Effect of Ultrasound on the Physico-chemical Properties of Poorly Soluble Drugs: Antisolvent Sonocrystallization of Irbesartan
Received: 15-Jun-2021 / Accepted Date: 23-Jul-2021 / Published Date: 28-Jul-2021 DOI: 10.4172/2471-9846.1000295
Abstract
In the present study, power ultrasound (US) using the antisolvent sonocrystallization technique (ASC) was used to improve the physicochemical properties of Irbesartan which a poorly water-soluble drug. The powders produced were characterized by Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectrophotometry (FTIR) and X-ray Diffraction (XRD). The effect of process variables on particle size, solubility and dissolution were studied. Flowability, compressibility and mechanical properties of the produced powders were also assessed. Using ASC led to considerable decrease in the particle size. SEM studies showed that the ASC produced particles were almost spherical with regular size. Thermal behavior, XRD patterns and FT-IR spectra of raw Irbesartan and powders obtained by ASC have shown no significant differences.
Keywords: Ultrasound; Spectrophotometry; Particle size; Sonocrystallization
Introduction
Oral drug delivery is considered the most convenient, economic and safest among other delivery routes with high patient compliance [1]. Drugs administered orally should dissolve first in gastrointestinal fluids then diffuse through membranes to reach the systemic circulation. However, the majority of the new chemical entities discovered by the pharmaceutical industry are poorly water-soluble lipophilic compounds. For these drugs, the dissolution rate is a rate-limiting step. Many technologies have been used to improve the solubility and dissolution rate of drugs, such as inclusion in cyclodextrins, solid dispersion, crystallization and antisolvent sonocrystallization (ASC). Crystallization from solution is divided into two distinct steps, nucleation, in which new crystals appear, and crystal growth, during which the existing crystals grow to larger sizes. The properties of the crystals produced are the result of the kinetic relationship between these two processes [2]. The driving forces for both nucleation and growth are the supersaturation and the interfacial tension between the solid and the liquid [3]. Ultrasonication (US) significantly influences the crystallization process as the resulting intensified micro-streaming enhances the mass transfer and increases the diffusion coefficient between the solvent and the antisolvent, leading to high levels of supersaturation [4]. It is also reported that the interfacial tension may be influenced by US [5,6]. In addition, the localized high pressure and the subsequent rapid local cooling rates have a significant effect in increasing supersaturation [7]. US can increase or decrease the growth rate of certain crystal faces leading to change in crystal habit [5,7]. Ultrasonic cavitation in liquid powder slurries produces related phenomena. Cavity collapse occurring near the solid surface drives high speed micro-jets of liquid into the surface that can reach 400 km/h [8-10], and induces shockwave damage to the surface [11]. However, it is reported that at US frequency of 20 kHz, the micro-jets are not observed with solid particles smaller than 200 μm [12]. Furthermore, cavitation and shockwaves can accelerate solid particles to high velocities [13]. The aforementioned events have important effects on the surface morphology leading to an increase in the surface area [8,11,14,15]. However, US waves of high power, usually within the MHz region, can result in high velocity particle collisions, sufficient for the formation of agglomerates [8,16]. The use of US during various crystallizing systems provides a non-destructive and relatively reproducible way to improve the end product properties, primarily crystal size and habit [7,17]. The induction time of nucleation is significantly reduced as well as the metastable zone width [18,19], which shows that US allows nucleation to occur at much lower super saturation levels [5-7]. Moreover, particle agglomeration is significantly prevented [5]. US can be used as an alternative to the addition of a seed crystal and thus improving the crystal purity [20], and for the isolation of the most thermodynamically favored polymorph when applied to a polymorphic system [7,21,22]. ASC is reported to decrease the oiling out phenomenon and to facilitate the crystallization of materials difficult to nucleate [18,23]. The modification in the size and habit of the product crystals may result in improved secondary physical properties such as compressibility, flow ability and packing; and in a more rapid filtration as well as the improvement of the yield of the process [24].
The aim of this present study is to improve the solubility, dissolution rate and extent of Irbesartan as a model for poorly soluble drugs. The resultant powder was characterized using Differential Scanning Calorimetry (DSC), X-Ray Diffraction (XRD), Fourier Transform Infrared (FT-IR) and Scanning Electron Microscopy (SEM). In addition, the effect of ASC on micrometric properties and powder secondary properties mainly flowability, compressibility, hardness and friability were also evaluated [25].
Materials and Methods
Irbesartan was supplied by Yellow Chem Pharma Products, Mumbai. Ingredient such as Ethanol, Methanol and hydrochloric acid was supplied by Central Drug House, Ltd. New Delhi, India. All other chemicals used in this study were analytical grade.
Method of preparation
A predetermined amount of raw Irbesartan was dissolved in 10 ml of organic solvent (ethanol) and then added progressively to distilled water (10 and 20 ml), as antisolvent, maintained at a constant temperature using a circulating water bath. The US horn (2.5cm in diameter) was immersed in the antisolvent at approximately 1.5 cm from the jacketed beaker's concave bottom. The mixture was simultaneously sonicated using a probe ultrasonicator (VCX 130 Vibra Cell) at 25±5°C, for different durations (10 min) with 20 KHz frequency and operated at amplitudes (50 %).
Similar experiments were carried out with mechanical agitation without US application to obtain the antisolvent crystallized samples (AnC). However, the obtained suspension was left for about 30 min before filtration in order to allow the formation of crystals. (Table 1) Shows the experimental setup used in this study.
| Sample | Drug amount (g) | US amplitude (%) | US time (min) | Processing temperature (°C) | Solvent nature |
|---|---|---|---|---|---|
| AnC | 0.150 | - | - | 25 | Ethanol |
| ASC 1 | 0.150 | 50 | 10 | 25 | Ethanol |
| ASC 2 | 0.125 | 50 | 10 | 25 | Ethanol |
| ASC 3 | 0.100 | 50 | 10 | 25 | Ethanol |
| ASC 4 | 0.750 | 50 | 10 | 25 | Ethanol |
Table 1: Experimental setup for the preparation of AnC and ASC samples.
Characterization
The process yield
The products obtained were filtered and weighed after drying, and the percentage yield was
Calculated, showed in the (Table 2)
| Batch No. | Drug | Yield (mg) | %yield |
|---|---|---|---|
| AnC | 150 mg in 10 ml ethanol | 116 | 77.33% |
| ASC1 | 150 mg in 10 ml ethanol | 110 | 73.33% |
| ASC2 | 125 mg in 10 ml ethanol | 120 | 96.00% |
| ASC3 | 100 mg in 10ml ethanol | 94 | 94.00% |
| ASC4 | 750 mg in 10ml ethanol | 625 | 83.33% |
Table 2: Batch Formulation
Yield (%) = (Wo/Wi) ×100
Where, Wo and Wi are the obtained and initial weights of Irbesartan, respectively.
Solubility Studies
The dynamic solubility of pure drug (IRB) and ASC was determined by adding excess solid (150 mg) to 100mL media (Different HCL strength, pH 1.2) placed in stoppered conical flasks, pre-equilibrated to 37±0.5 °C. The flasks were mechanically shaken in Orbital Shaker (HICON, New Delhi, India) at 100 rpm. At pre-determined intervals, sample was withdrawn, filtered through a 0.45μm syringe filter, diluted and analyzed by UV at 230nm (Table 3).
| HCl strength (N) | Time (min) | Pure drug (mg/mL) | ASC (mg/mL) | Ratio ASC/ Pure drug |
|---|---|---|---|---|
| 0.1 | 5 | 0.17 | 7.75 | 45.58 |
| 10 | 0.32 | 8.15 | 25.46 | |
| 30 | 0.41 | 8.66 | 21.12 | |
| 60 | 0.56 | 9.43 | 16.83 | |
| 120 | 0.89 | 10.70 | 12.02 | |
| 0.05 | 5 | 0.13 | 3.78 | 29.07 |
| 10 | 0.33 | 4.08 | 12.36 | |
| 30 | 0.46 | 4.49 | 9.76 | |
| 60 | 0.55 | 4.98 | 9.05 | |
| 120 | 0.76 | 5.69 | 7.48 | |
| 0.01 | 5 | 0.05 | 0.69 | 13.8 |
| 10 | 0.10 | 0.83 | 8.30 | |
| 30 | 0.17 | 0.89 | 5.23 | |
| 60 | 0.25 | 0.95 | 3.80 | |
| 120 | 0.31 | 1.13 | 3.64 |
Table 3: Solubility studies of pure drug (IRB) and ASC in different media
Determination of enhancement of solubility of Irbesartan
The saturated solutions of ASC were made in 10 ml solvents like water shake for 12 hours and kept aside for 24 hours. Then the solutions were filtered and the filtrate was analyzed in UV-Spectrophotometer at the respective 230 nm λmax of drug (Table 4).
| Formulation | Solubility |
|---|---|
| ASC | 0.294 mg/mL |
| Pure drug | 0.032 mg/mL |
Table 4: Solubility study of ASC and drug in water at room temperature.
X-Ray Diffraction Analysis
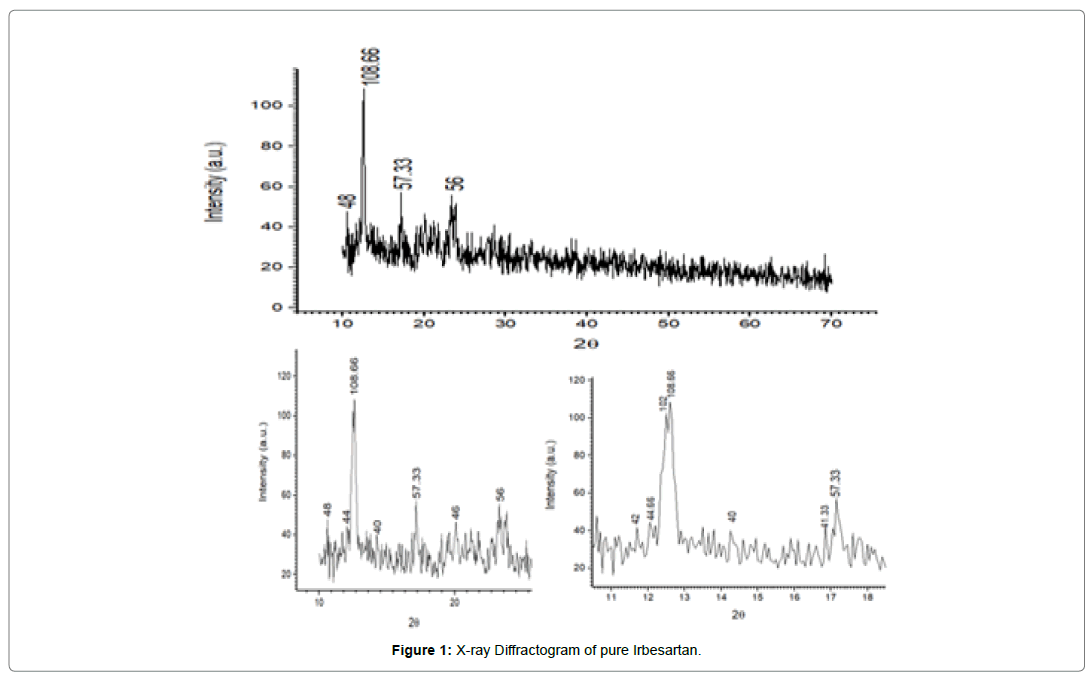
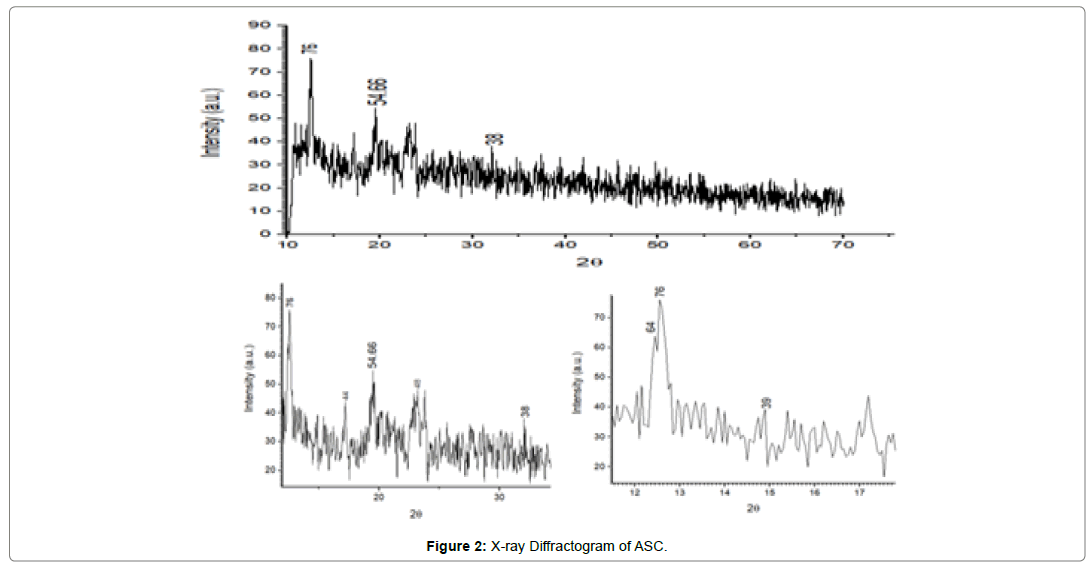
To investigate the effect of sonocrystallization on crystallinity of the drug carried out X-ray powder diffraction analysis. The XRD patterns of pure drug and ASC were recorded. Before scanning, samples were triturated and convert in to fine powder. Powder XRD patterns were determined by using X-ray diffraction (XRD), Philips Analytical X-RD (Model: PW 3710, Holland) using Ni- filtered, CuKα radiation, a voltage of 40 Kv voltages, and a current of 30 mA at room temperature. The samples were loaded on to the difffractometer and scanned over range of 2θ values form 10o to 80o at a scan rate of 0.05o/0.4 sec. The above experiments were performed in thrice for reproducibility and confirmation of the results, showed in (Figure 1& 2).
Fourier Transform Infrared Analysis
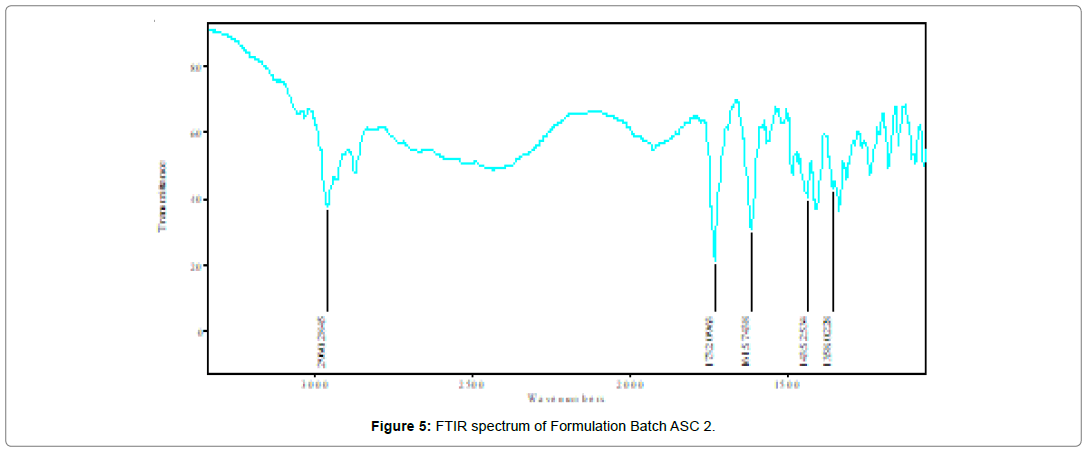
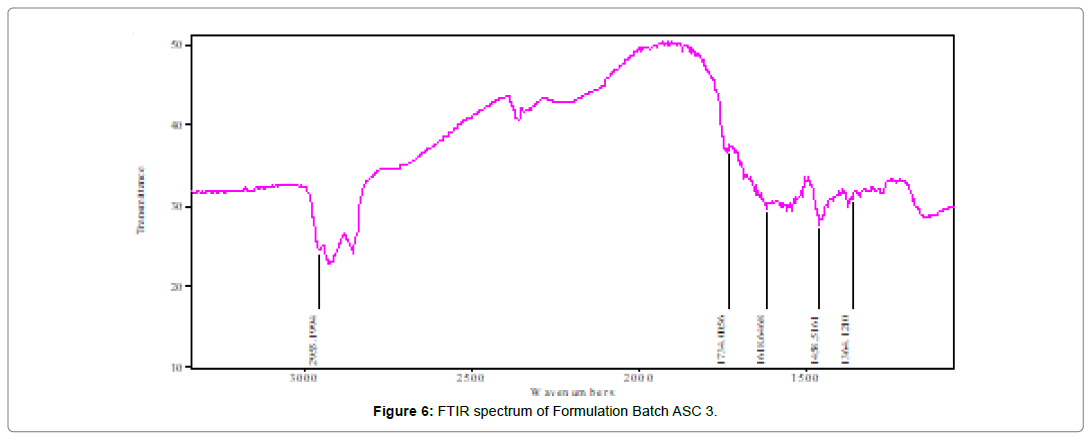
Fourier-transform Infrared (FTIR) spectrum informed about the group present in particular compound. Before I.R. spectra study all the formulation were dried in vaccum for 12 hours. After preparation of samples FTIR were performed using FTIR spectrophotometer (Shimadzu FTIR IRAffinity-1, Japan) and scanned under the wavenumber region of 4000–400 (cm-1). The above experiments were performed in triplicate to confirm the results and showed in (Table 5) (Figure 3-7).
| Functional group | Wave No. v (cm-1 ) | Reference Wave No. v (cm-1) |
||||
|---|---|---|---|---|---|---|
| AnC | ASC 1 | ASC 2 | ASC 3 | ASC 4 | ||
| N-H Stretching | 2960 | 2962 | 2954 | 2955 | 2954 | 3000-2800 |
| C=O Stretching | 1732 | 1731 | 1731 | 1734 | 1731 | 1740-1720 |
| C=C Stretching | 1615 | 1616 | 1619 | 1618 | 1620 | 1620-1610 |
| C-H Bending | 1435 | 1443 | 1454 | 1433 | 1435 | 1465-1430 |
| O-H Bending | 1358 | 1358 | 1365 | 1364 | 1365 | 1420-1330 |
Table 5: Functional group assignment and wave no. of AnC, ASC 1, ASC 2, ASC 3 and ASC 4 with the Reference Wave No. v (cm-1)
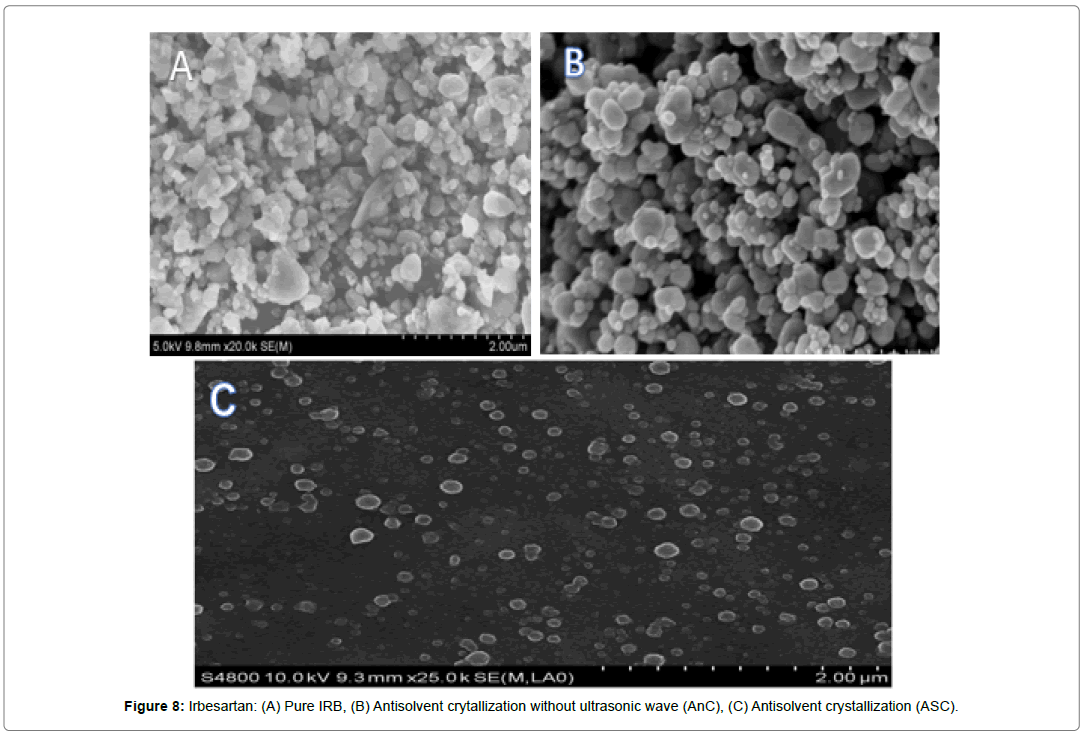
Scanning Electron Microscopy
The surface morphology of Drug, AnC, inclusion complex and ASC were investigated by scanning electron microscopy. Sample powder was attached to aluminum metal sample holder using doublesided adhesive tape and then made electrically conductive by coating with gold in a vacuum. The sample was observed by SEM (JEOL JSM 6490 LV, Japan) at various magnifications. The analysis was set at voltage was set at 5 kV and the current was 12 mA. The particles were examined for surface characteristics like shape, size, pores, pits and presence of aggregation.
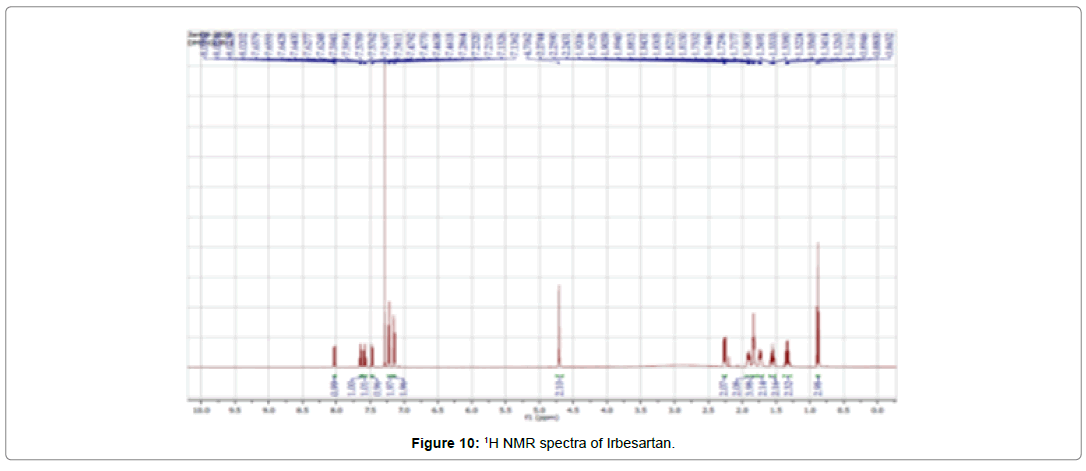
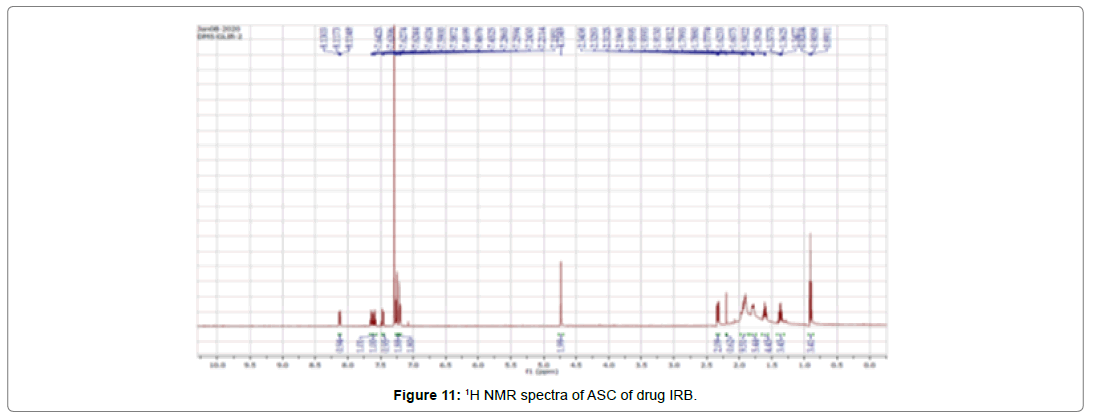
NMR spectroscopy
The 1H NMR spectrum of the Irbesartan is shown in (Figure 9-11), and the chemical shifts are given in (Table 6). The 1H NMR spectra of the irbesartan showed sigma value which corresponds to the structure of the irbesatran confirms the purity of irbesartan. In the ASC, the 1H NMR spectra shows the presence of the Irbesartan also.
| Group assignments | δ (ppm) |
|---|---|
| 3 H, CH3 | 0.7 |
| 2 H, CH2 | 1.25 |
| 2H, CH2 | 2.20 |
| 1H, C=N | 2.40 |
| 2 H, CH2 | 4.8 |
| Ar-H, C2, C3, C4 | 1.81 |
| benzene ring B, B1, B2, B3, B4 | 7.08 |
| benzene ring A, A1, A2, A3, A4 | 7.60 |
Table 6: 1H NMR chemical shifts of Irbesartan
Dissolution studies
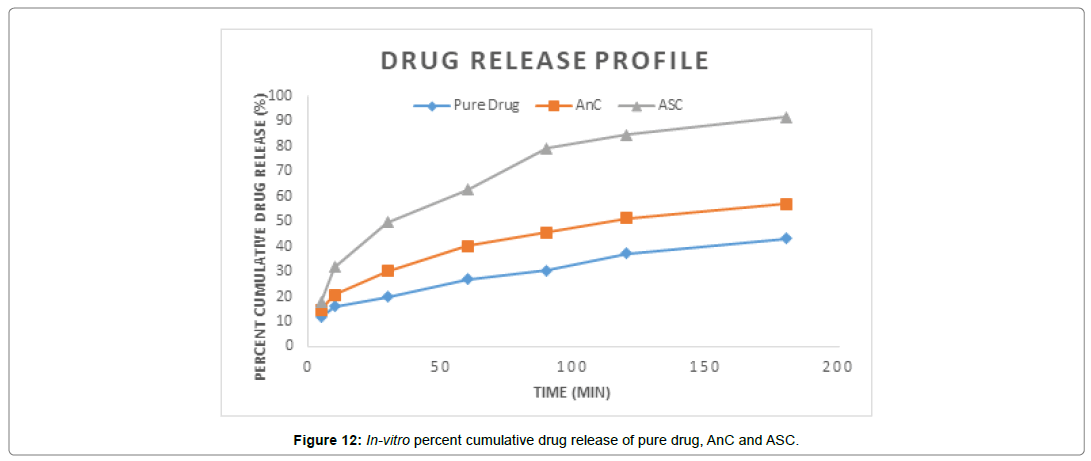
In vitro dissolution studies were performed using USP dissolution apparatus I (basket method) (Shimadzu UV-1700, Japan). Weigh the pure drug equivalent to 75 mg of drug and test sample (ASC formulations) separately were subjected for in-vitro dissolution study 900 ml of HCl medium (pH = 1.2) at 100 rpm, maintained at 37±0.5°C temperature of thermostatic controlled water bath and 100 rpm with the help of basket-type dissolution apparatus. At specific time intervals, 5 ml samples were withdrawn, filtered and analyzed for their concentration using UV spectrophotometer (Shimadzu UV-1700, Japan) at 230 nm. The withdrawn samples were replaced by equal amounts of the dissolution medium to maintain constant volume. Each determination was carefully performed to obtain the mean value and graph showed in figure when was plotted between percent cumulative drug release vs time and results showed in (Table 7) (Figure 12).
| Time (min) | Percent Cumulative Drug Release (%) | ||
|---|---|---|---|
| Pure Drug | AnC | ASC | |
| 0 | 0 | 0 | 0 |
| 5 | 11.693 | 14.756 | 17.679 |
| 10 | 16.148 | 20.603 | 31.740 |
| 30 | 19.767 | 30.069 | 49.559 |
| 60 | 26.867 | 40.092 | 62.505 |
| 90 | 30.348 | 45.522 | 79.071 |
| 120 | 37.169 | 51.229 | 84.640 |
| 180 | 43.016 | 56.937 | 91.600 |
Table 7: Percent Cumulative Drug Release profile of pure drug, AnC and ASC.
Results and Discussion
Antisolvent-based crystallization was applied to irbesartan with and without US. It is reported that in case of primary nucleation, Irbesartan presents a large metastable zone width [26]. In addition, at high drug concentrations (>25 mg/ml), an oiling out phenomenon took place, and hence, the concentration of 15 mg/ml was chosen for the preparation of crystallized powders without US as control. After mechanical agitation for approximately 10 min, the dispersed droplets solidified in about 30 min. However, poor reproducibility was observed. In contrast, when US was applied, rapid crystallization has occurred leading to direct separation of solid particles by filtration. The ASC powders were prepared successfully and reproducibly with different drug concentrations, when only 10 ml of antisolvent were used (1:1 solvent antisolvent ratio), the particles disappeared rapidly during the addition of the solvent. When the volume was raised to 30 ml (1:3 solvent-antisolvent ratio), a clear viscous phase was formed at the bottom of the jacketed beaker with no solid particle formation. (Table 2), demonstrated the good reproducibility of the process for the applied US intensity.
It was found that the yield of ASC powders was in the range of 73-96%. This may be due to the cavitation phenomenon which causes intensified micro-mixing, and thus generation of multiple simultaneous locations of supersaturation, resulting in higher nucleation rates and consequently higher yields [27]. (Table 4), the solubility of ASC formulations in different concentration of HCl (0.1N, 0.05 and 0.01) was found to be 10.70mg/mL, 5.69mg/mL and 1.13mg/ mL respectively. ASC showed highest solubility in 0.1N HCL (pH 1.2) i.e. 10.70mg/mL which was 12.02 folds more aqueous solubility than pure Irbesartan (0.89mg/mL). The solubility of Irbesartan was increased from 0.032 mg/ml for raw Irbesartan to 0.294 mg/ml for ASC 1 produced powder, equating to an increase of approximately 10.88%, as shown in (Table 4). This slight increase may be due to the decrease in particle size diameter of the resulting crystals and hence, increase in specific surface area. For study of crystalline change of drug employed XRD analysis. The result of XRD of pure IRB and ASC showed in (Figure 1& 2). The diffraction spectrum of ASC formulations showed numerous characteristic prominent sharp peaks that was nearly same as that of pure IRB diffractogram. From (Figure 3 to 7), FTIR analysis was used for drug excipient compatibility study. FTIR analysis study was done for interaction between the drug and polymer. I.R. spectra of physical blend of drug-polymer showed the prominent characteristic peaks as nearly same as pure IRB that confirms no interaction between drug and polymer. In (Figure 8), the surface morphology of IRB was investigated by scanning electron microscopy. SEM of particles showed regular sizes of ASC formulation. From (Figure 9 to 11), the 1H NMR spectra of the irbesartan showed sigma value which corresponds to the structure of the irbesatran confirms the purity of irbesartan. In the ASC, the 1H NMR spectra shows the presence of the Irbesartan also. In (Figure 12), the in-vitro drug release of pure drug, AnC and ASC was found to be 43.016%, 56.937 and 91.600. But the ASC showed highest 91.600% drug release among AnC and also in comparison to pure IRB (43.016%) within 180 min in 0.1N HCl pH 1.2. It reflects the drug bioavailability.
Conclusion
The solubility of the ASC powders has slightly increased by approximately 9% due to the increase in surface area. The US power has significantly improved the dissolution rate and extent of the slightly water-soluble drug, Irbesartan. The results show that the ASC is a no ndestructive technique and that the powder didn't undergo structural modification. In addition, the flowability and the compressibility were improved. However, the application is generally recommended for laboratory scale investigations. Multiple transducers and continuous sonocrystallization may constitute a design challenge for large scale application.
References
- Sastry SV, Nyshadham JR, et al. (2000) Recent technological advances in oral drug delivery-a review. Pharmaceutical science & technology today 3: 138-145.
- Myerson AS. Handbook of industrial crystallization, second ed. Butterworth-Heinemann, Boston.
- Yu LX (2004) Applications of process analytical technology to crystallization processes. Advanced drug delivery reviews 56: 349-369.
- Dhumal RS, Biradar SV, Paradkar A, York P, et al. (2008) Particle engineering using sonocrystallization: Salbutamol sulphate for pulmonary delivery. Int J Pharm 368: 129-137.
- Guo Z, Zhang ML, Wang H, Kougoulos J, et al. (2005) Effect of ultrasound on anti-solvent crystallization process. Journal of Crystal Growth 273: 555-563.
- Kordylla A, Koch S, Tumakaka F, Schembecker G, et al. (2008) Towards an optimized crystallization with ultrasound: Effect of solvent properties and ultrasonic process parameters. Journal of Crystal Growth 310: 4177- 4184.
- Ruecroft G, Hipkiss D, Ly T, Maxted N, Cains P W, et al. (2005) Sonocrystallization: the use of ultrasound for improved industrial crystallization. Organic process research & development 9: 923-932.
- Luque de C, Priego-Capote F, et al. (2007) Lesser known ultrasound-assisted heterogeneous samplepreparation procedures. Trac Trends in Analytical Chemistry 26: 154-162.
- Zheng L, Sun DW, et al. (2006) Innovative applications of power ultrasound during food freezing processes-a review. Trends in Food Science & Technology 17: 16-23.
- Suslick KS, Price GJ, et al. (1999) Applications of ultrasound to materials chemistry. Annual Review of Materials Science 29: 295-326.
- Cains PW, Martin PD, and Price CJ, et al. (1998) The use of ultrasound in industrial chemical synthesis and crystallization. 1. Applications to synthetic chemistry, Organic process research & development 2: 34- 48.
- Doktycz SJ, Suslick KS, et al. (1990) Interparticle collisions driven by ultrasound. Science 247: 1067.
- Gogate PR, Rajk KH, et al. (2008) Cavitational reactors for process intensification of chemical processing applications: a critical review, Chemical Engineering and Processing: Process Intensification 47: 515-527.
- Junior D, Krug F, Pereira M, Korn M, et al. (2006) Currents on ultrasoundassisted Extraction for Sample Preparation and Spectroscopic Analytes Determination. Applied Spectroscopy Reviews 41: 305-321.
- Saez V, Mason TJ, et al. (2009) Sonoelectrochemical synthesis of nanoparticles. Molecules. 14: 4284-4299.
- Gogate PR, Kabadi AM, et al. (2009) A review of applications of cavitation in biochemical engineering/biotechnology, Biochemical Engineering Journal 44: 60-72.
- Louhi-Kultanen M, Karjalainen M, Rantanen J, Huhtanen, Kallas J, et al. (2006) Crystallization of glycine with ultrasound. Int J Pharm 320: 23-29.
- Lyczko N, Espitalier F, Louisnard O, Schwartzentruber J, et al. (2002) Effect of ultrasound on the induction time and the metastable zone widths of potassium sulphate. Chemical Engineering Journal 86: 233-241.
- Kurotani M, Miyasaka E, Ebihara S, Hirasawa I, et.al. (2009) Effect of ultrasonic irradiation on the behavior of primary nucleation of amino acids in supersaturated solutions. Journal of Crystal Growth. 311: 2714-21.
- Tripathi R, Biradar SV, Mishra B, Paradkar AR, et al. (2001) Study of Polymorphs of Progesterone by Novel Melt Sonocrystallization Technique: A Technical Note, AAPS pharmscitech. 11: 1-6.
- Hatakka, Henry A, Hannu LA, Marjatta L, Ilkka HE, et.al (2010) Closed-Loop Control of Reactive Crystallization PART II: Polymorphism Control of L-Glutamic Acid by Sonocrystallization and Seeding, Chemical Engineering & Technology. 33: 751-756.
- Dennehy RD. (2003) Particle Engineering Using Power Ultrasound, Organic process research & development. 7: 1002-1006.
- Mansour A, Takrouri K, et al. (2007). A new technology for the crystallization of Dead Sea potassium chloride. Chemical Engineering Communications. 194: 803-810.
- Guo M, Muller FX, Augsburger LL, et al. (2002). Evaluation of the plug formation process of silicified microcrystalline cellulose. Int J Pharm. 233: 99-109.
- Espitalier F, Biscans B, Laguerie C, et al. (1997). Particle design Part A: Nucleation kinetics of ketoprofen. Chemical Engineering Journal. 68: 95-102.
- Patil MN, Gore GM, Pandit AB, et al. (2008) Ultrasonically controlled particle size distribution of explosives: A safe method. Ultrason Sonochem. 15: 177-187.
Citation: Dubey RK (2021) Effect of Ultrasound on the Physico-chemical Properties of Poorly Soluble Drugs: Antisolvent Sonocrystallization of Irbesartan. J Comm Pub Health Nursing 7: 295. DOI: 10.4172/2471-9846.1000295
Copyright: © 2021 Dubey RK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2934
- [From(publication date): 0-2021 - Dec 01, 2025]
- Breakdown by view type
- HTML page views: 2141
- PDF downloads: 793