Research Article Open Access
Effect on Acid Reflux Symptoms Occurring during Sleep of an Oral Adhering Disc Containing only Food Ingredients
Jeffrey Alan Burgess*, Peter Van Der Ven and Michael KarcherDepartment of Oral Medicine, University of Washington, Seattle, USA
- *Corresponding Author:
- Burgess JA
Department of Oral Medicine
University of Washington, Seattle, USA
Tel: 2064502640
E-mail: oral.care.research.assoc@gmail.com
Received date: August 10, 2017; Accepted date: August 30, 2017; Published date: August 31, 2017
Citation: Burgess JA, Der Ven PV, Karcher MK (2017) Effect on Acid Reflux Symptoms Occurring during Sleep of an Oral Adhering Disc Containing only Food Ingredients. J Gastrointest Dig Syst 7:524. doi:10.4172/2161-069X.1000524
Copyright: © 2017 Burgess JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The aim of this double-blinded, randomized, controlled study was to determine if OraCoat XyliMelts, an over the counter, dissolvable, adhering disc used to reduce excessive day time or night time dry mouth by increasing salivation, would also reduce reflux and heartburn symptoms occurring during sleep among subjects suffering from both GERD and xerostomia. Subjects submitted 14 days of baseline data by answering questions and were then randomized into one of two groups: one receiving a dry mouth gel (control) and a second receiving a disc (product of interest). Neither the identities of the two products nor the manufacturers of the products was revealed to subjects. Answers to the same set of questions were then collected for an additional 14 days as the supplied products were used. The accumulated data were evaluated for pre-post treatment changes within and between groups. Variables included reported heartburn, reflux, reflux taste, hoarseness, dry mouth, and antacid use. The results showed a significant decrease in the severity of the above symptoms of GERD with both disc and gel use, although improvement was greatest for those using the discs. Comparison between the gel and disc groups for most symptoms did not reveal significant differences. For heartburn, subjects using the discs perceived significantly less pain than subjects using the gel. We conclude that the use of certain products intended to reduce dry mouth during sleep, including OraCoat XyliMelts, may be an effective adjunctive remedy for reducing reflux and heartburn symptoms in patients with GERD and xerostomia.
Keywords
Esophageal reflux; Heartburn; Xerostomia; Mouth dryness
Introduction
Background
Gastro-esophageal reflux disease, commonly referred to as GERD, is estimated to occur in ten to 30% of the population in developed countries, with its frequency increasing [1]. Nocturnal GERD involves reflux of acid into the esophagus during sleep. The condition is estimated to occur once a month in up to 43% of individuals and once a week in 20% of the population [2,3]. GERD and its variants, is associated with multiple symptoms including heartburn, sleep disturbance, impaired salivation and swallowing, reduced esophageal motility, and erosion of teeth [4-6]. The two ‘classic’ symptoms that are typically associated with GERD are heartburn (epigastric pain) and acid reflux (an acidic taste at the back of the throat) [7,8]. As a natural antidote to GERD, salivary flow serves to neutralize, dilute, and wash down acid that escapes the stomach [9,10].
Hence, we hypothesized that stimulation of salivation during sleep by a slowly dissolving flavored disc that adheres to the oral mucosa could improve reflux and heartburn symptoms associated with GERD. There is no definitive test to diagnose GERD. Upper endoscopy and pH (acid) monitoring have demonstrated low predictive values and are not considered to be stand-alone criteria for identifying GERD [11]. Consequently, in selecting subjects for this on-line study, we relied on Guidelines for diagnosing GERD published by the American College of Gastroenterology and the American Gastroenterological Association [12,13]. These guidelines state that “a presumptive diagnosis of GERD can be established” in the clinical setting “by the typical symptoms of heartburn and acid regurgitation” and response to empiric trials of proton pump inhibitors (PPIs).
A significant problem associated with GERD is sleep disturbance. The research supporting the connection between GERD and sleep disturbance includes epidemiologic as well as clinical studies [14,15]. GERD-associated sleep disturbance may contribute to greater health care utilization, work loss, and decreased quality of life [16-21]. An epidemiological study by Mody et al. using data from the US National Health and Wellness Survey, notes that 19% of their over 60,000 surveyed respondents experienced heartburn at least two times a month [22].
Of those with night-time GERD, 68% also reported sleep difficulties, with 49% indicating difficulty getting to sleep and 58% indicating difficulty maintaining sleep. Of potential significance, subjects reporting night-time GERD had 1.5 times more overall sleep problems compared to those reporting daytime GERD. In addition, sleep difficulty was reported to be associated with increased health care utilization, impairment of daily activity, and loss of work productivity. Similar results have been published elsewhere [23]. Evidence suggests that reducing nocturnal GERD could provide a significant improvement in sleep, health care costs, and quality of life [24].
Other problems associated with nighttime GERD include oral disease, epigastric pain, and hoarseness. Oral problems arising as a direct result of GERD include tooth erosion and halitosis [5,25-27]. Tooth erosion is thought to result from the acidity of the gastric contents that reach the mouth during repeated GERD episodes, especially if they are inadequately buffered by saliva [28,29].
Saliva, or a lack thereof, may also have an effect on the esophageal mucosa exposed to refluxed acid, with this resulting in subsequent thoracic symptoms including chest pain (heartburn) and voice changes (hoarseness) [30]. A reduction in GERD could be expected to reduce the risk of acidic taste, dental pathology, bad breath, chest pain, and morning hoarseness.
Saliva is thought to play an important role in protecting the esophageal lining, by diluting, washing down, and buffering stomach acid that enters the esophagus through reflux [31-35]. Salivary flow has been shown to fall significantly during the night, according to an individual’s circadian rhythm [36]. This is significant because a number of studies report that stimulated night time production of saliva and its associated constituents can mitigate the symptoms of heartburn and reflux [32,37,38]. It has been proposed that increased salivation resulting from esophageal acidification may be mediated through an 'esophago-salivary' reflex [39]. Reflex salivation with associated increased bicarbonate content appears to be greatest when acid accumulates in the upper region of the esophagus [40].
The effect of acid infusion into the lower part of the esophagus does not appear to have the same effect on salivary flow. It has been reported that a significant increase in heartburn occurs when acid reaches the upper area of the esophagus [32,41]. Hence, in patients with reflux where the acid does not reach the upper area of the esophagus, a reflexive increase in saliva production may not be initiated. Thus, there may be benefit from stimulating the release of additional saliva whose buffering capacity could help protect the lower esophageal lining from refluxed acid.
Current Medication Management of GERD
GERD is managed by various medications including drugs that inhibit acid secretion, such as proton pump inhibitors (PPI’s) and histamine receptor antagonists (H2 blockers), and prokinetic drugs which increase tone in the lower esophageal sphincter [42,43]. Other remedies include OTC alkaline agents such as calcium carbonate, which simply neutralize acid. While the above drugs may help some patients, they may not be helpful in all cases. For example, it is reported that one-third of patients taking the PPI drugs do not respond to these drugs and side effects can be problematic [44].
PPI use has also been associated with an increased risk of bone fracture and Vitamin B12 deficiency [45]. PPI’s44 inhibit the liver P450 enzyme system and may interact with other drugs [46]. Further, the literature suggests that women planning on becoming pregnant or who are pregnant should consult their physician prior to taking PPI’s, and patients with kidney or liver problems or certain lung diseases such as COPD, diabetes, or a history of porphyria should also be cautious with the use of these drugs [47,48].
Prokinetic medications are only recommended for short term use and their potential side effect such as depression and severe muscle twitching, dizziness or lightheadedness, and potentially fatal heart arrhythmias limit their general usefulness [49]. Antacids are effective in managing acute GERD but are not considered reasonable for long term use due to potential side effects [50].
Another problem with antacid use is that for GERD occurring at night, it is not until sleep is disturbed that this class of medication is taken, which reduces the benefit in terms of sleep quality. Given the facts that many people do not gain relief from PPIs, that histamine-2 receptor antagonists are associated with multiple side effects and interactions with other drugs, and that the benefit of antacid use is limited by associated sleep disturbance and side effects can be an issue with chronic use, additional approaches to the treatment of GERD occurring during sleep need to be considered [15].
Study Rationale
The aim of this study was to determine if an orally dissolvable, adhering disc, previously shown to reduce excessive day time or night time dry mouth, would also reduce reflux and heartburn occurring during sleep. The discs are made from food ingredients and stimulate saliva via slowly released flavor when used as directed. Ho et al. report that among people with xerostomia, their use more than doubled saliva production when applied during the day [51].
Reflux symptoms are sometimes more prominent at night because acid can more readily enter the esophagus while patients are lying down. Anecdotal reports and research show that salivary stimulants can reduce the sensation of dry mouth. Research also suggests that salivation can ameliorate reflux and heartburn [24,31,32]. We hypothesized that the stimulation of salivation by a slowly dissolving intraoral disc could significantly reduce nocturnal reflux and heartburn symptoms associated with GERD in subjects who self-report xerostomia.
Subjects and Methods
Study design
This study was approved by the Western Institutional Review Board on September 16, 2014 (WIRB; 1019 39th Ave S, Ste 120, Puyallup, WA 98374). It was designed as a randomized, double-blinded, controlled trial involving two over the counter products currently on the market for use in the management of dry mouth symptoms. The product of interest was cleared by the FDA for investigation in the context of GERD on September 4, 2014 (Investigational New Drug Application number 123574 US FDA). Study information and results can be found on ClinicalTrials.gov (project identifier NCT02274636)
Both the participants and the research coordinator conducting the study were blinded as to which of the two products was the one of interest and delivery was independently randomized. The product of interest, OraCoat XyliMelts, was produced by OraHealth Corporation in Bellevue, Washington.
The ingredients are all-natural and commonly used in foods: xylitol for sweetness, mild mint for additional flavor, cellulose gum to slow dissolution and lubricate the mouth, an acacia gum adhesive layer, and a small amount of calcium carbonate to neutralize the acidity of acacia gum and render the product slightly basic. When a single disc is dissolved in 5 parts water, the resulting pH is 8.1 [52]. When used during the day by subjects with dry mouth having a mean salivary pH of 7.25, the use of two discs did not make saliva more basic [51].
Product users also report that discs slowly dissolve over six hours during sleep and that the flavor can still be sensed upon awakening after 8 hours of sleep [51,53]. The product used as a control was a water based gel containing cellulose hydrocolloid gums with sorbitol and xylitol sweeteners marketed by GlaxoSmithKline as a remedy for dry mouth. As it is a soluble gel introduced prior to sleep, it was presumed to be eliminated from the oral cavity fairly quickly via salivary stimulation.
Study population/subjects
Subjects were drawn from 372 individuals living in the United States who responded to an ad soliciting paid volunteers for an internet administered study involving substances recognized as safe for possible management of reflux symptoms. When individuals expressed interest in the study, a 21 item questionnaire was sent to them via email. For inclusion in the study, subjects had to have internet email access and be computer literate, be 18 years of age or older, have had a medical evaluation in the prior year, and have self-reported frequent symptoms of reflux or heartburn and dry mouth while sleeping.
Study exclusion criteria included a history of thoracic, esophageal or gastric surgery, current or past coronary artery disease, gallbladder disease, gastric or esophageal cancer, peptic ulcer disease, esophagitis, and eosinophilic, infectious, or esophageal motility disorders. Subjects were also excluded if another member of their household was already participating in the study. It was not necessary to have received a medical diagnosis of reflux, but 91% of final subjects reported this diagnosis. Subjects satisfying the inclusion criteria and qualifying for the study based on medical criteria were sent a comprehensive, WIRB approved consent form detailing the study intent, design, procedures, risks and discomforts, benefits, alternatives, confidentiality, legal rights, and other information.
Variables of interest
Study subjects were emailed a questionnaire every day during an initial two week baseline collection period and then a two week product intervention trial, for a total of four weeks participation. Questionnaire variables (listed below) were chosen because previous research suggests that reflux, characterized by a perception of a sour taste at the back of the mouth, is also associated with symptoms of heartburn, morning hoarseness, use of water during the night, and antacid use [8,54].
Procedures
Phase 1: Baseline
Upon receiving written consent via email response, subjects were entered into the 14 day baseline data collection phase of the study (Phase 1). During this period, they were instructed to continue with their normal daily activities including their usual diet and the use of ongoing OTC and/or prescribed medication for reflux, heartburn, and any other non-excluded medical conditions. Each subject was asked to answer the same nine questions delivered by email each morning by the research coordinator, relating to what occurred during their prior night’s sleep.
The specific questions were:
• Did you taste refluxed stomach acid during your sleep last night (yes/no);
• How severe was the reflux (mild, moderate, severe, very severe);
• Did you have heartburn when you slept (yes/no);
• How severe was the heartburn (mild, moderate, severe, very severe);
• Did you keep water by your bedside because of dry mouth occurring during sleep (yes/no);
• Did you have uncomfortable dry mouth when you slept or upon awakening (yes/no);
• Did you experience hoarseness of your voice in the morning (yes/ no);
• Did you need to take antacids during sleep (yes/no); and
• If so, how many did you take (number).
Subjects were asked not to view their prior email responses when replying in the morning and to maintain the same email thread for subsequent review, should it be necessary. When a subject replied with the exact same answers each day without variation during the baseline data-collection period he/she was disqualified from the study as this was considered to be statistically improbable.
Phase 2: Product trials
Upon completion of Phase 1, each subject’s data were quantified to see if he/she qualified for the second product phase of the study. To be accepted into Phase 2, subjects had to have reported reflux taste on eight of the fourteen mornings following their night’s sleep during baseline assessment and dry mouth on seven of the same mornings.
Those qualifying were then randomized into one of two groups: treatment or control. Each subject then received by mail either the adhering discs disguised in unmarked packaging (treatment), or the sweetened water based gel in an unmarked white tube (control), with printed instructions copied from the manufacturer’s instructions for their method of use at bedtime.
The dispensed product was then used by each subject every night for two weeks. During this second phase of the study, subjects answered the same nine questions as in Phase one delivered to them by email each morning. As in Phase one, subjects were asked not to view their prior email responses when replying.
In both study arms, subjects were allowed to miss one night’s responses but were disqualified if they failed to respond more than once. Some questions posed by subjects during the study relating to how the questionnaire should be completed indicated that there were instances of confusion regarding interpretation and intent. When subjects asked for clarification regarding completion of the questionnaire, consistent advice was provided by the research coordinator.
Statistics
As noted, variables of interest included reflux taste, reflux severity, heartburn sensation, heartburn severity, morning voice hoarseness, and antacid use each night. Baseline data for the above variables was collected from a total of 119 subjects who qualified for and consented to be in the study.
Based on the above qualifying criteria 53 subjects were selected to enter the two week intervention phase of the study, randomized to receive the disc or the gel. Comparisons were made within and between groups for all outcome variables. The severity data take the form of two clinical phases (baseline and treatment), each representing 14 nights of ordinal categorical data (no heartburn, mild heartburn, moderate heartburn, severe heartburn, and very severe heartburn).
The ordinal data suggest using a Mann-Whitney U test to compare the results from the treatment phase to the baseline phase for disc and gel groups, the results from the baseline phase of the disc group to the baseline phase of the gel group, and the treatment phase of the disc group to the treatment phase of the gel group [55]. The U test compares two groups of ordinal data by tabulating the pairwise comparisons (greater than, equal, or less than) between the data in each group and then comparing that tabulation to the known null distribution under the null hypothesis that a random member of either group is equally likely to be greater than a random member of the other group.
As with T-tests, U test alternative hypotheses can be one-sided or two-sided, depending on which outcomes are clinically relevant. The reflux taste and hoarseness data take the form of two clinical phases (baseline and treatment), each representing 14 nights of yes/no data stating whether each of the 26 subjects using the disc experienced the taste of acid during the night. The yes/no data suggest use of logistic regression with a random effect of subject. The nightly antacid use data take the form of two clinical phases (baseline and treatment), each representing 14 nights with computation of the mean number of antacids used. This suggests the use of a matched-pair T-test.
Results
Three hundred seventy-one individuals responded to the solicitation for participation in the study. Baseline data for the above variables was collected from a total of 119 subjects who qualified for and consented to be in the study. Based on the above described inclusion criteria post baseline, 53 subjects were selected to enter the two week intervention phase of the study, with 26 randomized to receive the disc and 27 to receive the gel. Forty-two qualifying subjects did not complete the first phase of the study.
Eight subjects did not qualify for the second phase of the study, while sixteen subjects were terminated from the study either because they did not comply with daily reporting or because someone in the household had already been in the study. Subjects in the two final intervention groups were not significantly different when compared on the basis of gender, age, or prior medical diagnosis of reflux. Mean age for the disc group was 46.1 (SD 12.1) and for the gel group was 41.3 (SD 13.4).
Heartburn
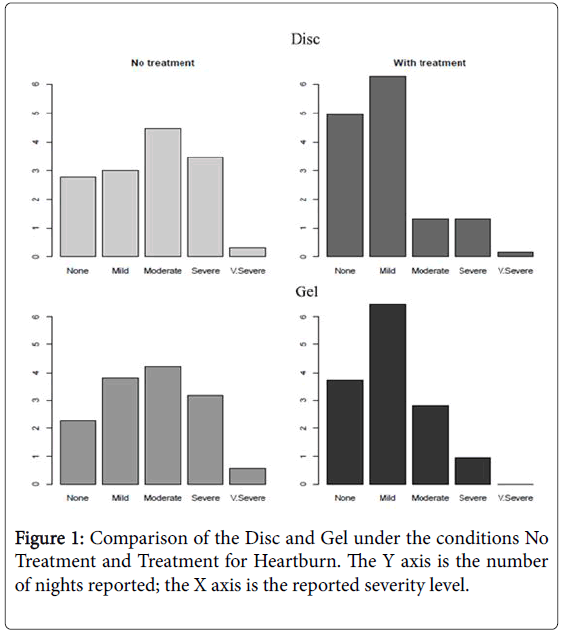
Subjects using the discs demonstrated a significant reduction in reported pain of heartburn when compared with baseline (one sided U-test p-value <0.001). Subjects using the gel also experienced a significant reduction in pain when compared to baseline (U-test pvalue <001). When these two remedies are compared via U-test, disc intervention demonstrates significantly greater improvement in heartburn pain than gel intervention (U-test p-value <0.01) (Figure 1).
Reflux severity
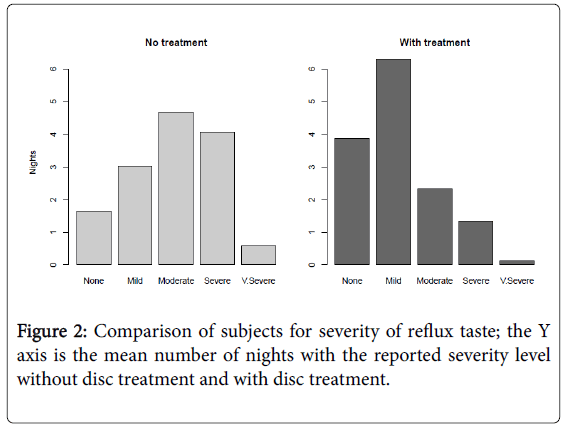
Subjects using the disc intervention reported a significant reduction in reflux severity when compared with baseline (one sided U-test pvalue <0.001) (Figure 2).
Subjects using the gel also experienced a significant reduction in reflux severity when compared to baseline values (U-test p-value <001). When the performance between the disc and gel treatments is compared, a statistically significant difference is not observed (U-test p>0.13).
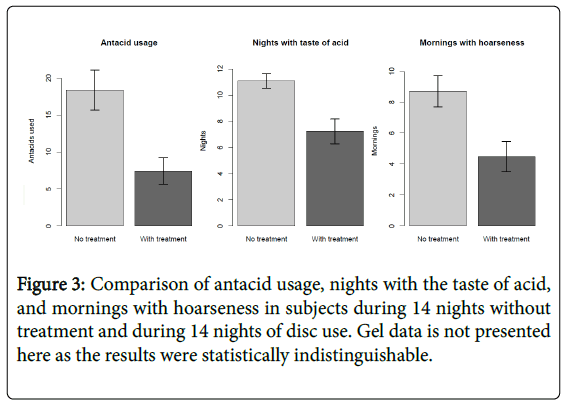
Using logistic regression with a random effect of subject, the disc treatment had a statistically significant (p <0.001) effect on taste of acid, with an 81% reduction in the odds of reporting taste of acid.
For the gel treatment, we also observed a statistically significant (p <.001) effect on acid taste, with an 82% reduction in the odds of reporting taste of acid. When we test for a difference between the two treatments, the difference is not statistically significant (p=0.71) (Figure 3).
Hoarseness
Using logistic regression with a random effect of subject, the disc treatment had a statistically significant (p<0.001) effect on reduced hoarseness, with an 87% reduction in the odds of reporting hoarseness.
For the gel treatment, there was a statistically significant (p <0.001) effect with a 92% reduction in the odds of reporting hoarseness. The difference between the two treatments was not statistically significant (p=0.41) (Figure 3).
Antacid use
Using a matched-pairs T-test, for the disc treatment, a mean of 60% fewer antacids were used compared to baseline (average 11 fewer used in 14 days, with standard deviation 9.8), with a p-value <0.001.
For the gel treatment, an average of 45% fewer antacids were used (average 11.5 fewer used in 14 days, with standard deviation 17.9), with p-value <0.001. A T-test comparing the two treatments did not show a significant difference (p=0.89) (Figure 3).
Discussion
The data indicate that nightly use of both discs and gel significantly reduces symptoms associated with GERD, including morning hoarseness, reflux acid taste, night time heartburn, and perceived reflux. Subjects who used the discs and gel for two weeks also demonstrated a significant reduction in antacid use during the night in comparison to two weeks of baseline use. The discs were found to be generally more effective in reducing symptoms than the gel, although most of the differences were not statistically significant. The exception was heartburn, where improvement was found to be significantly better for subjects using the discs than the gel. Significant side effects were not reported in either group during product use.
Subjects in the ‘control’ gel group were asked to use what was assumed would be an ineffective remedy. It was thought that the applied gel would wash out or be swallowed relatively quickly during the initial hours of sleep, thus providing only a narrow window of possible effectiveness. The unexpected result demonstrating significant reduction in symptoms for both disc and gel users, however, suggests that both of these products may have the effect of increasing salivation during sleep. Or alternatively, one or more constituents of the disc or gel, when swallowed, may have helped to protect the esophageal lining from acid irritation. Hence, rather than being an inert control, the gel, like the disc, appeared to function as an active agent.
As with any type of questionnaire methodology, reliance on subject memory of events can be problematic and the reliability of daily responses, in the absence of corroboration by direct measurement may be questioned. However, studies assessing questionnaire methodology suggest that memory issues may be lessened by regularly providing questions that are repeated in the exact same order and wording which was the approach used in this study [56]. Further, the specific questions used to assess the presence of symptoms were based on other previously validated self-reported questionnaires assessing potential GERD [57-60].
Subjects accepted into the study were not asked to discontinue any previously prescribed medication for reflux or heartburn during their participation. They were instructed that the disc or the gel were to be taken in conjunction with any other prescribed treatment. Our study protocol did not include a detailing by each subject of additional prescribed treatments (including the use of medication) during gel or disc use, hence it remains unknown if these additional prescribed interventions may have confounded or altered the findings. However, the use of any additional prescribed treatments by any subject was the same during the baseline and treatment phases, and the tested treatment showed a significant effect beyond any effect of the additional prescribed treatments.
Conclusions
This study suggests that two available OTC products used to manage dry mouth during sleep may provide an effective adjunctive remedy for reducing reflux and heartburn symptoms in patients with concomitant xerostomia. The discs and the gel were well tolerated and not associated with adverse reactions during use. Further, the data appear to support the hypothesis that an increase in salivation during sleep may be the reason for symptom reduction. However, more research is needed to confirm the mechanisms responsible for the therapeutic effects identified in the study.
The findings of this study are novel and potentially medically relevant for physicians treating GERD. And they are encouraging with respect to future research. Individuals who have dry mouth and experience symptoms of GERD during sleep may find benefit in using an OTC dry mouth product such as OraCoat XyliMelts.
Acknowledgements
The authors wish to thank OraHealth Corporation for providing the OraCoat XyliMelts discs and for coordinating the blinded distribution of the gel and disc products to subjects.
References
- Holtmann G (2001) Reflux disease: The disorder of the third millennium. Eur J Gastroenterol Hepatol 13: S5-11.
- Petersen H (1995) The prevalence of gastrooesophageal reflux disease. Scan J Gastroenterol 211: 5-6.
- Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ (1997) Prevalence and clinical spectrum of gastroesophageal reflux: A population-based study in Olmsted County, Minnesota. Gastroenterology 112: 1448-1456.
- Jung HK, Choung RS, Talley NJ (2010) Gastroesophageal reflux disease and sleep disorders: evidence for a causal link and therapeutic implications. J Neurogastroenterol Motil 16: 22-29.
- Ranjitkar S, Smales RJ, Kaidonis JA (2012) Oral manifestations of gastroesophageal reflux disease. J Gastroenterol Hepatol 1: 21-7.
- Dettmar PW, Castell DO, Heading RC (2011) Reflux and its consequences. Aliment Pharmacol Ther 33: 1-71.
- Karna Dev Bardhan, Vicki Strugala, Peter W Dettmar (2012) Reflux Revisited: Advancing the Role of Pepsin. Int J Otolaryngol.
- Vakil N, Zanten SV, Kahrilas P (2006) The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol 101: 1900-1920.
- Kahrilas PJ (2003) GERD pathogenesis, pathophysiology, and clinical manifestations. Cleve Clin J Med 70: S4-19.
- Patti MG (2016) Gastroesophageal Reflux Disease.
- Yuksel ES, Vaezi MF (2012) New Developments in Extraesophageal Reflux Disease. Gastroenterol Hepatol (NY). 8: 590-599.
- DeVault KR, Castell DO (2005) Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 100: 190-200.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, et al. (2008) American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Am Gastroenterol Assoc 135: 1383-1391.
- Jansson C, Nordenstedt H, Wallander MA (2009) A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol 7: 960-965.
- Gisbert JP, Cooper A, Karagiannis D (2009) Impact of gastroesophageal reflux disease on patient's daily lives: A European observational study in the primary care setting. Health Qual Life Outcomes 7: 60-68.
- Ferrus JA, Zapardiel J, Sobreviela E (2009) Management of gastroesophageal reflux disease in primary care settings in Spain: Sympathy i study. Eur J Gastroenterol Hepatol 21: 1269-1278.
- Gerson LB, Fass R (2009) A systematic review of the definitions, prevalence, and response to treatment of nocturnal gastroesophageal reflux disease. Clin Gastroenterol Hepatol 7: 372-378.
- Hungin AP, Hill C, Raghunath A (2009) Systematic review: Frequency and reasons for consultation for gastro-oesophageal reflux disease and dyspepsia. Aliment Pharmacol Ther 30: 331-342.
- Wallander MA, Johansson S, Ruigomez A (2007) Dyspepsia in general practice: incidence, risk factors, comorbidity and mortality. Fam Pract 24: 403-411.
- Kusano M, Kouzu T, Kawano T, Ohara S (2008) Nationwide epidemiological study on gastroesophageal reflux disease and sleep disorders in the Japanese population. J Gastroenterol 43: 833-841.
- Fass R, Quan SF, O'Connor GT, Ervin A (2005) Predictors of heartburn during sleep in a large prospective cohort study. Chest 127: 1658-1666.
- Mody R, Bolge SC, Kannan H, Fass R (2009) Effects of gastroesophageal reflux disease on sleep and outcomes. Clin Gastroenterol Hepatol 7: 953-959.
- Dubois RW, Aguilar D, Fass R (2007) Consequences of frequent nocturnal gastro-oesophageal reflux disease among employed adults: Symptom severity, quality of life and work productivity. Aliment Pharmacol Ther. 25: 487-500.
- Jung HK, Choung RS, Talley NJ (2010) Gastroesophageal Reflux Disease and Sleep Disorders: Evidence for a Causal Link and Therapeutic Implications. J Neurogastroenterol Motil 16: 22-29.
- Marsicano JA, de Moura-Grec PG, Bonato RC, Sales-Peres Mde C (2013) Gastroesophageal reflux, dental erosion, and halitosis in epidemiological surveys: a systematic review. Eur J Gastroenterol Hepatol 25: 135-141.
- Yoshikawa H, Furuta K, Ueno M (2012) Oral symptoms including dental erosion in gastroesophageal reflux disease are associated with decreased salivary flow volume and swallowing function. J Gastroenterol 47: 412-20.
- Jász M, Varga G, Tóth Z (2007) Dental erosion and gastro-esophageal reflux disease. Fogorv Sz 100: 3-10.
- Corrêa MC, Lerco MM, Cunha Mde (2012) Salivary parameters and teeth erosions in patients with gastroesophageal reflux disease. Arq Gastroenterol 49: 214-218.
- Vaezi MF (2006) Review article: The role of pH monitoring in extraoesophageal gastro-oesophageal reflux disease. Aliment Pharmacol Ther 23: 40-49.
- Alaraudanjoki V, Laitala ML, Tjäderhane L (2016) Influence of Intrinsic Factors on Erosive Tooth Wear in a Large-Scale Epidemiological Study. Caries Res 50: 508-516.
- Kao CH, Ho YJ, ChangLai SP (1999) Evidence for decreased salivary function in patients with reflux esophagitis. Digestion 60: 191-195.
- Helm JF, Dodds WJ, Hogan WJ (1987) Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology 93: 1393-1397.
- Pope CE (1994) Acid-reflux disorders. N Engl J Med 331: 656-60.
- Smith DJ, Joshipura K, Kent R (1992) Effect of age on immunoglobulin content and volume of human labial gland saliva. J Dent Res 71: 1891-1894.
- Burgess J (2015) Salivary Abnormalities in Dentistry. Emedicine.
- Dawes C (1972) Circadian rhythms in human salivary flow rate and composition. J Physiol 220: 529-545.
- Helm JF, Dodds WJ, Hogan WJ (1982) Acid neutralizing capacity of human saliva. Gastroenterol 83: 69-74.
- Yandrapu H, Marcinkiewicz M, Poplawski C (2015) Role of saliva in esophageal defense: implications in patients with nonerosive reflux disease. Am J Med Sci 349: 385-391.
- Shafik A, El-Sibai O, Shafik AA (2005) Effect of topical esophageal acidification on salivary secretion: identification of the mechanism of action. J Gastroenterol Hepatol 20: 1935-1939.
- Skoczylas T, Yandrapu H, Poplawski C (2014) Salivary bicarbonate as a major factor in the prevention of upper esophageal mucosal injury in gastroesophageal reflux disease. Dig Dis Sci 59: 2411-2416.
- Dutta SK, Agrawal K, Mahmoud MA (2010) Modulation of salivation and heartburn in response to the site of acid infusion in the human oesophagus. Aliment Pharmacol Ther 32: 795-800.
- Haag S, Holtmann G (2010) Onset of relief of symptoms of gastroesophageal reflux disease: post hoc analysis of two previously published studies comparing pantoprazole 20 mg once daily with nizatidine or ranitidine 150 mg twice daily. Clin Ther. 32: 678-690.
- Armstrong D, Paré P, Pericak D, Pyzyk M (2001) Symptom relief in gastroesophageal reflux disease: A randomized, controlled comparison of pantoprazole and nizatidine in a mixed patient population with erosive esophagitis or endoscopy-negative reflux disease. Am J Gastroenterol 96: 2849-2857.
- Vela MF (2014) Medical Treatments of GERD: The Old and New. Gastroenterol Clin North Am 43: 121-133.
- Lam JR, Schneider JL, Zhao W, Corley DA (2013) Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 310: 2435-2442.
- http://www.webmd.com/heartburn-gerd/h2-blockers-acid-reducers-for-gastroesophageal-reflux-disease-gerd
- http://livertox.nih.gov/H2ReceptorBlockers.htm
- http://www.medicinenet.com/nizatidine-oral/article.htm
- http://drugs.nmihi.com/prokinetics.htm
- http://www.mayoclinic.org/diseases-conditions/gerd/basics/treatment/con-20025201
- Ho J (2017) Effects of a novel disc formulation on dry mouth symptoms and enamel remineralization in patients with hyposalivation: An in vivo study. Dentistry 7-2.
- Tayee (2015) Evaluation of pH Values of Products Managing Xerostomia. Dental College of Georgia.
- Burgess J, Lee P (2012) XyliMelts time-release adhering discs for night-time oral dryness. Int J Dent Hyg 10: 118-121.
- Budylina SM (2015) Taste perception during clinical symptom complex of gastroesophageal reflux disease. Patol Fiziol Eksp Ter 59: 51-56.
- Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. The annals of mathematical statistics 18: 50-60.
- http://www.census.gov/srd/papers/pdf/rsm2006-13.pdf
- Bolier EA, Kessing BF, Smout AJ (2015) Systematic review: Questionnaires for assessment of gastroesophageal reflux disease. Dis Esophagus 28: 105-120.
- Vakil NB, Halling K, Becher A (2013) Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol 25: 2-14.
- Stanghellini V, Armstrong D, Mönnikes H (2007) Systematic review: Do we need a new gastro-oesophageal reflux disease questionnaire? Digestion 75: 3-16.
- Ponce J, Garrigues V, Agréus L (2012) Structured management strategy based on the Gastro-oesophageal Reflux Disease (GERD) Questionnaire (GerdQ) vs. usual primary care for GERD: Pooled analysis of five cluster-randomised European studies. Int J Clin Pract 66: 897-905.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14759
- [From(publication date):
August-2017 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 13842
- PDF downloads : 917