Effect of Tartrazine on Thyroid Gland of Male Rat and Ameliorating Role of Curcumin (Histological and Immunohistochemical Study)
Received: 15-Oct-2018 / Accepted Date: 25-Oct-2018 / Published Date: 01-Nov-2018
Keywords: Tartrazine; Curcumin; NF-kB p65; Thyroid gland
Introduction
Food additives play a vital role in increasing the taste, tint, quality and price of foods and classified into 6 main groups: Preservatives, nutritive, flavouring, colouring, texturizing and miscellaneous compounds [1]. Colour additives are defined as any dyes or pigment that can impart color to food, drugs or cosmetics to enhance the visual appeal [2]. Some of these substances are derived from the natural colors such as carotene and chlorophyll and others are synthetic such as indigotin, allura red and tartrazine [3].
Synthetic food additive tartrazine is a bright yellow azo dye that is more stable and a cheaper alternative to natural food dyes. It is known by other names such as FD and C Yellow No. 5 and E 102 Europe [4]. It is commonly seen in medication, cosmetics and food products such as candy, soft drinks (Mountain Dew), energy drinks, flavored corn chips, cereals (corn flakes), cake mixes, ice cream, ice pops, chewing gum, marzipan, jam, jelly, yogurt noodles, potato chips, biscuits and in some honey products [5]. It has been implicated to be responsible for allergic reactions [6] and has harmful effects in learning and memory functions in animals [2]. In toxicological studies on rats, some researchers revealed that it induces several adverse effects on kidney, liver and blood cells [1]. Some behavioral changes among children that intake coloured foods contained tartrazine such as irritability, restlessness and sleep disturbance [5].
The use of natural colors with or without synthetic colors as food coloring agents is considered essential for promoting health status and minimizing risks of adverse effects in consumers. Widespread usage of natural colors in the food industry occurred due to mounting demands by consumers for safer foods with natural additives. Currently, 13 natural food colors are approved for coloring foods one of which is curcumin [7]. Curcumin (curry powder) is the active ingredient of the dietary spice turmeric and is extracted from the rhizomes of Curcuma longa which belongs to ginger family. It has several pharmacological and biological properties such as being anti-inflammatory, antiviral, antimicrobial and antifungal agent [8]. In recent years, numerous studies have shown that oral administration of curcumin significantly ameliorated collagen-induced arthritis [9]. Moreover, curcumin shows chemopreventive actions [10] and were used in many cancers treatment including colorectal cancer, breast cancer, skin cancer, and oral cancer [11]. Curcumin was reported to have a protective effect against cyclosporine-induced nephrotoxicity in rats [12]. It is also used in Asian and African traditional medicine to treat several mild or moderate health problems such as aches; wounds; sprains; and liver disorders [13]. Thyroid gland is one of the most important endocrine organs and almost all cells of the body are target sites for its hormones (T3,T4) and considered as body’s primary regulator of metabolism [14]. Nuclear Factor Kappa B (NF-Kb) is a heterodimer protein complex, and it usually consists of two proteins, a p65 subunit and a p50 subunit and other subunits [15]. Caspase 3 is a cytosolic protein found in the cells as an inactive 32 kDa proenzyme. It is activated by proteolytic cleavage into two active subunits only when cells undergo apoptosis [16]. The present study was directed to evaluate the effect of tartrazine on thyroid gland of adult male albino rats from histological, immunohistochemical and biochemical point of view. Additionally, the possible protective effect of curcumin intake was also investigated.
Materials and Methods
Chemicals
Tartrazine: (CAS 1934-21-0, Purity 86.7%) was purchased in the form of powder from Sigma Aldrich (Germany). Its chemical formula: Trisodium salt: Trisodium 5-hydroxy-1-(4-sulfonatophenyl)-4-(E)-(4 sulfonatophenyl) diazenyl-1H-pyrazole-3-carboxylate,
Curcumin: (CAS 458-37-7, Purity: ≥ 95%) was purchased in the form of powder from El-goumhoria Co. for trading chemicals, medicines and medical appliances, Egypt. Its chemical formula: 1,7-bis (4-hydroxy-3methoxyphenyl)-1E,6E-heptadiene-3,5-dione. Molecular Formula: C21H20O6. Formula Weight: 368.4.
Animals
Forty five healthy adult male albino rats with average body weight (200-250 g) were used and housed in stainless steel cages at Animal House, Faculty of Medicine, Zagazig University. They were kept at room temperature, fed standard balanced diet and allowed water adlibitum. The experiment was performed in accordance with the (Guide for the Care and Use of Laboratory Animals). The experimental protocol was approved by the Ethical Committee of Zagazig University.
Experimental design
After an acclimatization period of 1 week, rats were randomly divided into four equal groups; I, II, III and IV.
Group I (Control group): Included 15 rats, further subdivided into three equal subgroups ((five rats each):
Subgroup Ia (Negative control group): Were left without intervention to measure the basic parameters.
Subgroup Ib (Positive control group; distilled water): Received 1 ml distilled water once daily by orogastric tube (the solvent of both tartrazine and curcumin) for thirty days.
Subgroup Ic: (Positive control group; curcumin group): Received curcumin dissolved into 1 ml distilled water at a dose of (100 mg/ kg once daily) by orogastric tube for thirty days.
Group II (treated group): Included 10 rats that were received tartrazine dissolved in 1 ml distilled water at concentration of 300 mg/kg given once daily by orogastric tube for thirty days [4].
Group III (protective group): Included 10 rats that were received tartrazine solution at the same dose as group II in concomitant with curcumin dissolved in 1ml distilled in a dose of (100 mg/kg water once daily) by orogastric tube [8] for thirty days [17].
Group IV (recovery group): Included 10 rats that were received tartrazine solution at the same dose as group II once daily for thirty days. At a day 31 of treatment, the rats were left without any treatment for further thirty days as a recovery period [18].
General observations of animals: During the experimental period, clinical signs and general appearances, which included the amount of food and water consumed, were checked daily. Mortalities of the rats were recorded as it occurred.
The body weight of each animal was assessed before and at the end of experiment. The percentage of body weight gain was calculated as follows according to previous reports [19].
Mean final body weight - Mean initial body weight × 100
Mean initial body weight
At the time of sacrifice, rats were anesthetized with intraperitoneal injection of sodium pentobarbital (Nembutal, 30 mg/kg body weight). Venous blood samples (2 ml each) were withdrawn from the retroorbital sinus for hormonal assay.
The thyroid glands were dissected out in two stages to avoid tissue damage. First, the neck was opened by a longitudinal incision, the fascia was removed, and the trachea was cut by a horizontal plane superior and inferior to the thyroid. It appeared as two small reddish oval masses on each side of the trachea [20,21]. Thyroid glands from each rat were quickly excised into specimens.
Histological Study
1 cm3 specimens were processed with paraffin technique [22] to investigate the histological and immunohistochemical results through the light microscopic examination.
The specimens were fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin and then the sections were cut at 4-5 μm by a microtome then stained with Haematoxylin and eosin (H and E). Also, the Periodic Acid and Schiff (PAS) staining procedure were used according to [22] to differentially stain the colloid inside the thyroid follicles [23].
1 mm specimens; were immediately fixed in 3% glutaraldehyde buffered with 0.1 M phosphate at 4°C (pH 7.4) then post fixed in 1% osmium tetroxide for 2 hrs at 4°C. The specimens were then dehydrated and embedded in epoxy resin. Semi-thin sections (1 μm thick) were stained with toluidine blue [24].
Immunnohistochemical study
Immunohistochemical detection of caspase-3 and NF-kB p65 was carried out using streptavidin-biotin complex immunoperoxidase system. Serial sections of paraffin-embedded specimens were deparaffinized on charged slides. The sections were incubated in 0.1% hydrogen peroxide for 30 min to block the endogenous peroxidase. The sections were then incubated with 1.5% non-immunized goat serum for 30 min at room temperature, and then incubated with diluted primary antibody (1:500 dilutions) [25] .
Primary antibody for Caspase 3: A rabbit polyclonal antibody of IgG type was carried out for localization of Caspase 3 (GTX 110543) [26] or NF-kB p65 (GTX102090) for 30 min at room temperature [27].
The sections were then washed 3-times with (PBS, pH 7.4) for 30 min and then incubated with biotinylated goat anti-mouse immunoglobulin serum for 60 min. After being gently washed with PBS, the sections were incubated with avidin-biotin peroxidase complex. Ultimately, sites for peroxidase binding were detected using DAB (3,30-diaminobenzidine) substrate) [28]. Tissue sections were then counterstained with hematoxylin and subjected to light microscopy analyses and morphometric measures. The kits were were purchased from Sigma-Aldrich, St. Louis.
Biochemical analysis of oxidant and antioxidant markers in thyroid tissue: Thyroid gland specimens were processed for determination the oxidative markers stress in tissues (Oxidant markers: Malondialdehyde (MDA) and Superoxide Dismutase (SOD); Antioxidant markers: Catalase (CAT) and reduced glutathione (GSH)). Those thyroid tissues were washed with ice-cold saline, blot-dried, suspended in phosphate buffer (pH 6) at 5-times then processed in a Potter-Elvehjem homogenizer. The raw homogenate was then frozen at 85°C until used in the various assays [29,30]. Measuring the levels of MDA was based on the method of thiobarbituric acid (TBA) [31]. The superoxide dismutase (SOD) activity (in μ/g tissue) was determined according to [32]. Catalase (CAT) activity (in μ/g tissue) was assessed by means of the method of Aebi [33]. Reduced glutathione (GSH) was determined according to the method of Tipple and Rogers [34]. All the abovementioned kits were purchased from Biodiagnostic and conducted at Biochemistry Department, Faculty of Medicine- Zagazig University.
Biochemical Hormonal assay study: The blood samples were obtained through heparinized capillary tubes and allowed to clot at room temperature in a water bath for 15 min. The supernatant serum was collected in a dry tube. The sera were quickly removed and kept at -20°C until the assay. Serum total T3 (triiodothyronine), T4 (thyroxine) and thyroid stimulating hormone (TSH) levels were measured using RIA Kit (Diagnostic Products Corporation, LA, USA).
Morphometric Study
The image analyzer computer system Leica Qwin 500 (Cambridge, UK, Leica Microsystems Imaging Solutions, Ltd) in the image analyzing unit of the Pathology Department, Faculty of Dentist, Cairo University, Egypt.
• Haematoxylin and eosin stained sections were used for the measurement of follicular epithelial height. This was performed in 5 non overlapping fields from 5 different sections of 5 different rats in each group at × 400.
• Periodic acid Schiff (PAS) reaction for the demonstration of the optical density of colloid of thyroid follicle.
• Immune stained sections were used for the measurement of optical density of immunoexpression in caspase-3 and NF-kB p65. This was performed in 5 non overlapping fields from 5 different sections of 5 different rats in each group at × 400.
Statistical analysis
Statistical analysis was performed using statistical Package for the Social Sciences (SPSS) version 20 for Windows software system (SPSS Inc, Chicago, IL, USA). Results were articulated as mean ± standard error (SE) and all statistical comparisons were made by means of a one-way ANOVA test. A P value <0.05 was considered significant. When the difference between groups was significant, post hoc analysis was carried out by applying the LSD.
Results
General observation, food intake and body weight
Treatment with tartrazine did not affect mortality and food intake when compared to the control group. As a function of growth, body weight of experimental animals was monitored in comparison with the control group. Our data showed that the body weight (%) of the rats of control group and curcumin protective group was non-significantly increased at the end of the experiment. However, there was a significant retardation (p<0.05) in it in rats of tartrazine treated and recovery groups. Results are presented in (Table 1).
| Group | (Ia) | (Ib) | (Ic) | (II) | III | IV | F | P. value |
|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Body weight (%) | 25.16 ± 0.46 | 24.99 ± 0.49 | 25.1 ± 0.47 | 10.33 ± 3.07a | 24.9 ± 16b | 23.87 ± 0.88a,c | 674.04 | <0.001 |
| Results: Values are expressed as mean ± standard deviation (SD) of n=10 rats/group, aSignificant as compared with the Ia,Ib and Ic groups, P<0.05. bSignificant as compared with II group, P<0.05. cSignificant as compared with III group, P<0.05. | ||||||||
Table 1: Statistical results of the body weight percent in the studied groups.
Histological Results
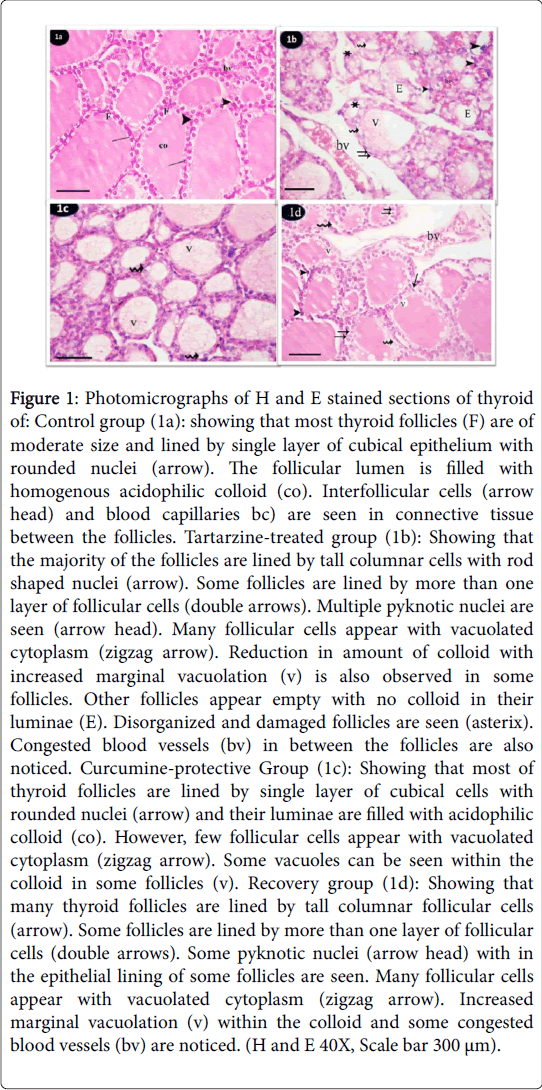
Both subgroups (a,b and c) of the control group revealed the same histological and immunohistochemical features. H and E stained sections of thyroid glands from the control group showed that thyroid parenchyma was composed of multiple and relatively rounded follicles. Mostly they are of moderate size and lined by single layer of cubical epithelium with rounded nuclei. The follicular lumen was filled with homogenous acidophilic colloid. Interfollicular cells and blood vessels are seen in connective tissue between the follicles (Figure 1a). Tartarzine-treated group showed that majority of the follicles were lined by tall columnar cells with rod shaped nuclei. Some follicles were lined by more than one layer of follicular cells. Multiple pyknotic nuclei were seen in follicular lining epithelium. Many follicular cells appeared with vacuolated cytoplasm. Reduction in amount of colloid with increased marginal vacuolation were also observed in some follicles. Other follicles appeared empty with no colloid in their lumina. Disorganized and damaged follicles were observed. Congested blood vessels in between the follicles were also noticed (Figure 1b). In curcumine-protective group sections Administration of tartarzine concomitantly with curcumin ameliorated thyroid follicles. Most of thyroid follicles were similar to that of the control group. They were lined by single layer of low cubical cells with rounded nuclei and their luminae were filled with acidophilic colloid. However, some vacuoles were seen within the colloid in some follicles. Few follicular cells appeared with vacuolated cytoplasm and some (Figure 1c). The recovery group showed that many thyroid follicles were lined by tall columnar follicular cells. However, some follicles were lined by low cuboidal epithelium. Some follicles were lined by more than one layer of follicular cells. Reduced amount of colloid with increased marginal vacuolation were also seen in some follicles. Some empty follicles and congested blood vessels were noticed (Figure 1d).
Figure 1: Photomicrographs of H and E stained sections of thyroid of: Control group (1a): showing that most thyroid follicles (F) are of moderate size and lined by single layer of cubical epithelium with rounded nuclei (arrow). The follicular lumen is filled with homogenous acidophilic colloid (co). Interfollicular cells (arrow head) and blood capillaries bc) are seen in connective tissue between the follicles. Tartarzine-treated group (1b): Showing that the majority of the follicles are lined by tall columnar cells with rod shaped nuclei (arrow). Some follicles are lined by more than one layer of follicular cells (double arrows). Multiple pyknotic nuclei are seen (arrow head). Many follicular cells appear with vacuolated cytoplasm (zigzag arrow). Reduction in amount of colloid with increased marginal vacuolation (v) is also observed in some follicles. Other follicles appear empty with no colloid in their luminae (E). Disorganized and damaged follicles are seen (asterix). Congested blood vessels (bv) in between the follicles are also noticed. Curcumine-protective Group (1c): Showing that most of thyroid follicles are lined by single layer of cubical cells with rounded nuclei (arrow) and their luminae are filled with acidophilic colloid (co). However, few follicular cells appear with vacuolated cytoplasm (zigzag arrow). Some vacuoles can be seen within the colloid in some follicles (v). Recovery group (1d): Showing that many thyroid follicles are lined by tall columnar follicular cells (arrow). Some follicles are lined by more than one layer of follicular cells (double arrows). Some pyknotic nuclei (arrow head) with in the epithelial lining of some follicles are seen. Many follicular cells appear with vacuolated cytoplasm (zigzag arrow). Increased marginal vacuolation (v) within the colloid and some congested blood vessels (bv) are noticed. (H and E 40X, Scale bar 300 μm).
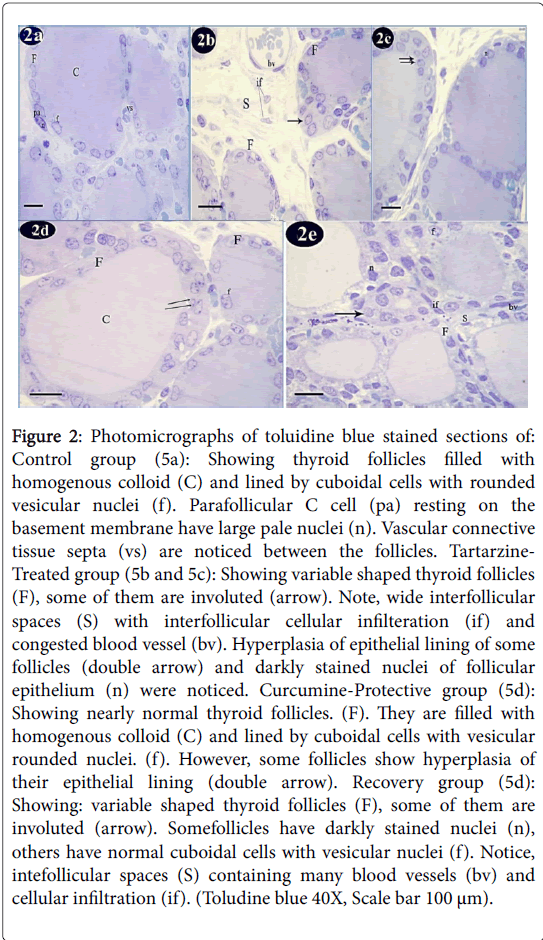
Toluidine blue staining of control group showed that thyroid follicles were lined by cuboidal cells with rounded vesicular nuclei. These follicles were filled with homogenous colloid. Parafollicular C cells were resting on the basement membrane and exhibited large pale nuclei. Vascular connective tissue septa were noticed between the follicles (Figure 2a). Tartarzine-treated group showed variable shaped thyroid follicles, some of them were involuted. Wide interfollicular spaces with interfollicular cellular infiltration and congested blood vessel were detected. Hyperplasia of epithelial lining of some follicles and darkly stained nuclei of follicular epithelium were also noticed (Figures 2b and 2c). Curcumine-protective group showed nearly normal thyroid follicles. They were filled with homogenous colloid and lined by cuboidal cells with vesicular rounded nuclei. However, some follicles revealed hyperplasia of their epithelial lining (Figure 2d). The recovery group revealed variable shaped thyroid follicles, some of them were involuted. Some follicles had darkly stained nuclei, others had normal cuboidal cells with vesicular nuclei. Intefollicular spaces containing many blood vessels and cellular infiltration were seen (Figure 2e).
Figure 2: Photomicrographs of toluidine blue stained sections of: Control group (5a): Showing thyroid follicles filled with homogenous colloid (C) and lined by cuboidal cells with rounded vesicular nuclei (f). Parafollicular C cell (pa) resting on the basement membrane have large pale nuclei (n). Vascular connective tissue septa (vs) are noticed between the follicles. Tartarzine- Treated group (5b and 5c): Showing variable shaped thyroid follicles (F), some of them are involuted (arrow). Note, wide interfollicular spaces (S) with interfollicular cellular infilteration (if) and congested blood vessel (bv). Hyperplasia of epithelial lining of some follicles (double arrow) and darkly stained nuclei of follicular epithelium (n) were noticed. Curcumine-Protective group (5d): Showing nearly normal thyroid follicles. (F). They are filled with homogenous colloid (C) and lined by cuboidal cells with vesicular rounded nuclei. (f). However, some follicles show hyperplasia of their epithelial lining (double arrow). Recovery group (5d): Showing: variable shaped thyroid follicles (F), some of them are involuted (arrow). Somefollicles have darkly stained nuclei (n), others have normal cuboidal cells with vesicular nuclei (f). Notice, intefollicular spaces (S) containing many blood vessels (bv) and cellular infiltration (if). (Toludine blue 40X, Scale bar 100 μm).
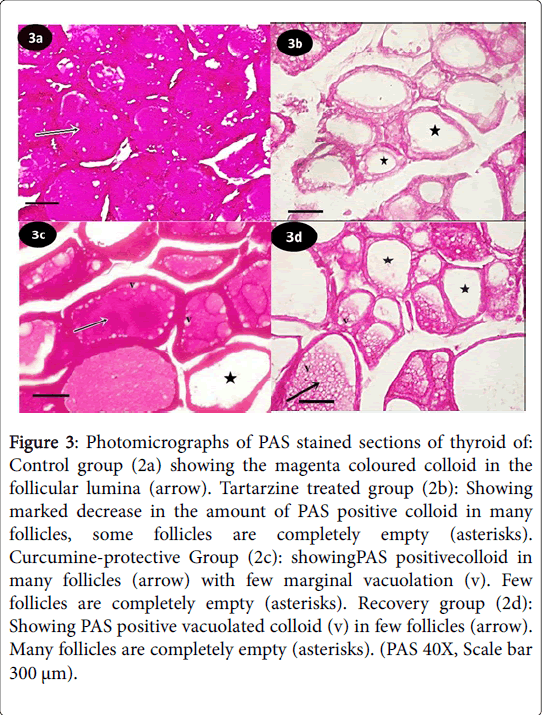
Periodic acid Schiff (PAS) reaction of control group showed the thyroid glands from control group exhibited the magenta colored colloid in the follicular lumina (Figure 3a). Tartarzine-treated group revealed marked decrease in the amount of PAS positive colloid in many follicles, many follicles were completely empty (Figure 3b). Curcumine-protective group showed PAS positive colloid in many follicles with few marginal vacuolation (v), few follicles were completely empty (Figure 3c). Recovery group revealed PAS positive vacuolated colloid in few follicles, many follicles were completely empty (Figure 3d).
Figure 3: Photomicrographs of PAS stained sections of thyroid of: Control group (2a) showing the magenta coloured colloid in the follicular lumina (arrow). Tartarzine treated group (2b): Showing marked decrease in the amount of PAS positive colloid in many follicles, some follicles are completely empty (asterisks). Curcumine-protective Group (2c): showingPAS positivecolloid in many follicles (arrow) with few marginal vacuolation (v). Few follicles are completely empty (asterisks). Recovery group (2d): Showing PAS positive vacuolated colloid (v) in few follicles (arrow). Many follicles are completely empty (asterisks). (PAS 40X, Scale bar 300 μm).
Immunohistochemical staining
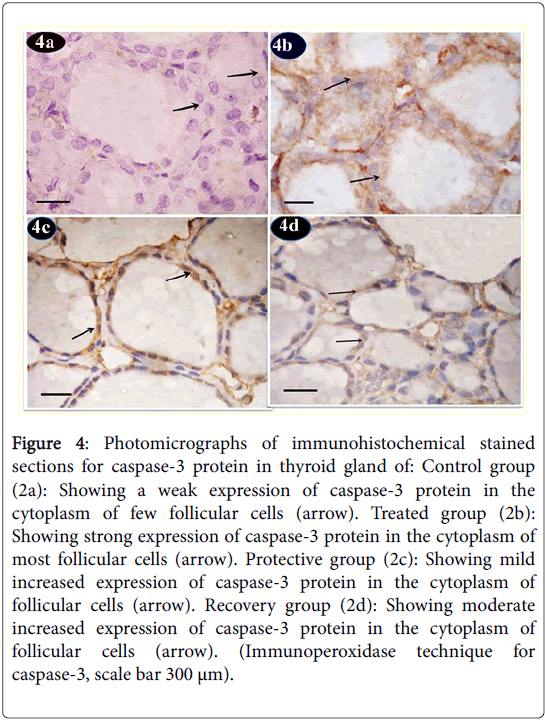
Caspase-3 protein immunoreactions showed in control group sections a weak expression in the cytoplasm of few follicular cells (Figure 4a). Tartarzine-treated group revealed a strong expression of caspase-3 protein in the cytoplasm of most follicular cells (Figure 4b). Curcumine-protective group showed mild increase in expression of caspase-3 protein in the cytoplasm of follicular cells (Figure 4c). Recovery group revealed moderate increase in expression of caspase-3 protein in the cytoplasm of follicular cells (Figure 4d).
Figure 4: Photomicrographs of immunohistochemical stained sections for caspase-3 protein in thyroid gland of: Control group (2a): Showing a weak expression of caspase-3 protein in the cytoplasm of few follicular cells (arrow). Treated group (2b): Showing strong expression of caspase-3 protein in the cytoplasm of most follicular cells (arrow). Protective group (2c): Showing mild increased expression of caspase-3 protein in the cytoplasm of follicular cells (arrow). Recovery group (2d): Showing moderate increased expression of caspase-3 protein in the cytoplasm of follicular cells (arrow). (Immunoperoxidase technique for caspase-3, scale bar 300 μm).
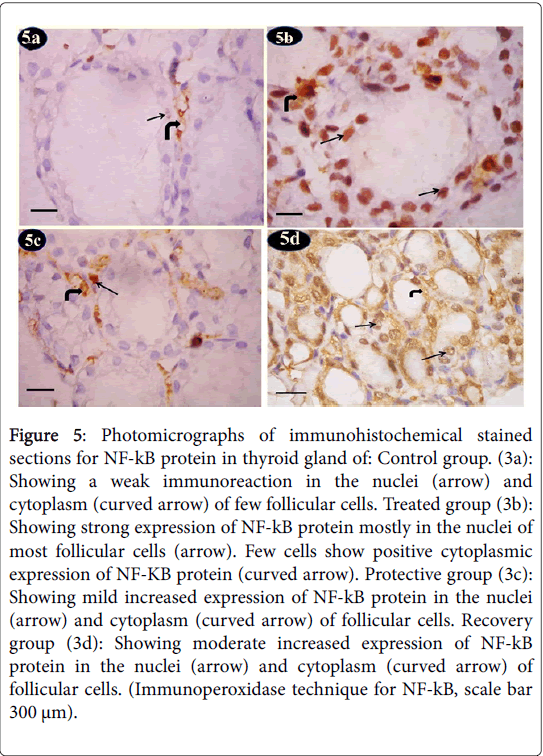
Nuclear factor kappa-b (NF-kB) showed control group a weak immunoreaction of NF-kB protein in the nuclei and cytoplasm of few follicular cells (Figure 5a). Tartarzine-treated group revealed a strong immunoreaction of NF-kB protein mostly in the nuclei of most follicular cells. Few cells showed positive cytoplasmic expression of NF-KB protein (Figure 5b). Curcumine-protective group showed mild increased immunoreaction of NF-kB protein in the nuclei and cytoplasm of follicular cells (Figure 5c). Recovery group exhibited moderate increased immunoreaction of NF-kB protein in the nuclei and cytoplasm of follicular cells (Figure 5d).
Figure 5: Photomicrographs of immunohistochemical stained sections for NF-kB protein in thyroid gland of: Control group. (3a): Showing a weak immunoreaction in the nuclei (arrow) and cytoplasm (curved arrow) of few follicular cells. Treated group (3b): Showing strong expression of NF-kB protein mostly in the nuclei of most follicular cells (arrow). Few cells show positive cytoplasmic expression of NF-KB protein (curved arrow). Protective group (3c): Showing mild increased expression of NF-kB protein in the nuclei (arrow) and cytoplasm (curved arrow) of follicular cells. Recovery group (3d): Showing moderate increased expression of NF-kB protein in the nuclei (arrow) and cytoplasm (curved arrow) of follicular cells. (Immunoperoxidase technique for NF-kB, scale bar 300 μm).
Biochemical Results
Statistical analysis of the mean of hormonal assay in comparison with the control group showed a significant increase in total T3 and T4 while a significant decrease in the TSH hormones in both tartarzine treated and recovery group(p<0.05). However, there was a nonsignificant difference between control and curcumin protective group (p>0.05) (Table 2).
| Group Parameters |
(Ia) | (Ib) | (Ic) | II | III | IV | F | P. value |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| T3 | 0.88 ± 0.32 | 0.90 ± 0.3 | 0.97 ± 0.35 | 1.63 ± 0.17a | 0.95 ± 0.28b | 1.56 ± 0.11a,c | 81.628 | <0.001 |
| T4 | 2.7 ± 0.43 | 2.65 ± 0.52 | 2.66 ± 0.44 | 4.85 ± 1.044a | 2.78 ± 0.27b | 5.14 ± 0.7a,c | 143 | <0.001 |
| TSH | 0.185 ± 0.075 | 0.189 ± 0.057 | 0.196 ± 0.064 | 0.069 ± 0.026a | 0.174 ± 0.074b | 0.11 ± 0.03a,c | 36.4 | <0.001 |
| Results: Values are expressed as mean ± standard deviation (SD) of n=10 rats/group. T3: Triiodothyronine-T4: Thyroxine-TSH: Thyroid Stimulating Hormone. aSignificant as compared with the Ia,Ib and Ic groups, P<0.05. bSignificant as compared with II group, P<0.05. cSignificant as compared with III group, P<0.05. | ||||||||
Table 2: Statistical results of the hormonal assay (T3,T4 and TSH) in the studied groups.
Statistical analysis of the mean of oxidative stress markers in thyroid tissues in comparison with the control group showed a highly significant increase in both MDA and SOD while a highly significant decrease in CAT and GSH in both tartarzine treated and recovery group (p< 0.001). However, there was a non-significant difference in all parameters between control and curcumin protective group (p>0.05) (Tables 3 and 4).
| Group parameters |
(Ia) | (Ib) | (Ic) | II | III | IV | F | P. value |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| MDA (nmol/g tissue) | 1.8 ± 0.18 | 1.8 ± 0.19 | 1.8 ± 0.15 | 2.8 ± 0.105a | 1.8 ± 1.7b | 2.5 ± 0.26a,c | 94.196 | <0.001 |
| SOD (u/g tissue) | 76.5 ± 1.29 | 76.5 ± 1.30 | 76.5 ± 1.34 | 110.75 ± 0.96a | 77.25 ± 1.7b | 106 ± 2.1a,c | 517.4 | <0.001 |
| CAT (u/g tissue) | 128 ± 2.16 | 128 ± 2.20 | 128 ± 2.17 | 84 ± 1.7a | 127 ± 2.5b | 97 ± 1. 29a,c | 495.011 | <0.001 |
| GSH (nmol/ tissue) | 57 ± 2.18 | 57 ± 2.19 | 57 ± 2.20 | 128 ± 2.16a | 56.5 ± 1.29b | 47 ± 10.99a,c | 42.784 | <0.001 |
| Results: Values are expressed as mean ± standard deviation (SD) of n=10 rats/group. MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Growth Stimulating Hormone. aSignificant as compared with the Ia,Ib and Ic groups, P<0.05. bSignificant as compared with II group, P<0.05. cSignificant as compared with III group, P<0.05. | ||||||||
Table 3: Statistical results of oxidative and antioxidant biomarkers levels in thyroid gland tissue in the studied groups.
| Group parameters |
Ia | Ib | Ic | II | III | IV | F | P. value |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Epithelial height (Mm) | 10.28 ± 0.7 | 10.7 ± 0.75 | 10.37 ± 0.56 | 14.27 ± 0.75a | 10.8 ± 1.41b | 12.27 ± 0.74a,c | 427.5 | <0.001 |
| Optical density of thyroid follicle | 67 ± 0.007 | 67 ± 0.008 | 68 ± 0.008 | 39 ± 0.7a | 66.9 ± 1.1b | 58.2 ± 0.4 | 887.064 | <0.001 |
| Caspase 3 (% area) | 8.19 ± 1.27 | 7.91 ± 1.19 | 7.97 ± 1.34 | 19.32 ± 1.1a | 8.59 ± 1.4b | 11.75 ± 1.54a,c | 104.4 | <0.001 |
| NFKß (optical density) | 4.16 ± 0.73 | 4.42 ± 0.82 | 4.22 ± 0.7 | 6.84 ± 0.64a | 4.12 ± 0.63b | 5.93 ± 0.53a,c | 131.2 | <0.001 |
| Results: Values are expressed as mean ± standard deviation (SD) of n=10 rats/group. aSignificant as compared with the Ia,Ib and Ic groups, P<0.05. bSignificant as compared with II group, P<0.05. cSignificant as compared with III group, P<0.05. | ||||||||
Table 4: Statistical results of the morphmetrical results in studied groups.
Morphometrical results
Statistical analysis of the mean of follicular epithelial height in comparison with the control group showed a significant increase in both tartarzine treated and recovery group (p<0.05). However, there was a non-significant increase in curcumin protective group (p>0.05).
Statistical analysis of the mean of optical density of follicular colloid in comparison with the control group showed a significant decrease in both tartarzine treated and recovery group (p<0.05). However, there was a non-significant decrease in curcumin protective group (p>0.05).
Statistical analysis of the mean of area % of the immune reaction of caspase-3 and NF-kB in comparison with the control group showed a significant increase in both tartarzine treated and recovery group (p<0.05). However, there was a non-significant increase in curcumin protective group (p>0.05).
Discussion
Today, food colorants are more strictly regulated than at any other time in history. Hence, any food colorant requires approval for use prior to its inclusion in food. Food dyes are broadly divided into two namely synthetic and natural food dyes [35].
Tartrazine as synthetic dye induces adverse effects in learning and memory functions in animals as well as behavioural changes. Tartrazine have toxic potential to harm lymphocytes, hepatocytes and renal functional unit. In human, tartrazine induces wide range of allergic reactions in sensitive or atopic individuals [36].
Curcumin as natural dye is a yellow-colored, polyphenol compound that has been shown to protect against reactive oxygen species (ROS)- mediated oxidative damage to cellular components and it has been reported to accelerate detoxification in liver [37].
Regardless of the variety of studies conducted to explore the oxidative effect caused by tartrazine in many organs such as kidney, liver and brain [37,2], there is still a lack of information concerning the oxidative damage induced by tartrazine consumption in thyroid gland. So, in this work toxic effects of tartrazine on thyroid gland were evaluated with a chronic exposure to the dye at a dose of 300 mg/kgb. w given daily up to 30 days. The ameliorative effect of curcumin against tartarzine toxicity also mentioned. Interestingly, the acceptable daily intake of tartarzine for human is 0-7.5 mg/kg-b.w [38].
Monitoring of body weight is considered as proper growth indicator of animal and human. Some researchers considered the loss of body weight is a reliable sensitive indicator for toxicity [37]. In this study, Tartrazine treated group showed a significant decrease in body weight throughout the experimental and recovery periods as compared with the control. This is in accordance with Borzelleca and Hallagan [39] who stated that Tartarzine in the diet reduces the palatability of food or otherwise resulting in avoidance. Furthermore, Tartarzine might result in generation of free radicals, which resulted in oxidative stress that caused metabolic disorders and general losses of body mass. While, in curcumine protective group, weight gain showed a nonsignificant increase ,this was in accordance with Crook [40] who mentioned that curcumin have improved metabolism by increasing capacities of antioxidants or acting directly as a scavenger of free radicals.
In this study, Biochemical assay of thyroid hormones showed significant increase in T3 and T4 and decrease in TSH of tartarzine treated group and recovery group as compared to control group and non-significant increases in T3 and T4 and decrease in TSH in curcumine treated group as compared to control group. These could be as a result of heavy presence of reactive oxygen species or free radicals insulting the follicular cells of the thyroid gland where T3 and T4 are stored. The increase in T4 and T3 especially forced a corresponding reduced level of TSH. The increased levels of TSH cause hyperstimulation of the thyroid gland resulting in increased T4 and T3. However, due to negative feedback mechanism, increase level of T4 and T3 stimulates reduced production of TSH from the pituitary [41].
Histological examination of sections of thyroid glands in tartarzinetreated group showed that the majority of the follicles were lined by tall columnar cells with rod shaped nuclei. Some follicles were lined by more than one layer of follicular cells. Multiple pyknotic nuclei were seen in follicular lining epithelium. Many follicular cells appeared with vacuolated cytoplasm. This was in accordance with Rus et al. [42] who reported that toxic potential of tartrazine to lymphocytes, hepatocytes and renal functional unit which seemed that tartrazine binds directly to DNA.
Reduction in amount of the PAS positive colloid with increased marginal vacuolation were also observed in many follicles. Other follicles appeared empty with no colloid in their lumina. This was in accordance with Aboul-Soud et al. [43] who reported that tartarzine might result in generation of free radicals, which resulted in oxidative stress that caused metabolic disorders. Reactive oxygen species have been reported to be an essential causative factor for histopathological alterations of the liver. With progression of cellular damage, hepatocyte swallowing follows and vacuolization occurs. These results were observed in hepatocytes of guinea pigs exposed for 3 weeks to Tz in drinking water.
Reactive oxygen species , such as hydrogen peroxide, superoxide anion and hydroxyl radical can, if not scavenged, result in oxidative stress whereby leading to peroxidation of lipids in membranes. Consequently, if the intracellular antioxidant defense system, both enzymatic and non-enzymatic is exhausted, residual ROS can cause damage [37].
Disorganized and damaged follicles were observed and this can be explained by intestinal bio-transformation of the tartarzine resulting in the production of metabolites which are super-reactive free radicals. These free radical and reactive oxygen species have been reported to cause distortion of organs such as the liver [44].
Congested blood vessels in between the follicles were also noticed. Membranes are particularly prone to effects of ROS, peroxidation of unsaturated fatty acids in biological membranes leads to a decrease of fluidity and disruption of membrane integrity and function [45]. Chakravarty et al. [46] added that tartrazine prevents red blood cell synthesis via inhibition of erythropoisis in the bone marrow.
Toluidine blue stained sections of tartarzine-treated Group showed variable shaped thyroid follicles, some of them were involuted. Wide interfollicular spaces with interfollicular cellular infilteration and congested blood vessel were detected. Hyperplasia of epithelial lining of some follicles and darkly stained nuclei of follicular epithelium were also noticed. Identical histopathological alterations have been observed in hepatocytes of guinea pigs exposed for 3 weeks to tartarzine in drinking water, infiltration of Kupffer cells, congested blood vessels with various areas of hemorrhage in liver exposed to tartarzine [47].
H and E, PAS and toluidine stained sections of thyroid glands of Curcumine-protective Group showed that most of thyroid follicles were similar to that of the control group. They were lined by single layer of low cubical cells with rounded nuclei and their luminae were filled with acidophilic colloid. Curcumin exerts its protective effects against severe oxidative damage via : (1) its powerful antioxidant property, whereby scavenges oxygen free radicals, and (2) its ability to increase intracellular GSH levels, which consequently lead to the efficient control of levels of lipid peroxidation [48].
Immunohistochemical Caspase-3 stained sections of thyroid gland of control group showed a weak expression of caspase-3 protein in the cytoplasm of few follicular cells. In Tartarzine-treated Group revealed a strong expression of caspase-3 protein in the cytoplasm of most follicular cells. In Curcumine-protective Group showed mild increase in expression of caspase-3 protein in the cytoplasm of follicular cells. In Recovery Group, revealed moderate increase in expression of caspase-3 protein in the cytoplasm of follicular cells. Curcumin protection against tartrazine-induced oxidative injuries and lipid peroxidation occurs via enhancement of the antioxidant defense system. The distinctive protective effect of curcumin is thought to be via expression of a gene subset since it has been shown to regulate gene expression of insulin-like growth factor, B-cell CLL/lymphoma [46].
In tartarzine-treated group there was a strong immunoreaction of NF-kB protein mostly in the nuclei of most follicular cells. Few cells showed positive cytoplasmic expression for it. Barnes and Karin [15] concluded that in unstimulated cells, NF-kB is found in cytoplasm and is bound to IkBa and IkBb, which prevent it from entering the nuclei. When these cells are stimulated, specific kinases phosphorylate IkB, causing its rapid degradation by proteasomes. The release of NF-kB from IkB results in the passage of NF-kB into the nucleus, where it binds to specific sequences in the promoter regions of inflammatory genes. In contrary to curcumin-protective group that showed mild increased immunoreaction of NF-kB protein in the nuclei and cytoplasm of follicular cells.
Concerning the recovery group, our study revealed that there was incomplete recovery from tartarzine effects. This result can be explained by binding of cellular copper and iron to the artificial food colorants, resulting in its tissues accumulation [48]. Soares et al. [49] also explained the incomplete recovery by the significant genotoxic effect of tartarzine that might generate carcinogenesis with its prolonged consumption.
Conclusion
We provided evidence for the potential of CUR, as a natural food coloring agent, to minimize or prevent oxidative stress, commonly taking place due to consumption of various potentially hazardous tartarzine coloring foods. CUR can thus delay the onset of formation of potentially damaging ROS, eventually leading to a better capacity in maintaining nutritional food quality and more efficient cellular redox state homeostasis.
Conflict of Interest
There is no potential conflict of interest among the authors.
References
- Amin KA, El-Shehri FS (2018) Toxicological and safety assessment of tartrazine as a synthetic food additive on health biomarkers: A review. Afr J Biotechol 17: 139-149.
- Gao Y, Li C, Shen J, Yin H, An X, et al. (2011) Effect of food azo dye tartrazine on learning and memory functions in mice and rats, and the possible mechanisms involved. J Food Sci 76: T125-T129.
- Ali FA, Abdelgayed AS, EL-Tawil SO, BaNeer MA (2016) Toxicological and histopathological studies on the effect of tartrazine in male albino rats. Int J Bio Biomol Agri Biotech Eng 10: 473-478.
- Boussada M, Lamine JA, Bini ID, Abidi N, Lasram M, et al. (2017) Assessment of a sub-chronic consumption of tartrazine (E102) on sperm and oxidative stress features in Wistar rat. Int Food Res 24: 1473-1481.
- Visweswaran B, Krishnamoorthy G (2012) Oxidative stress by tartrazine in the testis of wistar rats. Int J Pharm Biol Sci 2: 44-49.
- Moutinho IL, Bertges LC, Assis RV (2007) Prolonged use of the food dye tartrazine (fd&c yellow no 5) and its effects on the gastric mucosa of wistar rats. Braz J Biol 67: 141-145
- Carocho M, Barreiro MF, Morales P, Ferreira ICFR (2014) Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comp Rev Food Sci Food Safety 13: 377-399.
- Benzer F, Kandemir FM, Ozkaraca M, Kucukler, S, Caglayan C (2018) Curcumin ameliorates doxorubicinâ€induced cardiotoxicity by abrogation of inflammation, apoptosis oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol 32: e22030.
- Dou Y, Luo J, Wu X, Wei Z, Tong B, et al. (2018) Curcumin attenuates collagen-induced inflammatory response through the “gut-brain axisâ€. J Neuroinflammation 15: 1-6.
- Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: The story so far. Eur J Cancer 41: 1955-1968.
- Sa G, Das T (2008) Anti-cancer effects of curcumin: Cycle of life and death. Cell Div 3: 1-14.
- Huang J, Yao X, Weng G, Qi H, Ye X (2018) Protective effect of curcumin against cyclosporine a induced rat nephrotoxicity. Mol Med Rep 17: 6038-6044.
- El-Twab SMA, Abdul-Hamid M (2016) Curcumin mitigates lithium-induced thyroid dysfunction by modulating antioxidant status, apoptosis and inflammatory cytokines. J Basic Appl Zool 76: 7-19.
- Mohamed DA, Elnegris HM (2015) Histological study of thyroid gland after experimental exposure to low frequency electromagnetic fields in adult male albino rat and possible protective role of vitamin E. J Cytol Histol 6: 374.
- Barnes PJ, Karin M (1997) Nuclear factor-κB-a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066-1071.
- Fan TJ, Han LH, Cong RS, Liang J (2005) Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 37: 719-727.
- Hashish EA, Elgaml SA (2016) Hepatoprotective and nephroprotective effect of curcumin against copper toxicity in rats. Indian J Clin Biochem 31: 270-277.
- Al-Shinnawy MS, Elkattan NA (2013) Assessment of the changes in some diagnostic parameters in male albino rats fed on an azo dye. Int J Environ Sci Eng 4: 85-90.
- Le Grange D, Doyle PM, Swanson SA, Ludwig K, Glunz C, et al. (2012) Calculation of expected body weight in adolescents with eating disorders. Pediatrics 129: e438-e446.
- El-Bakry RH, Tawfik SM (2014) Histological study of the effect of potassium dichromate on the thyroid follicular cells of adult male albino rat and the possible protective role of ascorbic acid (vitamin C). J Microscopy Ultrastruct 2:137-150.
- Selim AO, El-Haleem MRA, Ibrahim IH (2012) Effect of sodium fluoride on the thyroid gland of growing male albino rats: Histological and biochemical study. Egypt J Histol 35: 470-482.
- Bancroft JD, Gamble A (2018) Theory and practice of histological techniques. (8th edtn). Churchil, Livingstone, New York, London.
- Mahmood T, Qureshi IZ, Iqbal MJ (2010) Histopathological and biochemical changes in rat thyroid following acute exposure to hexavalent chromium. Histol Histopathol 25: 1355-1370.
- Glauert AM, Lewis PR (2014) Biological specimen preparation for transmission electron microscopy. Princeton University Press, USA.
- Javois LC (1999) Immunocytochemical methods and protocols. Totowa: Humana Press, USA.
- Mancini M, Nicholson DW, Roy S, Thornberry NA, Peterson EP, et al. (1998) The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol 140: 1485-1495.
- Maguire O, O'Loughlin K, Minderman H (2015) Simultaneous assessment of NF-κB/p65 phosphorylation and nuclear localization using imaging flow cytometry. J Immunol Methods 423: 3-11.
- Sui X, Sui Y, Wang Y (2018) LARP7 in papillary thyroid carcinoma induces NIS expression through suppression of the SHH signaling pathway. Mol Med Rep 17: 7521-7528.
- Helewski KJ, Kowalczyk-Ziomek GI, Czecior E, Swietochowska E, Wielkoszynski T, et al. (2010) Administration of low doses of TNFa protects rat liver from ischemic damage and re-perfusion injury. J Physiol Pharmacol 61: 273-278.
- Negahdary M, Bezhgi M, Ajdary M (2015) Effects of silymarin on oxidative stress markers in rats treated with magnesium oxide nanoparticles. Annu Res Rev Biol 5: 254.
- Olszewska-Słonina DM, Mątewski D, Czajkowski R, Olszewski KJ, Wo Ÿniak A, et al. (2011) The concentration of thiobarbituric acid reactive substances (TBARS) and paraoxonase activity in blood of patients with osteoarthrosis after endoprosthesis implantation. Med Sci Monit 17: CR498-CR504.
- Nishikimi M (1975) Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun 63: 463-468.
- Tipple TE, Rogers LK (2012) Methods for the determination of plasma or tissue glutathione levels. Methods Mol Biol 889: 315-324.
- Sharma A, Goyal RP, Chakravarty G, Sharma S (2015) Effects of chocolate brown. Science Academic 57: 183-198.
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K (2002) The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat Res 519: 103-119.
- Amin H, Abdel-Hameid H, Abd-Elsttar AH (2010) Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chemi Toxicol 48: 2994-2999.
- Alison D, Collins P (2001) Colouring our foods in the last and next millennium. Int. J Food Sci Technol 35: 5-22.
- Borzelleca JF, Hallagan JB (1988) A chronic toxicity/carcinogenicity study of FD & C Yellow No. 5 (tartrazine) in mice. Food Chem Toxicol 26: 189-194.
- Crook AM (2006) Thyroid function. In clinical chemistry and metabolic medicine. (7th edtn), Hodder Arnold publishers, London.
- Sayari S, Molaei Z, Torabi Z (2018) The relationship between subclinical hypothyroidism and serum levels of uric acid and creatinine in children aged 2-14 years. Ann Pediatr Endocrinol Metab 23: 38-42.
- Rus V, Gherman C, Miclăuş V, Mihalca A, Nadăş GC et al. (2010) Comparative toxicity of food dyes on liver and kidney in guinea pigs: a histopathological study. Ann RSCB 15: 161-165.
- Aboul-Soud MA, Al-Othman, AM, El-Desoky GE, Al-Othman ZA, Yusuf K, et al. (2011) Hepatoprotective effects of vitamin E/selenium against malathion-induced injuries on the antioxidant status and apoptosis-related gene expression in rats. J Toxicol Sci 36: 285-296.
- Suzuki Y, Ishihara M, Segami T, Ito M (1998) Antiulcer effects of antioxidants, quercetin, alpha tocopherol, nifedipine and tetracycline in rats. Jpn J Pharmacol 78: 435-441.
- Chakravarty G, Goyal RP, Sharma S, Sharma A (2005) Haematological changes induced by a common non-permitted food colour malachite green (MG) in swiss albino mice. Indian J Environ Sci 9:113-117.
- El-Desoky GE, Abdel-Ghaffar A, Al-Othman ZA, Habila MA, Al-Sheikh YA, et al. (2017) Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur Rev Med Pharmacol Sci 21: 635-645.
- Al-Rubaei ZM, Mohammad TU, Ali LK (2014) Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak J Biol Sci 17: 1237-1241.
- Stevens LJ, Kuczek T, Burgess JR, Stochelski MA, Arnold LE, et al. (2013) Mechanisms of behavioral, atopic, and other reactions to artificial food colors in children. Nutr Rev 71: 268-281.
- Soares BM, Araujo TM, Ramos JA, Pinto LC, Khayat BM, et al. (2015) Effects on DNA repair in human lymphocytes exposed to the food dye tartrazine yellow. Anticancer Res 35: 1465-1474.
Citation: Abdel-Aziz HM, Alazouny ZM, Abdelfadeel KF, Abohashem AA (2018) Effect of Tartrazine on Thyroid Gland of Male Rat and Ameliorating Role of Curcumin (Histological and Immunohistochemical Study). J Biochem Cell Biol 1: 111.
Copyright: © 2018 Abdel-Aziz HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4815
- [From(publication date): 0-2019 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 3817
- PDF downloads: 998