by cause, in some cases the pain has an obvious cause that falls into one of five groups (spine-derived, nerve-derived, viscera-derived, angiogenic, and psychogenic); however, many of the patients with low back pain have no obvious cause and are classed as nonspecific low back pain without accompanying red flags or radiculopathy that might suggest complicated serious spinal disease. |
| The standard treatment for nonspecific low back pain is conservative therapy including medication, exercise therapy, physical therapy, and orthosis, depending on the pain and the dysfunction. While alternative therapies, such as manipulation, massage and acupuncture and cognitive behavioural therapy are also performed, a definitive treatment has not been established. Most low back pain currently presenting in Japan is treated with nonsteroidal anti-inflammatory drugs (NSAIDs), which are generally effective in alleviating pain [4], but also frequently associated with adverse gastrointestinal symptoms [5]. |
| We therefore developed a supplement for treating low back pain that contains agents considered to relax stiff muscles, stimulate blood flow, and/or affect intervertebral disc changes due to aging. The aim of the present study was to investigate the effect of this compound supplement on low back pain, mainly of the nonspecific chronic type, in a placebo-controlled, double-blind, parallel-group study. |
| Materials and Methods |
| Subjects |
| Adult men and women with subjective low back pain (including only those with constant or intermittent low back pain for 3 months or longer, and excluding those with transient symptoms) were recruited by FANCL Corporation for this study. Subjects were excluded based on the following criteria: suspected complication of serious spine disease, such as tumors, inflammation, and bone fractures; suspected complication of serious radiculopathy; receiving surgical treatment at a medical institution; taking regular oral medication or supplements that contain long pepper extract powder, theanine, proteoglycan, creatine, and/or α-lipoic acid; taking regular oral medication or supplements that could affect cartilage, muscle, or blood flow; currently pregnant or breastfeeding. |
| Investigational product |
| The investigational product was a tablet constituting 150 mg of long pepper extract powder (45 μg as piperines), 50 mg of theanine, 250 mg of creatine, 3 mg of α-lipoic acid, and 50 mg of salmon nasal cartilage extract powder (10 mg as proteoglycan), as well as folic acid, vitamin B6, and vitamin B12 (Koshilax, manufactured by FANCL Corporation, 89-1 Yamashita-cho, Naka-ku, Yokohama, Kanagawa, Japan). The placebo in this study was a tablet not containing any of these agents. These two tablets were indistinguishable from each other on appearance and were formulated such that four tablets constituted one daily dose. |
| Methods |
| We designed a double-blind, placebo-controlled, parallelcomparison study. Subjects were assigned an identification based on order of enrollment by a person not directly involved in the study, and were then randomly assigned to one of the two groups using Microsoft Excel (Microsoft Corp.). In the process of subject assignment, background factors were taken into consideration to avoid biased distribution in terms of the Japan Low Back Pain Evaluation Questionnaire (JLEQ) score [6] taken before starting, age, sex, occupation, form of employment, presence or absence of causative disease of low back pain, past history, status of attending a clinic or hospital, and exercise habit. Participants in the study (subjects) and people who conducted the study (investigators) were blinded, and neither the subject nor the investigators knew which tablet was taken. The order of assignment was sealed from the person in charge until the statistical analysis was completed. The administration period was set to consecutive 8 weeks, and 4 tablets of the investigational product or placebo were taken once daily after breakfast with a glass of water. Observations were conducted three times in total: before starting to take tablets, 4 weeks after the start (Week 4), and 8 weeks after the start (Week 8). In principle, subjects were prohibited to start new supplements, health foods, medication, or other treatment during the study period, and were advised not to change their lifestyle. In addition, physical condition and status of compliance were reviewed using a diary. |
| Endpoints |
| The JLEQ was set as the primary endpoint. This scoring system was developed in 2007 by the collaborative work of three medical associations, including the Japanese Orthopaedic Association, that took the lifestyle habits of Japanese people into consideration to evaluate the patients’ view of their health-related quality of life specific to diseases causing low back pain [6]. It is also used as an evaluation method for intervention in clinical studies of patients with chronic low back pain [7]. The JLEQ consists of self-assessment using a visual analog scale (VAS) [0 to 100 points] and 30 questions that measure daily motor function and health and mental condition by a 5-point scale [0 to 120 points], thus enabling multilateral evaluation including social life and psychological aspects. In the JLEQ evaluation, the self-assessment of pain using VAS and the total score of 30 questions (JLEQ score) are separately assessed. The JLEQ score reflects the average condition during the period from a few days to one month, and a higher score indicates a more serious condition. |
| Statistical analysis |
| Data are shown mean ± SEM and various statistical methods were employed for analysis according to the characteristics of the data. For non-parametric data, we used the Wilcoxon signed rank test (with correction based on Bonferroni inequality) for intragroup comparison and the Mann-Whitney U-Test for intergroup comparison. For parametric data, we used repeated measurement analysis of variance to assess intragroup change and the unpaired T test at each observation point for intergroup comparison. SPSS14.0 for Windows was used for all statistical analyses and the level of significance was set at 5% (twosided). |
| Ethical consideration |
| The study was conducted in accordance with the ethical principles derived from the Declaration of Helsinki and “Ethical guidelines for epidemiological studies”, after obtaining the approval of the FANCL clinical trial ethics committee. The principal investigator of this study provided a verbal explanation of the purpose, methods, and period of the study, as well as the investigational product, to the subjects who volunteered to participate in this study, and written, signed consent was obtained after confirming that the participant thoroughly understood the details of the study. The expenses required to conduct this study were provided by FANCL Corporation, and there were no other companies or organizations with conflicts of financial interests to declare. |
| Results |
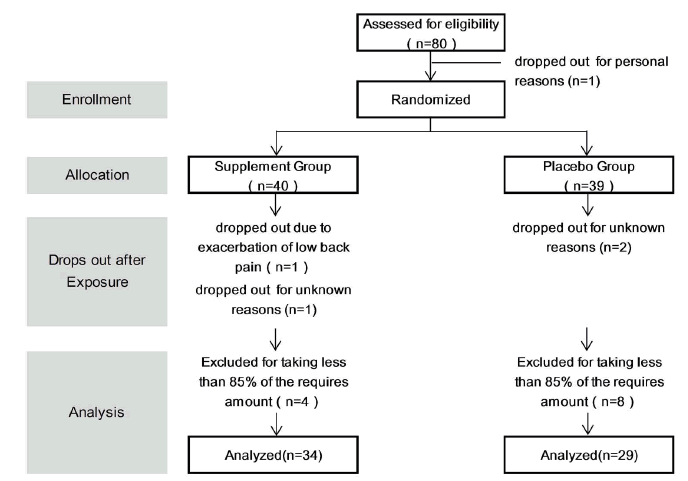
| This study was conducted from January 2013 to April 2013. Of the 80 subjects who volunteered, one subject dropped out for personal reasons after randomization, thus the supplement administration started with 79 subjects. One subject then dropped out due to exacerbation of low back pain, and 3 subjects did not undergo scheduled tests for personal reasons. Therefore, 75 subjects completed the study to the final tests. The results were tabulated every 4 weeks. Twelve subjects who had 20% or more missing data or took less than 85% of the required amount of the study tablet were excluded from the final analysis, which comprised the data of 63 subjects, 30 men and 33 women (Figure 1). The mean age was 44 years, ranging from 24 to 72 years. Table 1 detail the subject backgrounds, Figure 2 represents the JLEQ results, Figure 3 represents the changes in JLEQ score (%), and Figure 4 shows the VAS assessments of low back pain. |
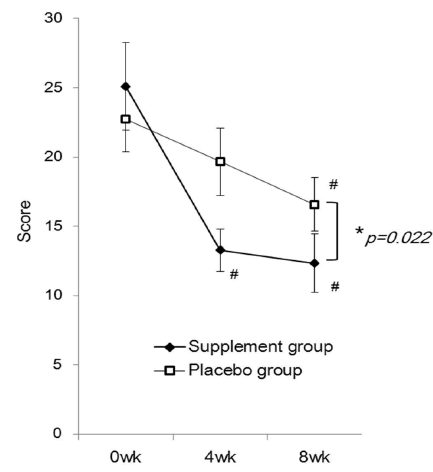
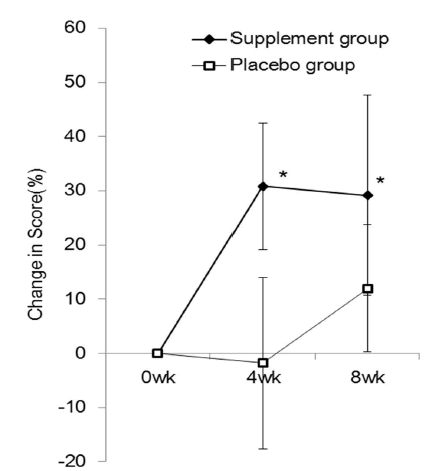
| The mean JLEQ score was significantly reduced in the group taking the investigational product (the supplement group) at 4 weeks (13.3 ± 1.5) and 8 weeks (12.3 ± 2.1) after the start of administration compared to those measured before taking the supplement (baseline, 25.1 ± 3.2) (Figure 2). At 8 weeks, the JLEQ score was also significantly lower in the supplement group compared to the placebo group. The change in JLEQ score (%) in the supplement group was 30.9 ± 11.7 at 4 weeks and 29.2 ± 18.4 at 8 weeks after the start of administration, showing approximately 30% improvement continuously from 4 weeks, and significantly higher improvement at both time points compared to the placebo group (Figure 3). With regard to the number of subjects who showed improvement in the score, 27 subjects showed improvement, 1 showed no improvement, and 6 showed aggravation at 4 weeks in the supplement group (n=34), while 20 subjects showed improvement and 9 showed aggravation in the placebo group (n=29). At 8 weeks after the start of administration, 29 subjects showed improvement and 5 showed aggravation in the supplement group, while 19 subjects showed improvement and 10 showed aggravation in the placebo group. |
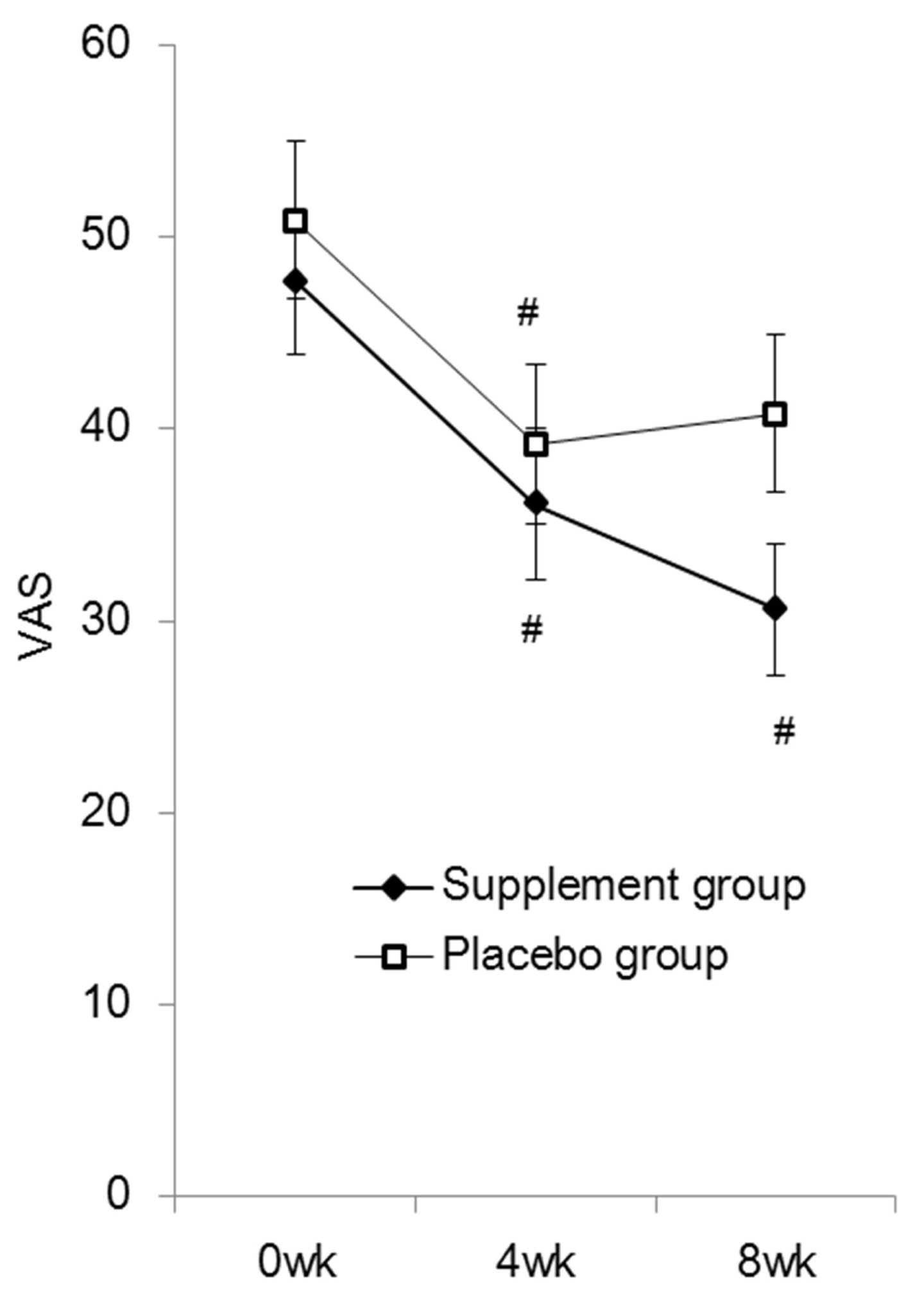
| The VAS score of low back pain in the supplement group was significantly reduced at 4 weeks (36.1 ± 4.0) and 8 weeks (30.6 ± 3.4) compared to baseline scores (47.6 ± 3.8). In the placebo group, although the score was also significantly reduced at 4 weeks (39.3 ± 4.1) compared to baseline (50.9 ± 4.1), the score was slightly elevated at 8 weeks (40.8 ± 4.1). Intergroup comparison at 8 weeks indicated a tendency for a lower score in the supplement group compared to those taking placebo, although the difference was not statistically significant (P=0.059) (Figure 4). No clinically significant adverse events were reported throughout the study period. |
| Discussion |
| The main findings of the present study as follows; (1) Approximately 30% of the JLEQ score of low back pain in the supplement group showed significantly improved compared to the placebo group; (2) In addition, VAS scores of low back pain were significantly reduced only in the supplement group compared to baseline; (3) No adverse events were associated with the investigational product during the study period. Therefore, these results suggest that the compound supplement tested herein, containing piperines, theanine, creatine, α-lipoic acid, and proteoglycan, provided some relief for patients with low back pain, and therefore could be useful in managing low back pain, particularly in elderly patients and safe alternative or complement to NSAID therapy. |
| Validation of compound supplement tested in this study |
| Concerning the compound supplement tested herein, long pepper extract powder reportedly accelerates recovery of normal skin temperature and stimulates blood flow [8]. Although capsicum and ginger are also well known to improve blood flow, their unique and strong flavor make long pepper a preferable choice. Theanine was included in the supplement with the expectation that its relaxing effect would provide muscle relaxation and blood flow improvement, based on increased alpha wave activity on electroencephalograms [9]. Creatine is expected to enhance energy production in the muscle, and α-lipoic acid increases the uptake of creatine [10]. Finally, proteoglycan was included to improve age-related reductions in cartilage metabolism [11]. |
| Possible mechanisms of pain relief by compound supplement |
| Capsaicin, the active ingredient of capsicum, activates the transient receptor potential vanilloid 1 (TRPV1), an ion channel-type receptor that is expressed in the sensory nerve system and acts via the sympathetic nervous system to increase the secretion of adrenaline from the adrenal gland to accelerate the production of body heat [12-16]. The piperines (long pepper extract powder) contained in the investigational product of this study also induce TRPV1 activation [17]. In addition, although there are no reports on the effectiveness of piperines in improving low back pain, application of a capsaicin patch for three weeks alleviated symptoms in patients with non-specific chronic low back pain [18,19]. While desensitization has to be considered in pain relief effects from localized treatment, oral intake could relieve low back pain due to elevation of the skin temperature. Since theanine has been known that facililation of vasodilator, nitric oxide (NO) production in endothelial cells and [20], it can be assumed that theanine can reduce the low back pain via reduced to algesic substances in the lumber area. Actually, there are reports that spa therapy and balneotherapy may be effective for treating patients with low back pain [21,22]. Also the blood concentration of an endogenous morphine, beta-endorphin levels were augmented under in vivo hyperthermia [23]. Although the precise mechanism underlying nutritional intake could improve low back pain is unknown, it is likely that both heat production and improving local blood flow effects acts synergistically on the analgesia. Since the subjects in these previous studies were mainly young adults without concurrent diseases and who perform office work that involves sitting at a desk for extended periods of time, it was therefore conjectured that the other ingredients in our formulation such as creatine and α-lipoic acid also acted in an integral manner to improve muscle stiffness and blood flow, leading to alleviation of low back pain symptoms within a relatively short period of time. But further studies needed to clarified these possibilities. |
| Methodological consideration |
| Pain can be deeply associated with psychological factors, and the analgesic effect of placebo has been proved in various studies [24], and in this study too, a certain level of effectiveness was observed in the placebo group. However, while the placebo effect tended to diminish with time, the positive results in the supplement group continued from 4 weeks after the start of administration until 8 weeks. These findings thus indicated a direct effectiveness of the investigational product. |
| Clinical significance and future direction |
| The supplement under investigation herein was introduced on the market as Koshilax, manufactured by FANCL Corporation, Yokohamashi, Kanagawa, Japan, as a commercial product for low back pain. After its launch a large-scale self-administered questionnaire survey was conducted by mail on 2,052 consumers who were using this product. The 757 eligible responses (response rate: 37%) were analyzed for the status of low back pain and the characteristics of the effect after taking the supplement, and the usefulness of the supplement was reconfirmed [25]. There was a tendency for a better effect in patients with a shorter duration of morbidity and in women, suggesting the possibility of different effects depending on individual patient backgrounds. In the future, a study design has to be carefully developed from a more clinical point of view, in terms of selecting the subjects and sample size. Further validation of the effect of the supplement assessed herein is necessary and more research is needed into the mechanisms underlying low back pain. |
References
- Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare (2012) Comprehensive Survey of Living Conditions 2010, General incorporated foundation, Health, Labour and Welfare Statistics Association, Minato-ku, Tokyo, Japan.
- Hagen KB, Jamtvedt G, Hilde G, Winnem MF (2005) The updated Cochrane review of bed rest for low back pain and sciatica. Spine 30: 542-546.
- Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, et al. (2007) Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society.Ann Intern Med 147: 478-491.
- Machado LA, Kamper SJ, Herbert RD, Maher CG, McAuley JH (2009) Analgesic effects of treatments for non-specific low back pain: a meta-analysis of placebo-controlled randomized trials.Rheumatology (Oxford) 48: 520-527.
- Schnitzer TJ, Ferraro A, Hunsche E, Kong SX (2004) A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain.J Pain Symptom Manage 28: 72-95.
- Shirado O, Doi T, Akai M, Fujino K, Hoshino Y, et al. (2007) An outcome measure for Japanese people with chronic low back pain: an introduction and validation study of Japan Low Back Pain Evaluation Questionnaire.Spine (Phila Pa 1976) 32: 3052-3059.
- Shirado O, Doi T, Akai M, Hoshino Y, Fujino K, et al. (2010) Multicenter randomized controlled trial to evaluate the effect of home-based exercise on patients with chronic low back pain: the Japan low back pain exercise therapy study.Spine (Phila Pa 1976) 35: E811-819.
- Yamada N, Nishihara C, Yoshimura H, Yamaguchi Y, Takagaki R, et al. (2009) [Effects of Piper longum L. on chills in Japanese young women: time-dependent changes in skin surface temperature and its recovery rate following the exposure to mild cold stress].Nihon ShinkeiSeishinYakurigakuZasshi 29: 7-15.
- Nobre AC, Rao A, Owen GN (2008) L-theanine, a natural constituent in tea, and its effect on mental state.Asia Pac J ClinNutr 17 Suppl 1: 167-168.
- Burke DG, Chilibeck PD, Parise G, Tarnopolsky MA, Candow DG (2003) Effect of alpha-lipoic acid combined with creatine monohydrate on human skeletal muscle creatine and phosphagen concentration.Int J Sport NutrExercMetab 13: 294-302.
- Takahashi T (2011) Bone, joint, muscle and nutrition – Material for anti-locomotive syndrome. Usefulness of proteoglycan from salmon nasal cartilage on joint pain, and metabolism of cartilage. Food Style 21 15: 52-54.
- Iwasaki Y, Morita A, Furuhata K, Watanabe T, Sugai E, et al. (2005) Ouyou Yakuri 69: 18-20.
- Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hökfelt T, et al. (1995) Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res 703: 175-183.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD,et al. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389: 816-824.
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat.Nature 398: 436-441.
- Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K (1988) Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats.Am J Physiol 255: E23-27.
- Iwasaki Y, Ishikawa A, Kobata K, Yamaguchi Y, Takagaki R, et al. (2007) Action of ethanolic or hot water extract of long pepper on the capsaicin receptor (TRPV1). The Japan Society for Bioscience, Biotechnology, and Agrochemistry .
- Keitel W, Frerick H, Kuhn U, Schmidt U, Kuhlmann M, et al. (2001) Capsicum pain plaster in chronic non-specific low back pain.Arzneimittelforschung 51: 896-903.
- Frerick H, Keitel W, Kuhn U, Schmidt S, Bredehorst A, et al. (2003) Topical treatment of chronic low back pain with a capsicum plaster.Pain 106: 59-64.
- Siamwala JH, Dias PM, Majumder S, Joshi MK, Sinkar VP, et al. (2013) L-theanine promotes nitric oxide production in endothelial cells through eNOS phosphorylation.J NutrBiochem 24: 595-605.
- Pittler MH, Karagülle MZ, Karagülle M, Ernst E (2006) Spa therapy and balneotherapy for treating low back pain: meta-analysis of randomized trials.Rheumatology (Oxford) 45: 880-884.
- Francon A, Forestier R (2009) Spa therapy in rheumatology. Indications based on the clinical guidelines of the French National Authority for health and the European League Against Rheumatism, and the results of 19 randomized clinical trials. Bull AcadNatl Med 193: 1345-1356.
- Kappel M, Gyhrs A, Galbo H, Pedersen BK (1997) The response on glucoregulatory hormones of in vivo whole body hyperthermia.Int J Hyperthermia 13: 413-421.
- Linton SJ (2000) A review of psychological risk factors in back and neck pain.Spine (Phila Pa 1976) 25: 1148-1156.
- Tarumizu C, Ohno T, Uotsu N, Teramoto S, Orimo H (2014) The effect of taking supplement on low back pain. The 14th Scientific Meeting of the Japanese Society of Anti-aging Medicine.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 14369
- [From(publication date):
July-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9970
- PDF downloads : 4399
