Effect of Various Factors on the Manufacturing of Geopolymer Mortar
Received: 20-Nov-2017 / Accepted Date: 29-Nov-2017 / Published Date: 05-Dec-2017
Abstract
In the recent years, research focus on the sustainable construction development. Due to high range of carbon dioxide emissions associated with manufacturing process of ordinary Portland cement that is finding alternatives procedures. This research studies the possibility of geopolymer mortar manufacturing from fly ash and Metakaolin then studies the fresh and hardened properties of this product. Two activating solutions were prepared for this purpose. The first is a mixture of sodium hydroxide/sodium silicate solution, fly ash, sand and water while the second is a mixture of sodium hydroxide/sodium silicate solution, Metalkaolin, sand and water. Test results indicate that the compressive strength is directly affected the fly ash and Metakaolin content and significantly affected the curing condition. A total of ten mixtures were evaluated by considering the effects of aggregate content, alkaline solution to fly ash and Metalkaolin ratio, sodium silicate to sodium hydroxide ratio, and curing method. Three optimal mixtures (M6, M7 and M8) were identified. Results show that the geopolymer mortar can be produced with compressive strength of 19.4 MPa at 28 days.
Keywords: Fly ash; Geopolymer mortar; Compressive strength; Sodium silicate activating solution
Introduction
Geopolymer is reaction of aluminosilicate materials with hydroxide or silicate solution (aqueous alkali) to produce alkali aluminosilicate compound, the term inorganic polymer is more common than geopolymer [1]. Geopolymer gives equivalent performance to normal cementitious binders in various ranges of utilizations, yet with more favorable position in bringing down emission of greenhouse [2]. Aluminosilicate materials show a wide differing of characteristics, upon on selection of raw materials, mixing procedure, curing methods and its temperature, alkaline solution concentration. Today, geopolymer is common reaching in an industry segment and considered an option and has been utilized as a part of various field applications like precast beams, boat ramp, pavement, bricks, retaining wall, water tanks, precast bridge decks [3]. Geopolymer concrete has specialized advantages on ordinary concrete, earlier gaining strength and higher compressive strength, less hydration heat, higher chemical resistance, good sulfate attack resistance, also good resistance to acid. Every one of these properties make geopolymer best and generally utilized [4].
Numerous materials can be utilized for assembling geopolymer as Metakaolin, fly Ash, slag, rice husk ash. Metakaolin as a source for aluminosilicate is utilized for production geopolymer. Metakaolin being produced by calcination of kaolinite clay at (500-800°C) relying on purity of this material [5]. Geopolymer binder action similarly as Portland cement. At room temperature its set and harden, and can increase sensible strength in brief time. Some proportions of geopolymer binders have been verified to be effective in construction, infrastructure applications with good properties as high mechanical performance, hard surface, thermal stability, good durability, and high acid resistance. Each present building part for example bricks, ceramic tiles and cement could be supplanted with geopolymer. Geopolymer does not needs extraordinary high production temperature, just low temperature handling of normally happening or specifically manual is applied to Kaoline or FA, mixture gives appropriate geopolymeric raw materials. These prompt critical lessening in the vitality utilization and emission of CO2. Around (0.6) less energy is required and (80-90%) less CO2 is created for generation of geopolymer than Portland cement. Therefore, it is great significance for environmental protection [5].
Yip et al. [6] studied the effect of the addition of calcium to Metakaolin Geopolymer concrete. The addition of 20% of calcium as a replacement improves in compressive strength, while by increasing calcium amount more than 40% compressive strength decreases. Within two days, strength development was close to complete. After 7 days, no significant increase in strength is shown.
Khater [7] studied the effect of Silicafume on the Geopolymer concrete produced from Metakaolin. Silicafume replacement started from 0% up to 10%. The specimen was cured at room temperature for (1, 7, 28 and 90 days). By increase curing time, the compressive strength evidently increased because hydration was progressive to form CSH in addition to produce Geopolymer gel lead to fine and homogeneous structure. Strength improving by increasing SF up to 7% then the strength decreased after this percentage up to 10%. Pozzolanic activity of Silicafume that have ability to enhance the properties like compressive strength, abrasion resistance and bond strength. Silicafume have ability to produce C-S-H (by consuming calcium hydroxide).
Al Bakri et al. [8] reported the effect of curing time and curing temperature on compressive strength of Fly ash based geopolymer concrete. When curing temperature and time increase, compressive strength increases. The maximum strength value optioned with curing temperatures range from 60-90°C within a period from 24-72 h.
Wang et al. [9] studied the effect of NaOH concentrations on Metakaolin geopolymer paste. NaOH concentrations effect on geopolymer pastes was investigated using different concentration of NaOH (8, 10, 12, 14 and 16 mol/L). Compressive strength of geopolymer increased with increasing NaOH concentration for 7 days age specimen. There results indicated that the compressive strength increases with NaOH concentration up to the studied value of 16 mol/L.
Kamarudin et al. [10] studied the effect of different concentration of NaOH on Kaolin Geopolymer concrete. This study focused on the development of compressive strength of Geopolymer from NaOH of different concentrations (6, 8, 10, 12 and 14 M) at age of 1, 2 and 3 days curing in an oven at a temperature of 80°C. After, curing of the specimen for 1 day and 2 days, there were no steady strength gains for different concentrations of NaOH solutions. Compressive strength is highest when NaOH concentration was 12 M for 3 day curing. The strength improved with an increase in NaOH concentration in solutions, the activation of binder become stronger and quicker. With increasing NaOH concentration solubility of aluminosilicate enhanced.
Demie et al. [11] studied the effect of curing temperature on compressive strength. The result referred to the compressive strength increased when the specimens were cured in oven up to 70°C for 48 h, while the specimens cured at curing temperature above 70°C results in a decrease in the compressive strength.
Rovnaník [12] studied the effect of curing temperature and curing time for Metakaolin based geopolymer on the development of dense structure. First group of specimens were cured at temperatures (10, 20, 40, 60 and 80°C) using electrical oven for 4 h and then kept at ambient temperature 20°C until the day of testing, only one specimen was stored in the fridge at (10°C) for the whole period before testing. The second group of specimens dealt with the effect of curing period at different temperatures. The specimens were cured at different time for 1, 2, 3 and 4 h at temperatures 40, 60 and 80°C. After that, the specimens are stored at an ambient temperature of 20°C until tested. Increasing temperature leads to accelerates formation of dense structure and accelerate geopolymerization reaction at early ages. The specimens cured at 60 and 80°C reached to final stage of strength at 24 h only, but there is reduction in strength as compared with specimens cured at ambient temperature (20°C), on the other hand the specimens cured at 10°C reach the same strength at 28 days.
Nath and Sarker [13] reported the effect of ambient curing at 23°C on compressive strength development of different geopolymer mixtures. Geoploymer manufacturing by using Fly ash only showed slowly to develop strength when cured in ambient condition (20-23°C). When granulated blast furnace slag (GBFS) was incorporated in the mixture as a part of total binder, the strength increased clearly. Compressive strength in 28-day increased up to 10 MPa for every 10% increment of slag content from control concrete. The increase in compressive strength by adding slag may be because of increase the calcium bearing compound in the dissolute binder which produced reaction product from both alkali activated fly ash and slag.
Liew et al. [14] reported the effect of curing regimes on Metakaolin geopolymer pastes. The results showed that curing at room temperature was unfeasible therefore, heat is relatively important in Metakaolin geopolymer synthesis. Low curing temperature, make the dissolution of the Metakaolin slow and thus the geopolymerization process was slow. The chemical reaction speeded up at higher temperature. The compressive strength improving at moderate elevated curing temperature (40 and 60°C). High temperature leads to quick polymerization and forming Geopolymer gels with poor structure, therefore compressive strength decreases.
Mechanism of geopolymerization
Geopolymerization is complex process accompanied by an exothermic production, stages of geopolymerization are [5]:
(a) Destruction to coagulation
(b) Coagulation to condensation
(c) Condensation to crystallization
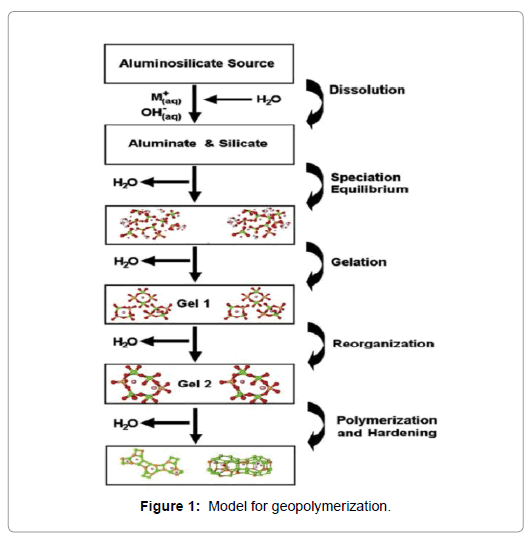
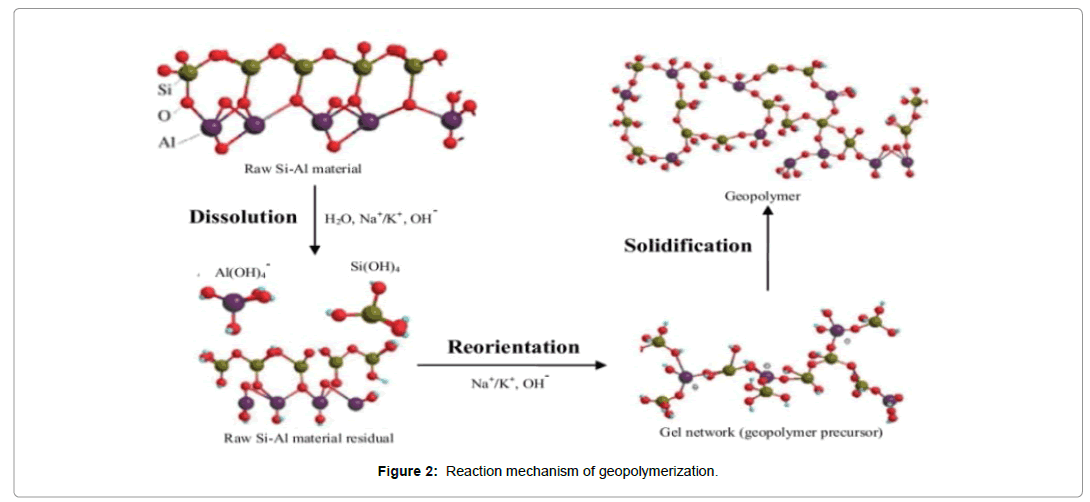
Generally, two main stages are carried out to produce geopolymer concrete: first stage includes aluminosilicate dissolution and formation species of polymer, second stage includs growth of polymeric particles through nuclei achieve critical size also crystals start to create [15] as shown in Figures 1 and 2. Dissolution stage begins when Si-Al from raw materials contacts alkaline solution, to produce Si and Al species. Many variables influence formation of Si and Al species, like alkaline metal type (Na+ or K+), alkaline solution concentration, rate, time mixing. Reorientation stage includes Si and Al diffused into the oligomers after dissolution. Oligomers into aqueous phase form many networks by condensation to gel formation, filtration of reactive Al and Si species from the raw materials is happening at Al+3 and Si+4 dissolving on surface of source Si-Al materials are removed. Solidification stage incorporates for formation of continuous gel including rearranging and reorganization, three stages, lead to produce geopolymer with amorphous, or semi-crystalline, three-dimensional aluminosilicate, network [15]. Table 1 shows the applications of geopolymeric materials based on silica-to Alumina [16].
| (Si:Al) ratio | Applications |
|---|---|
| 1 | Bricks Ceramics Fire protection |
| 2 | Low CO2 cements and concretes Radioactive and toxic waste encapsulation |
| 3 | Fire protection fibre glass composite Foundry equipments Heat resistant composites, 200°C to 1000°C Tooling for aeronautics titanium process |
| >3 | Sealants for industry, 200°C to 600°C Tooling for aeronautics |
| 20-35 | Fire resistant and heat resistant fibre composites |
Table 1: Applications of geopolymeric materials based on silica to alumina atomic ratio.
The purpose of this research is to produce new binding material (Geopolymer) that can bind components of conventional concrete and mortar (sand and gravel) instead of Portland cement depending on local materials (Metakaolin) in addition to imported materials (Fly ash).
Materials And Methods
The experimental work devoted to manufacture of geopolymer mortar by using metakaoline and fly ash. Many trial mixes were made to find out the optimum proportions. Many variables effect on this type of mortar are:
(a) Fly ash, Metakaoline content
(b) Ratio of Fly ash, Metakaoline to alkaline solution, NaOH and Na2SiO3
(c) Extra water content
(d) Ratio of NaOH to Na2SiO3
(e) Type and dose of superplasticizer
(f) Ratio of fine to total aggregate content
(g) Methods of curing: sunlight curing, laboratory curing, heat curing
Materials and their properties
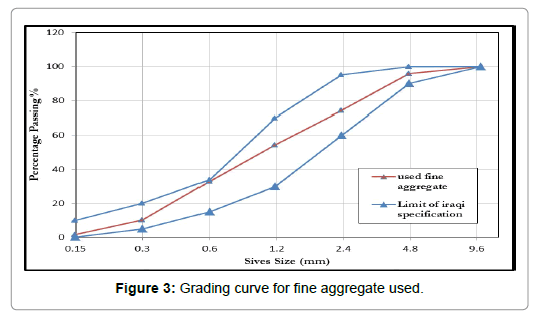
Fine aggregate: AL-Ekhaider sand was used as fine aggregate in this work with ultimate size of 4.75 mm and grading limited zone II. Sieve analysis and grading curve of fine aggregate show in Table 2 and Figure 3. Results demonstrate that fine aggregate grading and sulfate content were within requirements of I.Q.S (No. 45/1984) [17]. Table 3 demonstrates the chemical and physical properties of sand.
| Sieve size (mm) | Cumulative passing % | Limits of Iraqi Specification No.45/1984, zone (2) |
|---|---|---|
| 10 | 100 | 100 |
| 4.75 | 96.6 | 90-100 |
| 2.36 | 92.4 | 60-90 |
| 1.18 | 85.8 | 30–70 |
| 0.6 | 74.4 | 15-34 |
| 0.3 | 40.3 | May-20 |
| 0.15 | 6.9 | 0-10 |
Table 2: Grading of fine aggregate.
| Physical properties | Test Results | Limits of (IQS No.45/1984) |
|---|---|---|
| Specific gravity | 2.6 | - |
| Bulk Density (kg/m3) | 1729 | - |
| Sulfate content % | 0.343 | ≤ 0.5% |
| Absorption % | 2 | - |
Table 3: Physical properties of fine aggregate.
Mixing water: Tap water was utilized for washing, mixing and curing during the experimental work that provided from water supply network system.
High range water reducing admixture (Superplasticizer): GLENIUM 54 superplasticizer produced by BASF construction chemicals UAE LLC. It relies upon modified polycarboxylic ether which was utilized into test work. Dosage recommended by manufacturer is from 0.5 to 2.5 liters per 100 kg of cementitious materials. This type of admixture is according to ASTM C494- 05 (Table 4) [18].
| Property | Description |
|---|---|
| Form | liquid |
| Color | light brown |
| Relative density | 1.07gm/cm3 at 20ºC |
| PH | 05-Aug |
| Viscosity | 128+/-30 cps at 20ºC |
| Transport | not dangerous |
| Labeling | not hazard |
| Storage | stored in original containers above 5ºC |
| Packaging | available in 208 litter drums |
Table 4: Properties of Glenium 54.
Fly ash: Fly ash (FA) is fine and glassy powder that is recovered due to coal combustion through production of electricity from ISKENment-Turkey power station. It is viewed as coal combustion waste. Composition of FA relies on source, however FA incorporates significant amounts of (SiO2). It also contains (CaO), (Al2O3), (Fe2O3), Mg, K, Na, Ti, S which are also present in lesser amounts. FA atoms are spherical shape particle size ranged (0.5-100) μm. Two principle types of FA: Class F, Class C. Class F have been examined, it contains less than 20% CaO. Chemical composition of FA utilized in this examination is exhibited in Table 5, physical requirements are recorded in Table 6. Results demonstrate that FA utilized as a part of this examination adjusts requirement of ASTM C 618 [19], as appeared in Table 5. Some proportion about 2 ml of Glenium 54 was added into mix to determine % flow equal 110%. Casting of specimens and cured in the lab, then were subjected into airtight container for cured in oven, at (60 ± 5°C) for six days as appeared in Figure 3. Pozzolanic strength activity index was calculated by equation 1.
| Oxide | Content % |
|---|---|
| SiO2 | 57.63 |
| Al2O3 | 19.17 |
| Fe2O3 | 5.32 |
| CaO | 1 |
| MgO | 0.98 |
| SO3 | 0.07 |
| L.O.I | 2.77 |
Table 5: Chemical analysis of fly ash.
| Physical properties | Fly ash | ||
|---|---|---|---|
| Physical form | Powder | ||
| Color | Grey | ||
| Specific gravity | 2.33 | ||
| Surface area, m2/kg | 773 | ||
| Chemical properties | |||
| Oxide composition | Fly ash | Pozzolan class F | |
| SiO2+Al2O3+Fe2O3,min. percent | 70 | 89.73 | |
| SO3, max. percent | 5 | 0.67 | |
| Loss on ignition, max. percent | 6 | 2.77 | |
Table 6: Physical and chemical properties of fly ash.
P.I = (A/B) × 100 (1)
Where:-
P.I: pozzolanic strength activity index,
A: compressive strength, of 6 fly ash specimens, MPa,
B: Average compressive strength, of 6 reference specimens, MPa,
Procedure of mixing and compressive test of specimens were performed according to ASTM C305-02 [20] and ASTM C109/C 109M-05 [21] respectively.
Metakaolin: Kaolinite is a clay mineral with the chemical composition of Al2Si2O5(OH)4. Metakaolin is a dehydroxylated form of the clay mineral kaolinite associated with the reaction [22]:
Al2Si2O5(OH)4 → Al2O32SiO2 + 2H2O
Between 100-200°C, kaolinites lose most of their adsorbed water. In the range of 500-700°C, kaolinites become calcined by losing water through dehydroxilization. This process of changing kaolinite to Metakaolin is an endothermic process due to the large amount of energy required to remove the chemically bonded hydroxyl ions, which breaks down the crystal structure producing a transition phase (silica and amorphous alumina in reactive form) with high surface area.
Iraqi kaolin clay brought from Dewekhla region (Al-Anbar) was used. Kaolin was ground by air blast then burned in furnace up to 700 ± 20°C, for one hour, after that metakaolin was cooled for 24 h to the room temperature, depending on procedure mentioned by [5] and [23]. Table 7 shows the chemical analysis of metakaolin while Table 8 illustrates physical properties of metakaolin and Table 9 focuses chemical requirements of pozzolan ASTM C618 [19].
| Oxide | Content % |
|---|---|
| SiO2 | 51.59 |
| Al2O3 | 38.11 |
| Fe2O3 | 1.82 |
| CaO | 0.45 |
| MgO | 0.23 |
| SO3 | 0.14 |
| Na2O | 0.11 |
| K2O | 0.43 |
| L.O.I | 6.12 |
| Σ=99.00 |
Table 7: Chemical analysis of Metakaolin.
| Physical property | Result |
|---|---|
| Physical form | Powder |
| Color | Off-white |
| Specific gravity | 2.61 |
| Surface area, m2/kg | 1650, 1960 and 2300 |
Table 8: Physical properties of Metakaolin.
| Oxide composition | Pozzolan class N | Metakaolin |
|---|---|---|
| SiO2+Al2O3+Fe2O3, min. percent | 70 | 91.52 |
| SO3, max. percent | 4 | 0.14 |
| Loss on ignition, max. percent | 10 | 6.12 |
Table 9: Chemical requirements of natural Pozzolan according to the ASTM C618.
Strength Activity Index for Metakaolin: The strength activity index for Metakaolin is conducted according to the ASTM C311-05 [24].
Manufacturing of metakaolin geopolymer mortar
A. Preparation alkaline solution for geopolymer mortar
Solution of geopolymer moratr is constituted of sodium hydroxide and sodium silicate. Sodium hydroxide high purity, more than 98%, can be solved into distilled water to produce solution with appropriate concentration. NaOH concentration was varying from 5 to 16 Molar, mass of sodium hydroxide solids in solution varies relied upon solution concentration. The sodium silicate solution is commercially obtainable in various types. Throughout this work using solution of sodium silicate has a ratio of SiO2 to Na2O by mass which equals 2.4. The proportions, by mass of components, were SiO2=32.5%, Na2O=13.4%, and water=55.1%. After preparing NaOH as a solution, it was added to the Na2SiO3 solution. Alkaline liquid was combination of (NaOH) solution and Na2SiO3 solution, before using, must be alkaline liquid is prepare by mixing both solutions together at least 24 h. Extra water was usually used in geopolymer with different contents to improve workability. For more improvement, high range water reducing superplasticizer can be added which could result in strength improvements as well. The mixing of extra water with the superplasticizer was continued until a homogenous solution was produced. Finally, the alkaline liquid was added to the mixed extra water and superplasticizer together and mixing continues for not less than 2 min. Fifty trial mixes were made to select the optimum mix, depending on changing the components of mortar mixture for reaching to the best mix that giving higher compressive strength. Fine aggregate content, was kept constant for all mixes. The concentration of NaOH was 10 Molar, the mass ratio of NaOH to Na2SiO3 was 1:3.5 and the fineness of Metakaolin was 1650 m2/kg. The method of curing used for these trials was sunlight in August, when the temperature was ranging from 36 to 48°C as shown in Table 10 trial mixtures of geopolymer mortar.
| Mix type |
FA g/cm3 |
MK, g/cm3 | Fine Agg. g/cm3 | CaO % | NaoH to Na2Sio3 |
M of NaoH | Water by wt. of FA, MK |
Alkaline solution, by wt. of FA, MK | HRWR, ml/cm3 |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 300 | - | 600 | - | 0.4 | 8 | 0.2 | 1:2 | - |
| M2 | 300 | - | 600 | - | 0.4 | 8 | 0.2 | 1:2 | - |
| M3 | 300 | - | 600 | - | 0.4 | 12 | 0.2 | 1:3 | - |
| M4 | 270 | - | 600 | 30 | 0.5 | 12 | 0.2 | 1:3 | - |
| M5 | 270 | - | 600 | 30 | 0.45 | 12 | 0.2 | 1:3 | - |
| M6 | 240 | - | 600 | 60 | 0.45 | 8 | 0.2 | 1:2 | - |
| M7 | 210 | - | 600 | 90 | 0.45 | 10 | 0.2 | 1:2.5 | 12 |
| M8 | 300 | - | 600 | - | 0.45 | 12 | 0.1 | 1:3 | 12 |
| M9 | 300 | - | 600 | - | 0.45 | 12 | 0.1 | 1:3 | 10 |
| M10 | 300 | - | 600 | - | 0.45 | 12 | 0.3 | 1:2.5 | 14 |
| M11 | 300 | - | 600 | - | 0.45 | 8 | 0.3 | 1:2 | 12 |
| M12 | - | 300 | 600 | - | 0.45 | 10 | 0.1 | 1:2.5 | 12 |
| M13 | - | 300 | 600 | - | 0.45 | 8 | 0.1 | 1:2.5 | 12 |
| M14 | - | 300 | 600 | - | 0.45 | 8 | 3 | 1:2 | - |
| M15 | - | 270 | 600 | SF 10% | 0.45 | 8 | 0.3 | 1:2 | - |
Table 10: Trial mixtures of geopolymer mortar.
B. Studying the effect of different curing systems on compressive strength of geopolymer mortar
Type of curing is considered very important factor effecting on the strength of geopolymer mortar. After mixing, casting, Metakaolin geopolymer mortar specimens were demolded after 24 h and then the curing was started until the age of test. The followings are the used curing systems:
Sunlight curing: This method of curing was carried out by subjecting the specimens to the sunlight outside the laboratory after demolding. In Iraq, there is a high difference in temperature between winter and summer. To find out the effect of temperature of sunlight curing on strength of metakaolin geopolymer mortar; two groups of specimens were cast. The first was cured in January while the second were cured in August. The difference of temperature ranged from 12-19°C in January but changed from 36-48°C in August.
Laboratory curing: Specimens were placed inside the laboratory after removing from molds until the day of test. Also, two groups of specimens were made, one for winter and other for summer. The range of temperature inside the laboratory changed from 8-15°C in winter and ranged from 32-37°C in summer.
Oven curing: In this method of curing, the specimens were put into oven with temperatures ranged (70-100°C) and the curing process was continued until completing the age of test.
Water curing: Casted specimens were placed into a water tank after demolding and curing remained to the age of test. The temperature of water curing was kept at the range of 20 ± 2°C. This method is similar to that used for curing Portland cement.
Heating in oven and laboratory curing: After demolding the specimens heating in an oven was started. Temperature inside the oven was maintained at 60°C for 6 h, specimens were cured in laboratory temperature until the age of test. This system was adopted for curing only in winter when temperature deficiency.
Heating in oven and sunlight curing: In this method, heat curing process was started at first to accelerate the reaction of geopolymer mortar at 60°C for 6 h, then the specimens were put under sunlight in summer until the test day.
C. Mixing procedure of geopolymer mortar
Silica and aluminum oxide in metakaolin react with alkaline liquid solution (Na2SiO3 and NaOH) to form a paste of geopolymer that binds fine aggregate (sand) with other materials together to produce geopolymer mortar. Procedure of mixing have main effect on workability and strength of geopolymer mortar. Metakaolin, fine aggregate first were mixed together in dry form in a pan mixer for 3 min. Sand are prepared in saturated surface dry, superplasticizer, water was mixed with alkaline liquid for not less than 2 min. Then, liquid solution of mixture was added to the dry compounds into mixer pan for mixing continued usually for another 4 min. Casting in molds and compaction of geopolymer mortar by using vibrating table are then done. Compaction needed high skill because of the metakaolin based geopolymer mortar having zero flow while the compaction by rod was inappropriate technique.
Many trial mixes were conducted in this research to find out the optimum mix of metakaolin based geopolymer mortar. All component of mixture studied including metakaolin content, NaOH concentration, ratio of NaOH to Na2SiO3, G54 dose, ratio of metakaolin to alkaline solution. The optimum mix consisted of 500 kg of metakaolin, 80L of alkaline solution (NaOH to Na2SiO3=1:3.5), 3L of G54 superplasticizer, 40 kg of extra water and 720 of fine aggregate. The optimum mix used to study the fresh and hard properties of metakaolin geopolymer mortar.
D. Fresh properties
Flow test of geopolymer mortar was measured as traditional mortar test. Flow test conducted directly after mixing according to the ASTM C C1437-03 [25].
E. Hardened properties
The compressive strength test was done based on (ASTM C109/C 109M-05). Cubes with dimensions of 50×50×50 mm were tested by using hydraulic compression machine of 2000kN. Test was carried out at ages of 7 and 28 days under sunlight curing in January and August.
F. Utilize silicafume and aluminum oxide to produce modified geopolymer mortar
Silicafume was used to modify the metakaolin geopolymer mortar. Metakaolin was replaced by silicafume 10%. The specimens casting in cubes and cured under sunlight when temperature ranged from 36 to 48°C, till the day of testing and the compressive strength test conducted at 28 days. Alkaline solution was kept constant for all mixes (45% of binder weight) with NaOH: Na2SiO3 ratio equal 1:3.5, when the concentration of NaOH was 10 molar. Extra water was 10% of binder weight and 3 mL G54 superplasticizer for all mixes while the fine aggregate mass was 720 kg.
Manufacturing of FA geopolymer mortar: Fly ash based geopolymer mortar was produced for the comparison it with local metakaolin geopolymer mortar. Sunlight and heating curing were performed in August for curing fly ash geopolymer mortar. The tests conducted on fly ash geopolymer mortar included the flow and compressive strength tests. All these tests were conducted according to the same ASTM standard and same mixing, placing and curing for metakaolin geopolymer.
Fly ash-metakaolin geopolymer mortar: Fly ash used with metakaolin for modifying the Si/Al ratio; the compressive strength of geopolymer concrete was tested at 28 days. The optimum mix was used and the specimens (50 mm3 cubes) were cured by using heating, sunlight curing system in August when the temperature ranged between 36-48°C.
Results And Discussion
An attempt was made in the current study and numerous trials were conducted to produce geopolymer mortar see Table 1. Diverse types of curing studied in this research to find out the perfect way for curing fly ash and metakaolin geopolymer mortars such as the mixing types illustrated in Table 11. Compressive strength could be considered as scale for choosing the best type for curing that suitable with fly ash and metakaolin geopolymer mortar. Table 11 illustrates the effect of curing systems on the compressive strength of geopolymer mortar.
| Mix type | Curing system | Compressive Strength, MPa at age: | ||
|---|---|---|---|---|
| 7 days | 28 days | |||
| M1 | Sunlight | Summer Temp. (40-50°C) | 10.3 | 16.9 |
| M2 | Winter Temp. (15-20)°C | 9.5 | 15.6 | |
| M3 | Laboratory | Summer Temp. (30-35°C) | 8.2 | 13.53 |
| M4 | Winter Temp. (8-15°C) | 6.4 | 10.5 | |
| M5 | Water | 10.2 | 16.58 | |
| M6 | Oven Temp. (70-80°C) | 12.1 | 19.9 | |
| M7 | 12.06 | 19.4 | ||
| M8 | 11.3 | 19.05 | ||
| M9 | 10.9 | 16.2 | ||
| M10 | 11.6 | 17 | ||
Table 11: Effect of curing systems on compressive strength of geopolymer mortar.
Effect of curing temperature
Table 11 shows that the effect of curing temperature on the 7 and 28 days strengths of all mixes which cured at 70°C gives significantly increased strengths compared to those cured at 50°C for the same time. Lower curing temperature leads to mitigation of such expansion thus reflected negatively on the strength values.
Investigations on reactivity of FA under the effect of thermal curing reported that the good curing should be worked with temperatures in the range 30°C to 85°C [26]. Reaction kinetics resulting from different curing temperature were investigated with isothermal calorimetric tests on different materials (portland cement, activated slag and fly ash, and selected fly ash–slag blends) at 25°C, 35°C and 40°C observing the development of the polymerisation.
Effect of alkali ratio
An increase in alkali dosage (M+) resulted in an increase of the strength up to an M+ of 1.5%. Beyond this ‘optimum’ value, the strengths decreased, which is attributed to saturation of the gel with alkali ions resulting in less free water to be available for speciation of silica and alumina oligomers from the dissolution of FA [26]. There are many types of alkali activators that can be used to activate fly ash, metakaolin. For example, sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium silicate (Na2SiO3), potassium silicate (K2SiO3), calcium hydroxide (Ca(OH)2). The combined use of NaOH and Na2SiO3 is the most common practice in term of cost effectiveness to produce geopolymer with good compressive strength than the solely use of alkali silicate or hydroxide. The ratio of alkali activator/ash can be a critical parameter to the strength development. High alkali activator/ash increases the viscosity and difficulty in compaction. The workability and compressive strength decrease by further increment of the alkali activator/ash and Na2SiO3/NaOH.
According to the compressive strength results appeared in Table 11, sunlight curing in summer gives compressive strength equivalent to 16.9 MPa at 28 days when temperature ranged from 40 to 50°C, but compressive strength diminished obviously in winter when temperature was between 15 and 20°C. Increasing of temperature accelerates formation of hard structure particularly in early stage of geopolymerization reaction [12]. Distinction in compressive strength between summer and winter was around 8.42% at 7 days and 8.33% at 28 days. Compressive strength for laboratory cured mix was too close to sunlight curing. As was the case in sunlight curing, laboratory curing specimens had high compressive strength at high temperature (in summer) and also there was reduction in strength when temperature decreased (in winter). Curing with temperatures of (30-35°C) was viewed good mechanism for gaining strength and helping for geopolymerization that needs to heat with temperature of more than 35°C [1].
Difference in compressive strength between summer and winter was around 28.12% at 7 days and 28.85% at 28 days. Water curing for fly ash and metakaolin geopolymer mortars could be viewed as ineffectual for providing geopolymer mortar with moderate compressive strength. This type of curing was unsatisfactory and prompts to deceleration of reaction because of water inter inside mortar and makes expansion causing cracks into geopolymer structure. Optimum curing technique into oven at temperature (70-80°C) gave highest compressive strength 19.9 MPa at 28 days, increasing temperature of curing tend to increase geopolymerization process accordingly. There was increase in rate of improved strength at early ages with reduction in later ages. At low temperatures, such as 10°C, geopolymerization process was very slow, therefore compressive strength was very low in spite of only amorphous phase is available.
For mass production in precast factories sunlight curing system and oven curing in summer are viewed as reasonable systems for curing fly ash and Metakaolin based geopolymer mortars. Such a case is very convenient and economical in hot weather countries.
Effect of high-range water reducer dose
The increase in high-range water reducer content leads to an increase in compressive strength of geopolymer up to an optimum dose, 12 ml/cm3, at which strength reaches 12.1, 19.9 MPa at 7 and 28 days age respectively. After that limit, the strength decreases. By increasing the dose from 12 to 14 ml/cm3, mix M10, the reduction in compressive strength was 14.5% and that may be because of increasing of voids in the geopolymer mortar structure which have an adverse effect on compressive strength [27]. Moreover, decreasing high-range water reducer content from 12 to 10 ml/cm3, mix M9, caused the reduction by 15.5%. As the high-range water reducer dosage in the mix increases, the compressive strength increased. This was due to the more effective action of the high-range water reducer in facilitated and regulated compaction of the geopolymer mortar by enhancing workability [28].
Alkaline solution to fly ash, Metakaolin ratio
The results indicate that increasing the ratio of alkaline solution to Metakaolin from 40 to 45% of fly ash weight M5 causes an improvement in strength. For the alkaline Geopolymerisation reactions, more (Si) aids in the production of Si-O-Si bonds, and significantly increases the compressive strength of the Geopolymer. If the content of Si exceeds the suitable limit, the Geopolymerisation rate is negatively affected, leading to Geopolymer of low strength as shown in M4 when the alkaline solution to Fly ash ratio increased to 50% the compressive strength got a slight reduction that reaches to 36.3% when compared with mix M5 [10].
Effect of NaOH concentration
The concentration of NaOH plays an important role in compressive strength development of fly ash and Metakaolin geopolymer mortar. Increasing NaOH concentration leads to an improve in compressive strength. Geopolymerization process needs strong alkali to activate the (Si) and (Al) in fly ash and Metakaolin. By increasing NaOH concentration ability of solution to leach (Si) and (Al) in fly ash and Metakaolin is improved due to the formation of alumino-silicate gel at an early stage that resulted from increasing NaOH concentration [29].
Formation of gel has two opposing effects. First, the depletion of ions which prompts further leaching of ions from fly ash and Metakaolin particles. Second, the thickening of solution results in a lower mobility of ions particularly at the surface of Metakaolin and retards the leaching out of ions.
Effect the ratio of Na2SiO3/NaOH
The effect of NaOH to Na2SiO3 ratio is very clear as shown in Table 12. By increasing this ratio, compressive strength increased till the ratio reaches 1:3 by mass (maximum compressive strength). The improve in compressive strength by increasing NaOH to Na2SiO3 ratio returned to increase dissolution of silica and alumina of Metakaolin also the increase of sodium content in the mixture. Sodium is important for the formation of geopolymer as it acts as charge balancing ions. But when increasing NaOH to Na2SiO3 ratio to 1:4, compressive strength decreased because of the excess of sodium silicate which hinders water evaporation and structure formation. Also excess sodium content can form sodium carbonate by atmospheric carbonation, and this may disrupt the polymerization process. Therefore the optimum ratio of NaOH to Na2SiO3 was 1:3 by mass for the Metakaolin based geopolymer mortar.
| Alkaline solution by wt. of FA, MK |
NaOH in Molarity | Compressive Strength MPa at | |
|---|---|---|---|
| 7 days Age | 28 days Age | ||
| 01:02 | 8 | 12.1 | 19.9 |
| 01:02.5 | 10 | 12.06 | 19.4 |
| 01:03 | 12 | 11.3 | 19.05 |
Table 12: Effect of NaOH concentration on compressive strength for fly ash and Metakaolin geopolymer.
Conclusion
Local manufactured Metakaolin and fly ash were possible to be used for producing geopolymer mortar. Oven curing is considered the effective way for curing fly ash and Metakaolin geopolymer mortar. Sunlight curing system does not need energy, does not cause any environmental damage and without any cost. High ratio of alkali activator/ash and Na2SiO3/NaOH does not necessarily lead to high compressive strength. The optimum compressive strength was obtained when alkali activator/ash ratio was 0.45 regardless of the type of alkali activators. The effect of oxide molar ratios on the compressive strength was studied. Higher ratios do not lead to higher compressive strength.
The compressive strength of geopolymer using commercial grade Na2SiO3 was significantly higher than those using industrial grade Na2SiO3 regardless of the type of alkali hydroxide. Geopolymers can provide a desirable alternative to portland cement (PC) binders, not only for the environmental benefits arising from the avoidance of CO2 emissions associated with PC production, but also in terms of their performance and durability.
References
- Davidovits J (2011) Geopolymer chemistry and applications, (3rd edn.), Institut Geopolymere, France.
- Rodrigues FA, Joekes I (2011) Cement industry: Sustainability, challenges and perspectives. J Environ Chem Lett 9: 155-161.
- Aldred J, Day J (2012) Is geopolymer concrete a suitable alternative to traditional concrete. 37th conference on Our World in Concrete and Structures, Singapore.
- Lloyd NA, Rangan BV (2010) Geopolymer concrete with fly ash. Second international conference on sustainable construction materials and technologies 3: 1493-1504.
- Ibrahem AM, Wahab AA (2008) Effect of temperature on the pozzolanic properties of metakaolin produced from Iraqi kaolin clay. J AL- Fatih 4: 268-285.
- Yip CK, Lukey GC, Provis JL, van Deventer JSJ (2008) Effect of calcium silicate sources on geopolymerization. Cem Concr Res 38: 554-564.
- Khater HM (2013) Effect of silica fume on the characterization of the geopolymer materials. Int J Adv Str Eng 5: 12.
- Al Bakri M, Kamarudin AM, Bnhussain H, Nizar M IK, Rafiza AR, et al. (2012) The processing, characterization and properties of fly ash based Geopolymer concrete. Rev Adv Mater Sci 30: 90 -97.
- Wang J, Zhang C, Xu J, Qu P, Zhou Y, et al. (2012) The Effect of alkali on compressive of metakaolin based geopolymeric cement. Adv Mater Res 554-556: 327-330.
- Kamarudin H, Omar AK (2011) The effect of alkaline activator ratio on the compressive strength of fly ash-based geopolymer. Aust J Bas and Appl Sci 5: 1916-1922.
- Demie S, Nuruddin MF, Ahmed MF, Shafiq N (2011) Effects of curing temperature and superplasticizer on workability and compressive strength of self-compacting geopolymer concrete. National Postgraduate Conference (NPC), Perak, Malaysia.
- RovnanÃk P (2010) Effect of curing temperature on the development of hard structure of metakaolin based geopolymer. Constr Build Mater 24: 1176-1183.
- Nath P, Sarker PK (2012) Geopolymer concrete for ambient curing condition. Australasian structural engineering conference, Perth, Western Australia.
- Liew YM, Kamarudin H, Al Bakri AMA, Bnhussain M, Luqman M, et al. (2013) Effect of curing regimes on metakaolin geopolymer pastes produced from geopolymer powder. Adv Mater Res 626: 931-936.
- Duxson PA, Jimenez AF, Provis JL, Lukey GC, Palomo A, et al. (2007) Geopolymer technology: The current state of the art. J Mater Sci 42: 2917-2933.
- Davidovits J (1999) Chemistry of geopolymeric systems terminology. In proceedings second international conference, Geopolymer 99, Davidovits J, Davidovits R, James C (Eds.), Geopolymer Institute, Saint-Quentin, France: 9-39.
- Iraqi standard specification No. 45 (1984) Aggregate of natural sources using in concrete and building. Central organization for standardization and quality control, Baghdad.
- ASTM C494/C494M- 05 (2005) Standard specification for chemical admixtures for concrete. Am Soc Test Mater Int: 1-10.
- ASTM C618 (2002) Standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete. Am Soc Test Mater Int.
- ASTM C 305-02 (2002) Standard practice for mechanical mixing of hydraulic cement pastes and mortars of plastic consistency. Am Soc Test Mater Int: 1-3.
- ASTM C 109/C 109 M-05 (2005) Standard test method for compressive strength of hydraulic cement mortars (using 2 inch or [50-mm] cube specimens. Am Soc Test Mater Int: 1-9.
- Alvador S (1995) Pozzolanic properties of flash-calcined kaolinite: A comparative study with soak-calcined products. Cem Concr Res 25: 102-112.
- Frieh KJ, Abbas WA, Hamid MM (2012) Some properties of concrete containing high fraction volume of metkaolin. Eng Tech J 32.
- ASTM C311 (2005) Standard test methods for sampling and testing fly ash or natural pozzolans for use in Portland cement concrete. Am Soc Test Mater.
- ASTM C1437-15 (2004) Standard test method for flow of hydraulic cement mortar. Annual Book of ASTM Standards.
- Soutsos M, Alan P, Boyle B, Vinai R, Hadjierakleous A, et al. (2016) Factors influencing the compressive strength of fly ash based geopolymers. Constr Build Mater 110: 355-368.
- Laskar A, Bhattacharjee R (2012) Effect of plasticizer and superplasticizer on workability of fly ash based geopolymer concrete. Proceedings of international conference on advances in architecture and civil engineering. Paper ID SAM 134 2: 974-977.
- Nuruddin MF, Demie S, Shafiq N (2011) Effect of mix composition on workability and compressive strength of self-compacting geopolymer concrete. Canad J Civ Eng 38: 1196-1203.
- Rattanasak U, Chindaprasirt P (2009) Influence of NaOH solution on the synthesis of fly ash geopolymer. Min Eng 22: 1073-1078.
Citation: Hameed AM, Rawdhan RR, Al-Mishhadani SA (2017) Effect of Various Factors on the Manufacturing of Geopolymer Mortar. Arch Sci 1: 111.
Copyright: ©2017 Hameed AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 10827
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 9393
- PDF downloads: 1434