Research Article Open Access
Effect of the Storage Temperatures on BRL-encapsulated-alginate-PLO-alginate (APornA) Microcapsules
Yuan-Gang Liu1,2*, Yan Bai1, Shi-Bin Wang1,2*, Ai-Zheng Chen1,2 and Wen-Guo Wu1,2
1College of Chemical Engineering, Huaqiao University, Xiamen 361021, China
2Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen 361021, China
- Corresponding Authors:
- Yuan-Gang Liu
College of Chemical Engineering
Huaqiao University, Xiamen 361021, China
Tel: +86 592 6162326
Fax: +86 592 6162326
E-mail: ygliu@hqu.edu.cn
- Shi-Bin Wang
Institute of Biomaterials and Tissue Engineering
Huaqiao University, Xiamen 361021, China
E-mail: sbwang@hqu.edu.cn
Received date July 17, 2013; Accepted date August 23, 2013; Published date August 25, 2013
Citation: Yuan-Gang L, Bai Y, Shi-Bin W, Ai-Zheng C, Wen-Guo W (2013) Effect of the Storage Temperatures on BRL-encapsulated-alginate-PLO-alginate (APornA) Microcapsules. J Biomim Biomater Tissue Eng 18:110. doi: 10.4172/1662-100X.1000110
Copyright: © 2013 Yuan-Gang Liu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
To obtain long-term storage of cell-encapsulated alginate-PLO-alginate (APornA) microcapsules, the influences of storage temperatures on the microcapsule morphology, cell viability and metabolic activities were investigated. The results showed that the microcapsules exhibited the original morphological characteristics with smooth surface, good sphericity and overall integrity after 4 weeks preserved in liquid N2. 4°C and -20°C were presented to be the harsh conditions for cell propagation and the secretion of metabolic products compared to -80°C and -196°C. It can be concluded that lower temperature is beneficial for the cell recovery. These results supplied supplementary data for poly- L-ornithine (PLO) as a potential coating material in the future cell microencapsulated applications.
Keywords
Alginate; Poly-L-ornithine; Microcapsule; Storage temperature
Introduction
Cell microencapsulation technology, which is first reported by Lim and Sun in 1980 [1], has been a useful tool widely studied in cell culture [2], metabolite production [3], cell transplantation [4], artificial organs [5], and most recently, in tissue engineering [6-8]. Alginate-polycation microcapsules have attracted considerable attentions as efficient micro-vessels or immuno-protection devices because the preparation method is facile and most importantly, these systems own liquid cores, which can supply sufficient space for cell growth and propagation, and some properties, such as immuno-isolation, biocompatibility and biodegradability. Among these microcapsules, major researches are concerned about alginate-poly-L-lysine-alginate (APA) and alginatechitosan- alginate (ACA) microcapsules [9,10]. Other coating materials mainly include poly(methylene-co-guanidine) [11], poly-L-ornithine (PLO) [12] and poly-L-arginine [13].
PLO, which structure is similar to poly-L-lysine (PLL), has attracted researchers’ interests in the last few years. Compared to PLL, PLO is proven to have better characteristics beneficial for cell encapsulation. For example, Darrabie et al. [14] reported that PLO coating could reduce swelling and increase the mechanical strength of alginate microcapsules as compared to PLL coating, and PLO coating more effectively restricted higher molecular weight components than PLL from permeating the microcapsules, which resulted in the increased structural integrity and biocompatibility of PLO coating microcapsules. Tam et al. [15] also found that the use of PLO rather than PLL resulted in microcapsules with less immune cell adhesion and better biocompatibility in vivo, and a greater hydrophilicity and adequate diffusion of polycation into the gel to form a membrane that resisted swelling and rupture when subjected to osmotic stress. Therefore, PLO may be used as a suitable polycation instead of PLL in the future studies.
Despite the current progresses in alginate-PLO-alginate (APornA) microcapsules, much work is needed to be fully explored for the potential uses. For the long-term storage of microencapsulated cells, cryopreservation has emerged as an attractive method. Maintaining frozen cells at liquid nitrogen can minimize damage to frozen cells [16]. Many publications have concerned the cryopreservation strategies for APA or ACA microcapsules [17,18], but there are no reports concerning the storage of APornA microcapsules. Herein we investigated the influence of different storage temperatures on the cellencapsulated APornA microcapsules. We studied the effect of common storage temperatures (4°C, -20°C, -80°C, and liquid N2) on BRLencapsulated APornA microcapsules. Different parameters, including the microcapsule morphology, cell viability, and metabolic activities were investigated. It is anticipated that the thawed microcapsules will have excellent morphology, cell viability and functions, which may be positive for the future applications of APornA microcapsule-based cell therapy or tissue engineering.
Materials and Methods
Materials
Sodium alginate (viscosity ~250 cP), PLO (M.W. 15,000~50,000) and DMSO (dimethyl sulfoxide) were purchased from Sigma Chemical (USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), trypsin (1:250), EDTA Na2 (disodium ethylenediaminetetraacetate dihydrate) were supplied by Amresco (USA). Newborn calf serum and RPMI 1640 (Roswell Park Memorial Institute) were bought from Gibco (USA). Ampicillin trihydrate and Streptomycin sulfate were supplied by BBI (Canada). Urea Assay Kit and Protein Assay Kit were from Sichuan Xincheng Biotechnology Co. Ltd. and Shanghai GeneStar Biotechnology Co. Ltd. respectively (China). All other reagents were of analytical grade and all solutions were sterilized by filtration (0.22 μm) before use.
Buffalo rat liver (BRL) cell line was kindly provided by the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China.
Preparation of BRL-encapsulated APornA microcapsules
BRL cells in logarithmic phase were digested with trypsin solution, and then the cells were mixed with 2.0% (w/v) sodium alginate (which had been previously filtered through 0.80, 0.45, and 0.22 μm membrane) to obtain a final cell concentration 1×106/mL. The preparation of BRL-encapsulated APornA microcapsules was similar with the cell microencapsulation process which had been reported elsewhere [17,19]. Briefly, the above cell suspension was extruded into a 1.5% (w/v) CaCl2 solution using a high-voltage electrostatic droplet generator to form alginate beads. The optimized parameters were: voltage 6.3 KV, distance between the pinhead and the CaCl2 solution surface 20 mm, 7# flat pinhead with inner diameter of 0.51 mm, and push speed 50 mm/h. The resulting beads were subsequently coated with 0.1% (w/v) PLO solution for 6 min, followed by a coating with 0.15% sodium alginate for another 5 min. After liquefying the core with 3% (w/v) sodium citrate for 6 min, the collected microcapsules were washed with saline three times and then the resulting microencapsulated BRL cells were incubated at 37°C in a humidified air with 5% CO2.
Storage of BRL-encapsulated microcapsules
After incubation for 24 h, the BRL-encapsulated APornA microcapsules were collected for storage. The composition of the cell freezing medium included 20% newborn calf serum, 10% DMSO and 70% RPMI 1640 medium. First, the cell-loaded microcapsules were dispersed in the cell freezing medium to obtain a suspension (the volume ratio of microcapsules to the freezing medium was 1:9). Then the microencapsulated cell suspension was transferred to cryovials (2 mL, Coring, USA) with 1 mL per vial under aseptic conditions. To find the optimal storage temperature for microencapsulated BRL cells, four different conditions including 4°C, -20°C, -80°C, and liquid N2 were investigated. The freezing protocol was as follows: 4°C 30 min, -20°C 30 min, -80°C overnight, and then in liquid N2. The microcapsule morphology was observed under light microscopy after 1 week and 4 weeks.
Thawing of microencapsulated cells
After storage for 1 week, 2, 3, and 4 weeks, respectively, frozen vials were taken out and placed in a 37°C water bath for cell thawing. Microcapsule suspension was finally transferred into 96-well plate (Costar, Coring, USA) in fresh culture medium and cultured at 37°C in a humidified air with 5% CO2. After 24 and 72 h respectively, cell viability and metabolic activity of the thawed microencapsulated cells were tested as indexes to evaluate the influence of different temperatures on the storage of BRL-encapsulated APornA microcapsules.
Cell viability and metabolic activity of the thawed microencapsulated cells
Cell viability was examined by MTT assay which method was popular in other publications [20,21]. Metabolic activities of the thawed microencapsulated cells including albumin (ALB) and urea contents were characterized according to the protocols of the Urea Assay Kit and Protein Assay Kit supplied by the companies.
Statistical analysis
Origin 7.0 was used for statistical analysis. All the samples were performed in triplicate and the values were expressed as the mean ± S.D.
Results and Discussion
The integrity and stability of the cell-loaded microcapsules are key requirements in the development of biocompatible devices. The microcapsules should have sufficient film strength to ensure that the encapsulated cells playing a response function. On the contrary, the microcapsule will be easy to collapse, crush and distort and then loses lose the immune isolation effect after implantation if the membrane strength is poor. To characterize the integrity and stability of the microcapsules, some parameters, such as light micrographs, volume expansion rate, membrane thickness and breakage rate are used [22]. Among these parameters, light micrograph is still the direct and simple way. Therefore, post-thawed morphology of the cell-loaded microcapsules was examined using an inverted optical microscopy (IX51, Olympus, Japan) to evaluate the influence of different storage temperatures on BRL-encapsulated APornA microcapsules.
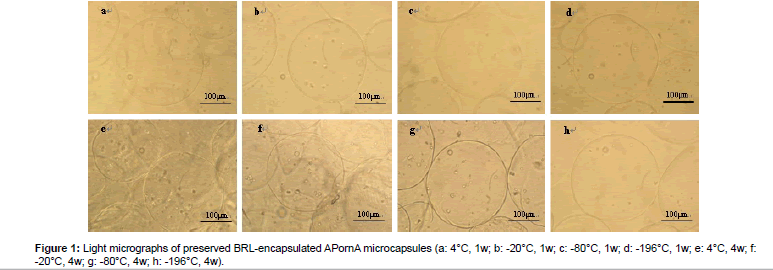
Figure 1 shows the light micrographs of preserved BRL-encapsulated APornA microcapsules under different storage temperatures. As can be seen from (Figures 1a-1d), there were no obvious morphological differences. These microcapsules had smooth surface, good sphericity, and overall integrity. However, the differences became obvious after 4 weeks preserved in the freezing medium (Figures 1e-1h). The surface of the microcapsules turned to be rough, irregular, and shrinking especially in Figures 1e and 1f. We also found that a few microcapsules were even broken with different extents (Figures 1e and 1g). While in Figure 1h, microcapsules nearly kept the original morphological characteristics with smooth surface, good sphericity and overall integrity. Interestingly, the lower the storage temperature was, the better morphological characteristics would be. The preserved BRLencapsulated APornA microcapsules could be well preserved in liquid N2 whenever the time was 1 week or 4 weeks.
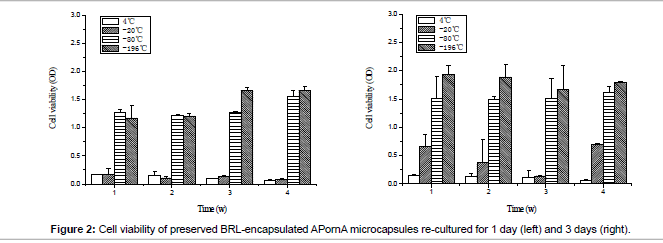
Cell viability of preserved BRL-encapsulated APornA microcapsules is shown in Figure 2. MTT was used to characterize the living cells during this process. When the storage temperature was 4°C, there was no obvious difference between re-cultured for 1 day and 3 days, cell viability was the lowest and the remaining living cells were hardly to propagate. At -20°C, cells grew slowly. When re-cultured for 1 day, the cell viability was nearly the same as 4°C, after that the cells started to grow faster. Compared with 4°C and -20°C, the cells at -80°C and -196°C presented excellent proliferations, and -196°C showed better situation than -80°C.
The metabolic activities are important indexes to evaluate the influence of storage temperature on the functions of the encapsulated cells. Referring the indexes of hepatic functions reported in the published literature [23], herein, albumin (ALB) secretion and urea biosynthesis of the thawed microencapsulated cells were characterized.
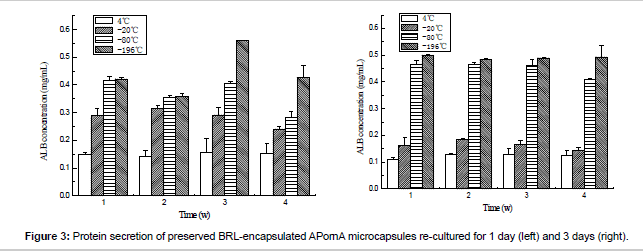
Figure 3 gives the details of the ALB secretion of the thawed microencapsulated BRL cells. Opposite tendencies could be observed when the storage temperature changed. When the re-culture time was increased from 1 day to 3 days, the content of ALB secretion decreased for 4°C and -20°C groups, while increased for -80°C and -196°C groups. These results meant that the catabolism function would partly be damaged when the BRL cells were preserved under 4°C and -20°C.
The urea concentration can be observed from Figure 4. As same as ALB secretion, cyropreserved under -80°C and -196°C presented higher urea secretion content, which meant that the BRL cells maintained better urea biosynthesis function than preserved under 4°C and -20°C. Based on the results above, we can find that -80°C and -196°C were better than 4°C and -20°C. The mechanism was analyzed as follows: Cell survival is strongly influenced by the storage temperature. Lower storage temperatures are associated with extended viability of the preserved samples. When the temperatures are 4°C and -20°C, the metabolic activity is still active, which results in cell death and loss of cell viability after storage because the nutrition in the cell freezing medium is limited. At the same time, the existence of water or unfrozen water will lead to the swelling of the microcapsules, which may destroy the microcapsule surface morphology. While under -80°C and -196°C, the metabolic activity nearly or completely ceases, which depends on the glass transition phase of the medium, thus the cell remains high viability after thawed. Furthermore, one point must be mentioned that compared with the unencapsulated cells, the encapsulated cells can be protected by the microcapsules during the process of cryopreservation, which has been proved by other researchers [24].
Conclusion
The influence of different storage temperatures on BRLencapsulated APornA microcapsules was investigated. Combining with the morphology, cell viability and metabolic activities results, we can conclude that the temperature has a significant impact on the storage of BRL cells. Lower temperature is beneficial for the cell recovery. Compared with 4°C and -20°C, -80°C and -196°C showed better storage effects. These results indicated that the cell storage using APornA microcapsules will be useful in the future microcapsule-based cell therapy or tissue engineering.
Acknowledgement
Financial supports from NSFC (31000441 and 31170939), Natural Science Foundation of Fujian Province (2013J01189), Program for Prominent Young Talents in Fujian Province University (JA12004), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry) and the Fundamental Research Funds for the Central Universities (JB-JC1009, JBSJ1009) are gratefully acknowledged.
References
- Lim F, Sun AM (1980) Microencapsulated islets as bioartificial endocrine pancreas. Science 210: 908-910.
- Yu W, Song H, Zheng G, Liu X, Zhang Y, et al. (2011) Study on membrane characteristics of alginate-chitosan microcapsule with cell growth. J Memb Sci 377: 214-220.
- Wojcieszynska D, Hupert-Kocurek K, Jankowska A, Guzik U (2012) Properties of catechol 2,3-dioxygenase from crude extract of Stenotrophomonas maltophilia strain KB2 immobilized in calcium alginate hydrogels. Biochem Eng J 66: 1-7.
- Kerby A, Jones ES, Jones PM, King AJ (2013) Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy 15: 192-200.
- Pan XP, Li LJ (2012) Advances in cell sources of hepatocytes for bioartificial liver. Hepatobiliary Pancreat Dis Int 11: 594-605.
- Sakai S, Inamoto K, Liu Y, Tanaka S, Arii S, et al. (2012) Multicellular tumor spheroid formation in duplex microcapsules for analysis of chemosensitivity. Cancer Sci 103: 549-554.
- Huang X, Zhang X, Wang X, Wang C, Tang B (2012) Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J Biosci Bioeng 114: 1-8.
- Lin YH, Yang YW, Chen YD, Wang SS, Chang YH, et al. (2012) The application of an optically switched dielectrophoretic (ODEP) force for the manipulation and assembly of cell-encapsulating alginate microbeads in a microfluidic perfusion cell culture system for bottom-up tissue engineering. Lab Chip 12: 1164-1173.
- Zhang X, Wang W, Xie Y, Zhang Y, Wang X, et al. (2006) Proliferation, viability, and metabolism of human tumor and normal cells cultured in microcapsule. Appl Biochem Biotechnol 134: 61-76.
- Lu D, Zhang Y, Niu S, Wang L, Lin S, et al. (2012) Study of phenol biodegradation using Bacillus amyloliquefaciens strain WJDB-1 immobilized in alginate-chitosan-alginate (ACA) microcapsules by electrochemical method. Biodegradation 23: 209-219.
- Rokstad AM, Brekke OL, Steinkjer B, Ryan L, Kolláriková G, et al. (2013) The induction of cytokines by polycation containing microspheres by a complement dependent mechanism. Biomaterials 34: 621-630.
- Chen A, Bai Y, Wang S, Liu Y, Chen Z (2012) Molecular biocompatibility evaluation of poly-L-ornithine-coated alginate microcapsules by investigating mRNA expression of pro-inflammatory cytokines. J Biomim Biomater Tissue Eng 14: 53-64.
- Wang S, Liu Y, Weng L, Ma X (2003) Effect of molecular weights of poly-L-arginine on membrane strength and permeating property of poly-L-arginine group microcapsule. Macromol Biosci 3: 347-350.
- Darrabie MD, Kendall Jr WF, Opara EC (2005) Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials 26: 6846-6852.
- Tam SK, Bilodeau S, Dusseault J, Langlois G, Hallé JP, et al. (2011) Biocompatibility and physicochemical characteristics of alginate–polycation microcapsules. Acta Biomater 7: 1683-1692.
- Meryman HT (2007) Cryopreservation of living cells: principles and practice. Transfusion 47: 935-945.
- Murua A, Orive G, Hernández RM, Pedraz, JL (2009) Cryopreservation based on freezing protocols for the long-term storage of microencapsulated myoblasts. Biomaterials 30: 3495-3501.
- Kanmani P, Kumar RS, Yuvaraj N, Paari KA, Pattukumar V, et al. (2011) Effect of cryopreservation and microencapsulation of lactic acid bacterium Enterococcus faecium MC13 for long-term storage. Biochem Eng J 58-59: 140-147.
- Ma Y, Zhang Y, Liu Y, Chen L, Li S, et al. (2013) Investigation of alginate-e-poly-L-lysine microcapsules for cell microencapsulation. J Biomed Mater Res A 101: 1265-1273.
- Thorat ND, Khot VM, Salunkhe AB, Ningthoujam RS, Pawar SH (2013) Functionalization of La0.7Sr0.3MnO3 nanoparticles with polymer: Studies on enhanced hyperthermia and biocompatibility properties for biomedical applications. Colloids Surf B Biointerfaces 104: 40-47.
- Nakamura H, Liao L, Hitaka Y, Tsukigawa K, Subr V, et al. (2013) Micelles of zinc protoporphyrin conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer for imaging and light-induced antitumor effects in vivo. J Control Release 165: 191-198.
- Liu Y, Tong Y, Wang S, Deng Q, Chen A (2013) Influence of different divalent metal ions on the properties of alginate microcapsules and microencapsulated cells. J Sol-Gel Sci Technol 67: 66-76.
- Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, et al. (2012) Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 33: 8304-8315.
- Pravdyuk AI, Petrenko YA, Fuller BJ, Petrenko AY (2013) Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology 66: 215-222.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21816
- [From(publication date):
September-2013 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 17234
- PDF downloads : 4582