Research Article Open Access
Effect of Somatic Cell Count on Bovine Milk Protein Fractions
Ramos TM1*, Costa FF2, Pinto ISB3, Pinto SM4 and Abreu LR4
1Department of Animal and Food Sciences, University of Delaware, Christina Mill Drive, Newark, Delaware, United States of America
2Department of Food Science, Federal University, Juiz de Fora, Brazil
3Department Animal and Food Sciences, Embrapa Gado de Leite, Juiz de Fora, Brazil
4Department of Food Science, Federal University of Lavras, Lavras, Brazil
- *Corresponding Author:
- Ramos TM
Department Animal and Food Sciences
University of Delaware, 1045
Christina Mill Drive, Newark, Delaware
19711, United States of America
Tel: 302-831-2501
E-mail: thaisramos85@yahoo.com.br
Received date: July 07, 2015; Accepted date: August 18, 2015; Published date: August 25, 2015
Citation: Ramos TM, Costa FF, Pinto ISB, Pinto SM, Abreu LR (2015) Effect of Somatic Cell Count on Bovine Milk Protein Fractions. J Anal Bioanal Tech 6:269 doi:10.4172/2155-9872.1000269
Copyright: © 2015 Ramos TM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The objective of this study was to evaluate the influence of somatic cell count (SCC) on the physicochemical properties and protein fractions of milk. Milk was collected and analyzed for somatic cell count, fat, lactose, acidity, total solids, ash, total nitrogen, soluble nitrogen at pH 4.6, and soluble nitrogen in trichloroacetic acid (TCA) 12%. Milk was divided into four groups according to the value of SCC, each constituting a treatment, as follows: Treatment 1 (<300,000 cells/ml), Treatment 2 (300,000 to 750,000 cells/ml), Treatment 3 (750,000-1,000,000 cells/ ml), and Treatment 4 (>1 million cells/ml). The electrophoretic profile of milk was also evaluated using microfluidic electrophoresis for separation and quantification of milk proteins. An increase in the concentration of SCC resulted in a significant increase in the amount of fat, soluble nitrogen and soluble protein (casein) fractions, and a reduction of α-casein, β-casein, and κ-casein. There was a higher proteolytic activity associated with high SCC. Changes in protein fractions of milk caused by high SCC had strong implications regarding the potential of milk as raw material for manufacturing products as the industrial yield of milk is mainly associated with the casein fraction.
Keywords
Casein; SCC; Mastitis; Electrophoresis
Abbreviations
SCC: Somatic Cell Count; TN: Total Nitrogen; NCN: Noncasein Nitrogen Content; CP: Crude Protein; SN: Soluble Nitrogen; NPN: Non-Protein Nitrogen; CN: Casein Nitrogen; TP: True Protein; SP: Soluble Protein; β-CN: β-Casein; α-CN: α-Casein; κ-CN: κ-Casein; TA: Titratable Acidity; La: α-Lactalbumin; β-Lg: β-Lactoglobulin
Introduction
Caseins are milk proteins secreted by cells of the mammary gland. They constitute approximately 78-82% of bovine milk proteins and are divided into four main groups: αS1-casein, αS2-casein, β-casein and κ-casein, forming supramolecular structures known as micelles [1,2]. The protein composition of cow's milk is an important factor for the profitability of the dairy industry. An increase in the proportion of casein, in particular α- and β-CN, results in better product yield, especially in cheese [3]. The caseins are phosphoproteins containing a variable number of phosphate radicals linked to serine (P-Se) and are concentrated in different regions of polypeptide chains. Based on the location of these phosphate radicals, the resulting molecule regions are more hydrophilic or more hydrophobic, and consequently, the caseins are more susceptible to proteolysis.
Proteolysis in milk is an important quality criterion that can have beneficial or detrimental effects, depending on processing. Milk protein proteolysis can be attributed to both indigenous proteases and also proteases produced by psychrotrophic bacteria during the cold storage of milk [4]. Proteolytic bacterial enzymes act mostly on the κ-casein, resulting in the destabilization of the casein micelles and coagulation of milk in a manner analogous to chymosin [5,6]. Proteolysis of bovine milk can also occur naturally [7]. During this process, native thermostable protease activity is mainly related to the activity of plasmin, a serine protease derived from its inactive precursor plasminogen [8].
Plasmin is an alkaline proteolytic enzyme which participates in the hydrolysis of casein. It is of great importance in natural milk proteolysis [7]. Plasmin is the active form which is produced from the zymogen called plasminogen. The conversion of plasminogen to plasmin occurs by the action of specific plasminogen activators, which are also proteases [9]. The increase in plasmin activity is caused by somatic cells from its inactive precursor plasminogen, which is converted into plasmin, in a process initiating in the mammary gland and continuing throughout the storage period [7,8,10,11]. Increased SCC in milk results in elevated activation of plaminogen into plasmin, that in turn leads to high breakdown of some proteins chains, primarily β-casein, because protein fraction partially diffuses into solution at low temperature, which facilitates enzyme attack, producing small fragments, such as γ-caseins and other small peptides that diffuse to the aqueous phase of the milk [12,13]. This protease has specificity for Lys-x and Arg-x bonds [13-15].
The level and activity of plasmin in milk can vary and depends on biological factors, such as stage of lactation and somatic cell count [16]. The milk somatic cells, mainly composed of neutrophils and macrophages, have a wide range of proteolytic and lipolytic enzymes, which are released during the intracellular mechanism, killing microorganisms in subclinical mastitis, and may significantly contribute to proteolysis and lipolysis of the milk constituents [17,18]. Therefore, concentrations of many enzymes or their activity in the milk are increased during mastitis [19-21]. The enzymes of primary concern for the dairy industry are those with proteolytic activities, because the increase of proteolysis in milk and milk products has a negative impact on the quality and technological properties.
Proteolysis associated with increased somatic cell count in milk promotes the breakdown of casein micelles [22], one of these is the indigenous milk proteinase plasmin, which is associated primarily with the casein micelles [23], where it is capable of hydrolysing all caseins except κ-casein [24-26], in which contributes to increased susceptibility to defects in dairy products such as technological problems related to proteolytic enzymes include the gelling of UHT milk (Ultra High Temperature) [27,28], generation of free amino acids during cheese ripening and development of undesirable flavors and a bitter taste in milk and dairy products [29,30]. Even ultrahigh temperature (UHT) treatment of milk is insufficient to inactivate plasmin completely, but typical retort sterilisation does inactivate plasmin completely [25]. The use of milk with elevated SCC has detrimental technological implications, such as low yield, and decreased shelf life of products, changes in the characteristics of milk, and milk products, and interference in manufacturing technologies, especially in cheese.
Cooling is important and a way of improving milk quality. However, extended refrigeration time leads to modifications in composition and physical properties of milk. Among the many changes that occur during the cooling process, includes the dissociation of caseins, specifically the β-casein, which can solubilize up to 18% of its total fraction, solubilization of colloidal calcium phosphate, and as a consequence decrease in size of the micelles.
The separation and quantification of major milk proteins are fundamental in dairy research. Therefore, accurate and rapid methods are profoundly important. The microfluidic chip technique is faster, and uses considerably fewer chemicals and materials traditional techniques [31].
The aim of this study was to elucidate the behavior of protein fractions of milk with different somatic cell counts; specifically β-casein, it can be broken by plasmin with potentially bitter peptide formation and reducing the total solids.
Materials and Methods
Milk and milk proteins
Sample collection: Raw milk samples were collected from isothermal stirred bulk tanks with an internal temperature no more than 5°C. The samples were collected by specially educated technicians from the bulk tank milk of raw milk suppliers. The samples were labeled and transported according to the procedures established by the laboratory responsible for testing. The samples milk was collected in a dairy located in the city of Lavras, MG, Brazil.
Analysis of milk: The analyses were developed into different steps. Milk Quality Analyze Laboratory (LABUFMG) at Federal University of Minas Gerais (UFMG), Belo Horizonte MG, Brazil, developed Somatic Cells Counting (SSC) analysis. Both physicochemical and microbiological analyses were performed at Federal University of Lavras (UFLA), Lavras MG, Brazil. Thereafter, the frozen samples were transported to the Brazilian Agricultural Research Corporation (EMBRAPA) Laboratory, Juiz de Fora MG, Brazil, to do the electrophoresis profile of the proteins analysis.
Milk proteins: For analysis of proteins were used purified α-lactoalbumina (α-La), β-lactoglobulin (β-Lg), αs-casein (αs-CN), β-casein (β-CN) and κ-casein (κ-CN) were obtained from Sigma- Aldrich (USA). Solutions (10 mg mL-1) of each individual protein were prepared by adding each individual protein purified water (Ultrapure Milli-Q; Millipore Corp., USA) and stirring until dissolved. Mixed protein standards were prepared by combining each of the individual protein solutions (1 mL) and making the final volume up to 10 mL to give a mixed protein standard with an individual protein concentration of 1 mg mL-1.
Microbiological examination
Mesophilic bacteria count: decimal dilutions of raw milk samples were taken and plated on Plate Count Agar - PCA mesophilic bacteria to viable counts after incubation at 32°C for 48 hours. Count of psychrotrophic and proteolytic psychrotrophic: Dilutions decimal of raw milk samples were plated on agar Calcium Caseinate (Merck®) for the bacterial count of psychrotrophic and proteolytic psychrotrophic viable, with incubation at 7°C ± 0.5°C for 10 days.
Analysis of chemical composition and SCC
Fat, lactose, total solids and somatic cell counts were determined by infrared absorption (Bentley CombSystem 2300).
Physical-chemical analysis
Total nitrogen (TN), noncasein nitrogen content (NCN) corresponding to the milk soluble fraction at pH 4,6, and NPN content corresponding to the non-precipitated fraction with 12% trichloroacetic acid were determined by the Kjeldahl method following the AOAC [32]. Nitrogen was then multiplied by a standard factor (6.38) so that the results are expressed as total protein. Ash was determined by an AOAC (Association of the Official Analytical Chemists) technique using carbonization of the samples in a direct flame and subsequent calcination in a muffle at 550°C for 4-6 hours.
Titratable acidity
The acidity was determined by titration with a 0.1N NaOH solution using phenolphthalein as an indicator, and the result was expressed in grams of lactic acid or percentage of compounds having acidic character [32].
Microfluidic chip electrophoresis
Milk samples were subjected to ultracentrifugation in triplicate (40,000 × g) at 4°C for 60 min using a CR21 Himac ultracentrifuge (Hitachi, Japan). After centrifugation, the supernatant (soluble phase) was separated for analysis of protein profiles. Separation of individual milk proteins was performed using the microfluidic chip electrophoresis system (Agilent 2100 Bioanalyser - Technologies GmbH, Waldbronn, Germany) and the associated Protein 80 kit (Agilent Technologies, Germany). These kits contain the chips and proprietary reagents such as the gel matrix solution, proteins in a concentrated solution, a marker protein buffer solution and a protein molecular mass ladder solution to perform the electrophoresis [31,33-35].
The TPS buffer consisted of 0.1 mol L-1 tris chloride acid (Amresco, USA), pH 8.8, containing 2 mol L-1 urea (USB, Germany), 15% glycerol (Invitrogency, New Zealand) and 0.1 mol L-1 dithiothreitol (DTT) (Bioangency, Brazil). It was prepared according to the SOP (Standard Operating Procedure) available
from the Food Standards Agency (FSA) of the United Kingdom [31,35]. The SEP buffer solution, pH 3.0, used to separate the proteins consisted of 6.0 mol L1 urea (USB, Germany), 20 mmol L1 trisodium citrate dehydrate (Synth, Brazil), 0.1 mol L-1 citric acid (Merck, Brazil) and 0.05% (w/w) hydroxypropylmethyl cellulose (Sigma-Aldrich, USA) [31,36].
Segundo Costa et al. [31], milk was diluted in a 1 : 4 ratio with TPS buffer, SEP buffer and pure water (Ultrapure Milli-Q; Millipore Corp., USA) to compare and select the more efficient diluting agent. Samples were allowed for at least 2 h at 4°C for protein solubilization before application in microfluidic chip electrophoresis which was performed using an Agilent 2100 Bioanalyzer system (Agilent Technologies, Germany). The gel matrix, solutions and samples for electrophoresis were prepared according to the Bioanalyser protocols (Agilent Technologies, Germany). In Eppendorf tubes (0.5 mL total volume) 4 μL of samples (milk; milk+TPS buffer; milk+SEP buffer; milk+pure water; and milk added with each individual protein+SEP buffer) were mixed with 2 μL of 2-mercaptoethanol (Sigma-Aldrich, USA), heated (95°C, 5 min), cooled in an ice bath, briefly spun in a centrifuge (3000 g) and then 84 μL of Milli-Q water was added to give a total volume of 90 μL. All chips were loaded with ten samples with three replicates each
Quantification was carried out considering the area under the electropherogram using the Agilent 2100 Expert software associated with the instrument. The results were expressed as percentages (%) according to all the proteins identified in the electropherograms.
Experimental design
Bovine milk with mesophilic bacterial counts below 40,000 cfu/ ml and psychrotrophic counts below 2000 cfu/mL were collected and analyzed for somatic cell count. Milk samples were grouped according to SCC in four groups, each representing one treatment as follows:
Treatment 1: (<400.000 cells/mL);
Treatment 2: (400.000-750.000 cells/mL);
Treatment 3: (750.000-1.000.000 cells/mL);
Treatment 4: (>1.000.000 cells/mL).
Statistical analysis
Results were analyzed by ANOVA and the Tukey test at 5% probability using the R statistical package (R Development Core Team, Vienna, Austria).
Results and Discussion
Composition of milk with different somatic cell counts
The chemical composition of milk with different somatic cell counts is shown in Table 1. There were no significant differences (p>0.05) in concentrations of total solids, solids-not-fat, ash, acidity, lactose and total protein among treatments. The concentrations of total solids, although not statistically significant, presented tendence to increase as SCC increased. Research conducted by Fernandes [37] found an elevation in total solids with higher SCC. However, Marques [38] and Klei et al. [39] reported that the total solids content of milk with high SCC did not change. Moslehishad et al. [40], Lee et al. [41], and Salah El-Tahawy [42] showed higher percentage of total solids with an increase in SCC. Theses and other reports indicate inconsistent variation for this attribute in relation to SCC, once some compounds have their values increasing whereas others have theirs decreasing.
| Treatments1 | Analysis2 | |||||||
| SCC | TS | SNF | Ash | Fat | Ta | Lactose | CP | |
| <300 | 271.5 ± 33.09a | 12.27 ± 0.85a | 8.99 ± 0.69a | 0.68 ± 0.06a | 3.28 ± 0.27a | 0.15 ± 0.86a | 4.58 ± 0.12a | 3.16 ± 0.62a |
| 300-750 | 528.7 ± 241.69b | 12.56 ± 0.63a | 9.02 ± 0.40a | 0.70 ± 0.04a | 3.54 ± 0.30b | 0.15 ± 0.35a | 4.63 ± 0.06a | 3.16 ± 0.43a |
| 750-1000 | 796.33 ± 49.52c | 12.71 ± 0.30a | 9.17 ± 0.23a | 0.71 ± 0.03a | 3.54 ± 0.12b | 0.15 ± 0.74a | 4.49 ± 0.16a | 3.18 ± 0.14a |
| >1000 | 1145 ± 95.9d | 12.80 ± 0.30a | 9.22 ± 0.32a | 0.72 ± 0.01a | 3.58 ± 0.12b | 0.14 ± 0.59a | 4.51 ± 0.20a | 3.10 ± 0.44a |
a-dMeans within a columns with different superscripts differ (P<0.05)
Values are given as means ± standard deviation
1Treatments: <300=Tank milk with somatic cell count of <300000 cells/ mL; 300-750=Tank milk with somatic cell count of 300000 to 750000 cells/mL; 750-1000=Tank milk with somatic cell count of 750000 to 1000000 cells/ mL; >1000=Tank milk with somatic cell count of >1000000 cells/mL
2Analysis: SCC: Somatic cell count; TS: Total solid (%); SNF: Solid-non-fat (%); Ash (%); Fat (%); TA=Total Acidity (grams of lactic acid /100 grams of sample); CP: Crude protein (%)
Table 1: Chemical composition of bulk tank milk with different somatic cell counts.
Protein results, despite the non-significant differences (p>0.05), had a slightly increment with higher concentrations of SCC. However, the experimental results do not agree with the effects of high SCC milk on the total protein of milk, measured by the concentration of total nitrogen, as reported by several studies. The effect of mastitis on the total concentration of milk protein is variable [43]. Research [39,40] has shown that a higher milk somatic cell count results in higher levels of total protein. On the other hand, Verdi et al. [44], Rogers [45], and Albenzio et al. [10] reported no change in the total protein content of the milk, which possessed high SCC compared to milk with lower values, while Lee et al. [41] stated that total protein was lower in milk from cows with high SCC. Overall, total protein in milk with high SCC can remain unchanged or undergo small changes, because the content of casein decrease is accompanied by an increase in whey proteins, resulting in a negligible change in total milk protein.
There is an inverse relationship between the values of lactose and SCC but with no significant difference (p>0.05). Some authors [10,46] agree that there is a reduction in the concentration of lactose in milk with high SCC. Inflammation of the mammary gland results in lesser synthesis of lactose [46,47]. During mastitis, the NaCl concentration in milk more elevated, resulting in an augment in its osmotic potential, making the milk in the lumen hyper-osmotic relative to the surrounding blood. Because these two mediums must be iso-osmotic for the synthesis of milk, there is a physiological compensation by reducing the lactose content of the milk [48], which explains the results obtained.
Higher fat content (p<0.05) was observed between groups of SCC (<300, 300-750, and 750-1000) when associated with higher values of SCC. Similar results were reported by Miller et al. [49], Mitchel et al. [50], Marques et al. [38]. However, Munro et al. [51] and Moslehishad et al. [40] found no significant difference (p>0.05) for the values of milk with different fat content, and Najafi et al. [52] observed an inverse relationship between fat content and SCC values. These results indicate that there can be no standard established relative to fat content and SCC.
Although Nafaji et al. [52] reported that high SCC milk reduces the acidity by reducing its solid content, the mean values of acidity did not differ (p>0.05). The average milk composition related to crude protein (CP), total nitrogen (TN), soluble nitrogen (SN), non-protein nitrogen (NPN), casein nitrogen (CN), true protein (TP), soluble protein (PS), casein, and the ratio of CN/TP are reported in Table 2. The content of total protein (TP), total nitrogen (TN), true protein (TP), and the relationship between CN/TP was not affected by the milk SCC (p>0.05). Santos et al. [53] found similar results regarding the content of total protein (TP), non-protein nitrogen (NPN) and true protein. In contrast, Ma et al. [54] demonstrated that milk with a lower (45,000 cells/ml) SCC concentration had lower CP than that observed in milk with increased SCC (849,000 cells/mL).
| Analysis2 | |||||||||
| Treatments1 | CP | TN | SN | NPN | CN | TP | SP | Casein | Casein/TP |
| <300 | 3.16 ± 0.62a | 0.49 ± 0.09a | 0.09 ± 0.1a | 0.023 ± 0.01a | 0.39 ± 0.09a | 3.13 ± 0.62a | 0.63 ± 0.06a | 2.49 ± 0.57a | 0.26 ± 0.08a |
| 300-750 | 3.16 ± 0.43a | 0.49 ± 0.06a | 0.10 ± 0.01b | 0.026 ± 0.01b | 0.38 ± 0.07a | 3.13 ± 0.39a | 0.69 ± 0.09b | 2.42 ± 0.44a | 0.25 ± 0.04a |
| 750-1000 | 3.18 ± 0.14a | 0.50 ± 0.02a | 0.11 ± 0.05c | 0.028 ± 0.01c | 0.37 ± 0.07b | 3.15 ± 0.15a | 0.76 ± 0.03c | 2.36 ± 0.44b | 0.24 ± 0.01a |
| >1000 | 3.10 ± 0.44a | 0.48 ± 0.04a | 0.12 ± 0.04c | 0.029 ± 0.01d | 0.36 ± 0.06b | 3.07 ± 0.30a | 0.74 ± 0.02c | 2.29 ± 0.38b | 0.25 ± 0.02a |
a-dMeans within a column with different superscripts differ (P<0.05)
Values are given as means ± standard deviation
1Treatments: <300=Tank milk with somatic cell count of <300000 cells/ mL; 300-750=Tank milk with somatic cell count of 300000 to 750000 cells/mL; 750-1000=Tank milk with somatic cell count of 750000 to 1000000 cells/ mL; >1000=Tank milk with somatic cell count of >1000000 cells/mL
2Analysis: CP=Crude protein (%); TN=Total nitrogen (%); SN=Soluble nitrogen (%); NPN=Non-protein nitrogen (%); CN=Casein nitrogen (%); TP=True protein (%); SP=Soluble protein (%)
Table 2: Nitrogen components of tank milk with different somatic cell counts.
Occurred significant difference (p<0.05) in the contents of soluble nitrogen (SN), non-protein nitrogen (NPN), soluble protein (PS), and casein. The levels of casein were reduced (p<0.05). It is well known that during mastitis, casein synthesis is usually reduced, similar to results found by Santos et al. [53] and O`Connell et al. [55]. Nevertheless, some authors found no significant reduction in casein when correlated with high SCC [10,40,56-59].
Reports [39,44] have previously described that the CN/TP is reduced with lower SCC. This finding accounts for the reduction of casein without changing the total protein (Table 2).
TP concentrations were not significantly different (p>0.5) between treatments, while the levels of casein showed differences (p<0.05) between milk below 750,000 SCC (treatments 1 and 2) and milk above 750,000 SCC (treatments 3 and 4). The reduction of casein and CN/ TP probably occurs by partial degradation of casein, particularly of β-casein by more intense proteolytic activity of plasmin in high SCC milk. The values of soluble nitrogen (SN) and soluble protein (PS) increased (p<0.05) with increasing SCC. The higher values of the soluble fractions seem to be a clear indication of a intense proteolytic activity of plasmin, coupled with a low integrity of the casein micelle, due to the solubility of β-casein in cold milk.
Moslehishad et al. [40] found no significant difference in the content of total nitrogen (TN) and casein (CN) at three levels of SCC (<200, 200-800 and >800).
Separation and identification of major milk proteins by microfluidic chip electrophoresis
As a starting point, the analysis of the milk proteins of raw bovine milk was carried out using deionized water and two different buffers for the treatment of milk samples before the standard procedure recommended by the manufacturer of the electrophoresis equipment microfluidics. The two buffers compared were a total protein solubilization buffer (TPS buffer) and a separating milk protein buffer (SEP buffer). The first one is recommended for the preparation of milk samples before application in microfluidic chip electrophoresis [31,35] while the latter is commonly used for the separation of protein fractions of milk during the sample preparation for analysis by CE [60].
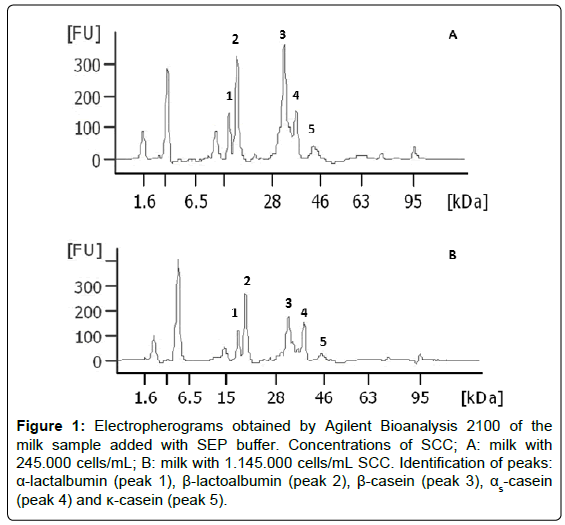
In order to identify the peaks corresponding to each of the protein fractions, the addition of individual protein standards to the sample of milk was carried out. The identification was confirmed by the observation of an increased signal of each one of the individual proteins added (Figure 1). Thus, the Figure 1, presented here in only for illustrative purposes, shows results from the percentage of total protein fractions of the samples with the highest and the lowest SCC respectively. The elution order is: α-lactalbumin (peak 1), β-lactoglobulin (peak 2), β-casein (peak 3), αs-casein (peak 4) and κ-casein (peak 5). The electropherograms are presented as fluorescence units (FU), the molecular weight (kDa) and migration time (FU × kDa; FU × Time). By comparing the signals detected in milk samples submitted at low and high SCC (Figure 1A and 1B, respectively), variations in the quantification of protein fractions are observed.
Figure 1: Electropherograms obtained by Agilent Bioanalysis 2100 of the milk sample added with SEP buffer. Concentrations of SCC; A: milk with 245.000 cells/mL; B: milk with 1.145.000 cells/mL SCC. Identification of peaks: α-lactalbumin (peak 1), β-lactoalbumin (peak 2), β-casein (peak 3), αs-casein (peak 4) and κ-casein (peak 5).
The literature Costa et al. [31] showed the addition of both the SEP and TPS buffers in the treatment of milk samples made it possible to separate different peaks corresponding to the major milk proteins with a good resolution. These results are explained because the milk caseins are dissociated by the addition of urea [61] and both buffers contained urea, the TPS buffer had a concentration of 2 mol L-1 and the SEP buffer had a concentration of 6 mol L-1 of urea, respectively [31].
Data of the average percentage of individual protein fractions of milk are displayed in Table 3. The microfluidic electrophoresis revealed that the greatest number of somatic cells significantly elevated the products generated by casein hydrolysis in milk. Figure 1 presents, in descending order, significant reductions (p<0.05) of percentage of β-casein (peak 3), α-casein (peak 4), and κ-casein (peak 5) of milk associated with SCC, which in turn produced higher concentration of the soluble fractions of milk (Table 2).
| Protein Fraction | Treatments1 | |||
| <300 | 300-750 | 750-1000 | >1000 | |
| α-lactalbumin | 7.88 ± 1.46a | 7.26 ± 0.57a | 7.89 ± 0.65a | 6.97 ± 0.80a |
| β-lactoglobulin | 17.64 ± 1.39a | 17.34 ± 1.29a | 16.77 ± 0.46a | 15.58 ± 1.55a |
| β-casein | 31.85 ± 1.37a | 27.08 ± 1.68b | 22.1 ± 1.30c | 16.35 ± 2.03d |
| αs-casein | 19.00 ± 1.57a | 18.08 ± 1.63a | 18.58 ± 0.90a | 13.00 ± 1.25b |
| κ-casein | 5.54 ± 1.52a | 5.19 ± 0.71a | 5.27 ± 1.27a | 3.47 ± 1.22b |
a-dMeans within a row with different superscripts differ (P<0.05)
Values are given as means ± standard deviation
1Treatments: <300=Tank milk with somatic cell count of <300000 cells/ mL; 300-750=Tank milk with somatic cell count of 300000 to 750000 cells/mL; 750-1000=Tank milk with somatic cell count of 750000 to 1000000 cells/mL; >1000=Tank milk with somatic cell count of >1000000 cells/mL
Table 3: Distribution of protein fraction from the soluble phase of tank milk with different levels of somatic cells after ultracentrifugation count.
Quantitative determination of major milk proteins by microfluidic chip electrophoresis
Approximately 80% of total nitrogen in bovine milk consists of casein. The bovine casein can be classified into four types of proteins with different properties: αs1, αs2, β and κ, comprising 38%, 10%, 34% and 15% of total casein, respectively [48]. It may be observed (Figure 1) a reduction in the β-casein fraction (the fraction most affected by the enzymatic action of plasmin), in the order of 15%, 18%, 26%, and 30% due to the elevation of SCC, it can be noted as well a total variation (between treatment 1 and 4) of approximately 48%. The migration of the β-casein from the aggregate micellar form to dispersed molecules in the soluble phase of milk is more intense at lower temperatures [33,62], becoming in turn, more susceptible to the enzymatic action of plasmin, decreasing its concentration and increasing the concentration of lower molecular weights peptides. As these peptides are of high solubility, they are carried by the whey during the cheese making process, significantly reducing milk yield. In addition, the texture of the cheese changes, because the reduction in the concentration of the β-casein in the micelle causes changes in the physicochemical properties of the cheese mass. High SCC causes serious technological problems in manufacturing dairy products. For example, in cheese manufacturing process, changes in the CN/SP of the milk (Table 2) due to the elevation of SCC, increases the clotting time, particularly by affecting the access of the enzyme to the κ-casein, and reducing the development of the proper pH. Moreover, the time to reach the draining point is lengthened, because the soluble components have higher water holding capacity, and high SCC refrain development of acidity, facts that reduce syneresis. These characteristics affect not only the manufacturing process but also significantly impair the standardization of each type of cheese.
The high proteolytic activity in milk from diseased udders likely leads to a reduction in the concentration of both α-CN and β-CN, with a simultaneous elevation of γ-CN concentration, with evidence that the hydrolysis of casein occurs within the udder previous to the milking process [63]. With respect to α-CN and κ-CN fractions, concentrations did not suffer significant interference, except in SSC over 1,000,000 (Table 3). However, Moslehishad et al. [40] studied the influence of three levels (<200 to >800) of somatic cells to examine the electrophoresis profile of milk using polyacrylamide gel electrophoresis (SDS-PAGE) and achieved significant reductions (p<0.05) of α-casein and β-casein fractions, with higher SCC.
Casein is considered to be the more important protein, as far as economical issue is concerned, due to its relation to the production of milk products. Mastitis can significantly affect the quality of dairy products. Rogers and Mitchell [64] reported that an increase in SCC impaired the sensory characteristics of nonfat yogurt. Munro et al. [51] found that yogurt obtained from milk with high SCC showed a color change, characterized as slightly yellow. Also, Oliveira et al. [65] showed a decrease in sensory quality of yogurt after 20 days of cold storage, especially in consistency and flavor attributes when milk with >800,000 cells/mL was used. Fernandes [37] observed elevated viscosity of yogurt obtained from milk with SCC >800,000 cells/mL after 10 days of storage. High SCC can also be correlated to a reduced quality of butter, and Auldist and Hubble [43] reported that SCC alter the composition of butter and elongate churning time. Sensory properties are also affected and butter deteriorates faster during storage. In the production of milk powder, Auldist et al. [46] reported that milk powder with high SCC has lower heat stability and that, other properties deteriorate more rapidly in comparison to milk powder with low SCC, which is highly probably due to more intense lipolysis and proteolysis [66-68].
High somatic cell counting significantly affect the protein fractions, particularly β-CN, with remarkable reduction of protein values, that directly affect the dairy industry, by causing economical losses, decreasing stability of fluid milk that leads to low thermal stability, lower yield and poorer sensory properties of milk products [69,70].
Conclusions
Milk with high somatic cell count undergoes several chemical changes. In general, total solids and solids not fat had a slight increase, whereas fat content had a significant increment. Lactose was reduced. The more intense changes occurred in the proteins. Crude protein had a small elevation in milk with SCC around 700,000 and decreasing with SCC above 1,000,000. Percentage of casein reduced and that of soluble proteins decreased which led to a considerable reduction of the Ratio casein/soluble proteins. The percentage of true proteins was lower and NPN had a remarkably increment in milk with higher SCC. Regarding the casein fractions, high SCC caused reduction in β and α and κ in descendent order. In the particular case of the β-casein, it reduced approximately 48% from milk with SCC lower than 300,000 to milk above 1,000,000. One may conclude with high certainty that the quality of milk is directly and negatively affected by high somatic cell counting.
References
- Eigel WN, Butler JE, Ernstrom CA, Farrell HM, Harwalker VR, et al. (1984) Nomenclature of proteins of cow’s milk: fifth revision. J Dairy Sci 76: 1599-1631.
- Dalgleish DG, Morris ER (1988) Interactions between carrageenans and casein micelles: electrophoretic and hydrodynamic properties of the particles. Food Hydrocoll 2: 311-320.
- Pabst K (1994) Organic milk, is the change worth while. Tierzuchter 46: 22-25.
- Ismail B, Nielsen SS (2011) Plasmin System in Milk. In: Fuquay JW, Fox PF, McSweeney PLH (Eds) Encyclopedia of Dairy Sciences. pp. 929-934.
- Fairbairn DJ, Law BA (1986) Proteinases of psychrotrophic bacteria: their production, properties, effects and control. J Dairy Res 53: 139-177.
- Recio I, Frutos M, Olano A, Ramos M (1996) Protein changes in stored ultra-high temperature-treated milks studied by capillary electrophoresis and high-performance liquid chromatography. J Agric Food Chem 44: 3955-3959.
- Fox PF, McSweeney PLH (2003) Advanced Dairy Chemistry: Volume 1: Proteins Parts A&B. Springer US, USA.
- Bastian ED, Brown RJ (1996) Plasmin in milk and dairy products: an update. Int Dairy J 6: 435-457.
- Lahteenmaki K, Kuusela P, Korhonen TK (2001) Bacterial plasminogen activators and receptors. FEMS Microbiol Rev 25: 531-552.
- Albenzio M, Caroprese M, Santillo A, Marino R, Muscio A, et al. (2005) Proteolytic patterns and plasmin activity in ewes' milk as affected by somatic cell count and stage of lactation. J Dairy Res 72: 86-92.
- Fox PF, Mcsweeney PLH (1996) Proteolysis in cheese during ripening. Food Reviews International 12: 457-509.
- Crudden A, Fox PF, Kelly AL (2005) Factors affecting the hydrolytic action of plasmin in milk. Int Dairy J 15: 305-313.
- Fox PF, Kelly AL (2006) Indigenous enzymes in milk: Overview and historical aspects - Part 2. Int Dairy J 16: 517-532
- Sgarbieri VC (2005) Revisão: Propriedades estruturais e físico-químicas das proteínas do leite. Brasilian Journal of Food Technology. Campinas. 8: 43-56.
- Souza MJ, Ardo Y, Mcsweeney PLH (2001) Advances in the study of proteolysis during cheese ripening. Int Dairy J 11: 327-345.
- Larsen LB, Hinz K, Jorgensen AL, Moller HS, Wellnitz O, et al. (2010) Proteomic and peptidomic study of proteolysis in quarter milk after infusion with lipoteichoic acid from Staphylococcus aureus. J Dairy Sci 93: 5613-5626.
- Santos MV, Ma Y, Barbano DM (2003) Effect of somatic cell count on proteolysis and lipolysis in pasteurized fluid milk during shelf-life storage. J Dairy Sci 86: 2491-2503.
- Philpot NW, Nickerson SC (1991) Mastitis: counter attack. Babson Bro, USA.
- Kitchen BJ (1981) Review of the progress of dairy science: bovine mastitis: milk compositional changes and related diagnostic tests. J Dairy Res 48: 167-188.
- Fox PF, Morrissey PA (1981) Enzymes and food processing. In: Birch GC, Blakeborough N, Parker KJ (Eds) Enzymes and Food Processing. Applied Science Publishers, London, UK, 213-238.
- Andrews AT, Olivercrona T, Bengtssonolivercrona G, Fox PF, Bjorck L, et al. (1991) Indigenous enzymes in milk. In: Fox PF (Ed) Food Enzymology. Elsevier Applied Science, New York, USA, 1: 53-129.
- Datta N, Deeth HC (2001) Age gelation of UHT milk-a review. Food and Bioproducts Processing 79: 197-210.
- Politis I, Barbano DM, Gorewit RC (1992) Distribution of plasminogen and plasmin in fractions of bovine milk. J Dairy Sci 75: 1402-1410.
- Eigel WN (1977) Effect of bovine plasmin on alpha-S1-B and kappa-A caseins. J Dairy Sci 60: 1399-1403.
- Grufferty MB, Fox PF (1988) Milk alkaline proteinase. J Dairy Res 55: 609-630.
- Le Bars D, Gripon JC (1993) Hydrolysis of as 1-casein by bovine plasmin. Lait 73: 337-344.
- Rauh VM, Anja S, Mette B, Richard I, Marie P, et al. (2014) Plasmin activity as a possible cause for age gelation in UHT milk produced by direct steam infusion. Dairy J 38: 199-207.
- Rauh VM, Johansen LB, Ipsen R, Paulsson M, Larsen LB, et al. (2014) Plasmin activity in UHT milk: relationship between proteolysis, age gelation, and bitterness. J Agric Food Chem 62: 6852-6860.
- Fernandes AM, Moretti TS, Bovo F, Lima CG, Oliveira CAF (2008) Effect of somatic cell counts on lipolysis, proteolysis and apparent viscosity of UHT milk during storage. Int J Dairy Technol 61: 327-332.
- Lemieux L, Simard RE (1992) Bitter flavour in dairy products. II. A review of bitter peptides from caseins: their formation, isolation and identification, structure masking and inhibition. Lait 72: 335-385.
- Costa FF, Brito MAVP, Furtado MAM, Martins MF, de Oliveira MAL, et al. (2014) Microfluidic chip electrophoresis investigation of major milk proteins: study of buffer effects and quantitative approaching. Analytical Methods 6: 1666-1673.
- AOAC (2005) Official methods of analysis of the Association of the Official Analytical Chemists. AOAC, Gaithersburg.
- Costa FF, Resende JV, Abreu LR, Goff HD (2008) Effect of calcium chloride addition on ice cream structure and quality. J Dairy Sci 91: 2165-2174.
- Costa FF, Brito MAVP, Guimaraes MFM, Furtado MAM, de Oliveira MAL, et al. (2010) Protein distribution in a supernatant of milk ultra-centrifuged using lab-on-a-chip microfluid electrophoresis. In: 16 Latin-American Symposium LACE 2010, 2010, Florianópolis.
- Dooley J, Brown H, Wellum S, Burch B, Jasionowicz P (2010) Determining the milk content of milk-based food products. Food Standards Agency-FSA Final Report Q01117.
- Anema SG (2009) The use of lab-on-a-chip microfluid SDS electrophoresis technology for the separation an quantification of milk protein. Inter Dairy J 19: 198-204.
- Fernandes AM (2003) Avaliação do iogurte produzidos com leite contendo diferentes níveis de células somáticas. Tese (Mestrado em Zootecnia) - Faculdade de Zootecnia e Engenharia de Alimentos, Universidade de São Paulo, Pirassununga.
- Marques LT, Balbinoti M, Ficher V (2002) Variations in the Milk chemical compisition according to somatic cell count. Panamerican congress on milk quality and mastitis control, 2, Ribeirão Preto, Brazil.
- Klei L, Yun J, Sapru A, Lynch J, Barbano D, et al. (1998) Effects of milk somatic cell count on cottage cheese yield and quality. J Dairy Sci 81: 1205-1213.
- Moslehishad M, Hamid E, Mehdi A (2010) Chemical and electrophoretic properties of Holstein cow milk as affected by somatic cell count. Inter J Dairy Technol 63: 512-515.
- Lee SC, Yu JH, Jeong CL, Back YJ, Yoon YC (1991) The influence of mastitis on the quality of raw milk and cheese. Korean Journal of Dairy Science 13: 217-223.
- El-Tahawy AS, El-Far AH (2010) Influences of somatic cell count on milk composition and dairy farm profitability. Int J Dairy Technology 3: 463-469.
- Auldist MJ, Hubble IB (1998) Effects of mastitis on raw milk and dairy products. Aust J Dairy Technol 53: 28-36.
- Verdi RJ, Barbano DM, Dellavalle ME, Senyk GF (1987) Variability in true protein, casein, nonprotein nitrogen, and proteolysis in high and low somatic cell milks. J Dairy Sci 70: 230-242.
- Rogers SA, Slattery SL, Mitchell GE, Hirst PA, Grieve PA (1989) The relationship between somatic cell count, composition and manufacturing properties of bulk milk III. Individual proteins. Aust J Dairy Technol 44: 49-52.
- Auldist MJ, Coats S, Sutherland BJ, Mayes JJ, McDowell GH, et al. (1996) Effects of somatic cell count and stage of lactation on raw milk composition and the yield and quality of Cheddar cheese. J Dairy Res 63: 269-280.
- Silva PLF, Pereira AR, Machado PF, Sarries GA (2000) Effects of somatic cell levels on milk components II-lactose and total solids. Braz J Vet Res Anim Sci 37: 1678-4456.
- Fox PF, Guinee TP, Cogan, TM, McSweeney PLH (2000) Fundamentals of cheese science. Springer, New York.
- Miller RH, Emanuelsson U, Persson E, Brolund L, Philipsson J, et al. (1983) Relationships of milk somatic cell counts to daily milk yield and composition. Acta Agric Scand 33: 209-223.
- Mitchell GE, Fedrick IA, Rogers SA (1986) The relationship between somatic cell count, composition and manufacturing properties of bulk milk. 2. Cheddar cheese from farm bulk milk. Aust J Dairy Technol 41: 12-14.
- Munro GL, Rieve GPA, Kitchen BJ (1984) Effects of mastitis on milk yield, milk composition, processing properties and yield and quality of milk products. Aust J Dairy Technol 39: 7-16.
- Najafi MN, Mortaza SA, Koocheki A, Khorami J, Rekik B (2008) Fat and protein contents, acidity and somatic cell counts in bulk milk of Holstein cows in the Khorasan Razavi Province, Iran. Int Dairy J 62: 19-26.
- Santos MV, Oliveira CAF, Lima YVR, Botaro BG (2006) Somatic cell removal by microfiltration does not affect composition and proteolysis of milk. Ciência Rural 36: 1486-1493.
- Ma Y, Ryan C, Barbano DM, Galton DM, Rudan MA, et al. (2000) Effects of somatic cell count on quality and shelf-life of pasteurized fluid milk. J Dairy Sci 83: 264-274.
- O'Connell JE, Grinberg VY, de Kruif CG (2003) Association behavior of beta-casein. J Colloid Interface Sci 258: 33-39.
- Pirisi A, Piredda G, Podda F, Pintus S (1996) Effect of somatic cell count on sheep milk composition and cheesemaking properties. Somatic Cells and Milk of Small Ruminants. EAAP Publication, Wageningen Pers, Wageningen, The Netherlands 77: 245-251.
- Pellegrini O, Remeuf F, Rivemale M, Barillet F (1997) Renneting properties of milk from individual ewes: influence of genetic and non-genetic variables, and relationship with physicochemical characteristics. J Dairy Res 64: 355-366.
- Nudda A, Feligini M, Battacone G, Macciotta NPP, Pulina G (2003) Effects of lactation stage, parity, ß-lactoglobulin genotype and milk SCC on whey protein composition in Sarda dairy ewes. Italian Journal of Animal Science 2: 29-39.
- Albenzio M, Caroprese M, Santillo A, Marino R, Taibi L, et al. (2004) Effects of somatic cell count and stage of lactation on the plasmin activity and cheese-making properties of ewe milk. J Dairy Sci 87: 533-542.
- Gouldsworthy AM, Banks JM, Law AJR, Leaver J (1990) Casein degradation in Cheddar cheese monitored by capillary electrophoresis. Milk Science International 54: 620-623.
- Hames BD, Rickwood D (1998) In: 4th edn, Gel Electrophoresis of Proteins: A Practical Approach, Oxford University Press, pp. 98-145.
- Trejo R, Harte F (2010) The effect of ethanol and heat on the functional hydrophobicity of casein micelles. J Dairy Sci 93: 2338-2343.
- Zafalon LF, Nader FA, Carvalho MRB, Lima TMA (2008) Influence of bovine subclinical mastitis on milk protein fractions. Arq Inst Biol São Paulo 75: 135-140.
- Rogers SA, Mitchell GE (1994) The relationship between somatic cell count, composition and manufacturing properties of bulk milk. 6. Cheddar cheese and skim milk yoghurt. Aust J Dairy Technol 49: 70-74.
- Oliveira CAF, Fernades AM, Neto COC, Fonseca LFL, Silva EOT, et al. (2002) Composition and sensory evaluation of whole yogurt produced from milk with different somatic cell counts. Aust J Dairy Technol 57: 192-196.
- Aslam M, Hurley WL (1997) Proteolysis of milk proteins during involution of the bovine mammary gland. J Dairy Sci 80: 2004-2010.
- Barbano DM, Clark JL (1990) Kjeldahl method for determination of total nitrogen content of milk: collaborative study. Journal AOAC International 73: 849-859.
- Considine T, Healy A, Kelly AL, Mcsweeney PLH (2002) Proteolytic specificity of cathepsin G on bovine α- and แบ?-caseins. Food Chemistry 79: 59-67.
- Lima MCG, Sena MJ, Mota RA, Mendes ES, Almeida CC, et al. (2006) Somatic cell count and physicochemical and microbiological analyzes of raw milk produced in the wild type c region of Pernambuco state. Arq Inst Biol 73: 89-95.
- Walstra P, Vliet VT (1986) The physical chemistry of curd making. Netherlands Milk and Dairy Journal 40: 241-259.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17098
- [From(publication date):
October-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12355
- PDF downloads : 4743