Effect of Pre-operative Oral Gabapentin on Postoperative Pain in Opioiddependent Patients Undergoing Orthopedic Surgeries of the Lower Extremity: A Randomized Double-blind Placebo-controlled Trial
Received: 10-Dec-2018 / Accepted Date: 03-Jan-2019 / Published Date: 11-Jan-2019 DOI: 10.4172/2167-0846.1000336
Abstract
Background: The aim of this study is to investigate the effect of a single dose of gabapentin, 1200 mg and one hour before surgery on postoperative pain in opioid-dependent patients undergoing lower extremity orthopedic surgeries.
Methods: In this randomized double-blind clinical trial, 64 opioid-dependent patients, candidates of elective lower extremity orthopedic surgery at Chamran Hospital, Shiraz, Southern Iran, were recruited and randomly assigned to intervention (G) - 1200 mg single oral dose of gabapentin an hour before surgery - and placebo (P) groups. Twenty four hour pain intensity (NRS) after surgery at 2, 4, 6, 12, 18, and 24 hour post-operative, time to the first request for analgesic and total amount of prescribed morphine were measured. The side effects of gabapentin and morphine were recorded at the mentioned time intervals. The patients’ global satisfaction of the pain management was also assessed.
Results: The patients in group G requested analgesic significantly later than the patients in group P (p=0.003). In the first postoperative hour group G received significantly lower amounts of morphine (p=0.038). Otherwise, no significant difference was detected between the two groups regarding the pain intensity and the amount of administered morphine. The frequency of drug-related side effects and the patients' global satisfaction were not significantly different in the two groups of the study.
Conclusions: Oral gabapentin (1200 mg single bolus) can reduce the need for morphine and pain intensity only shortly (about an hour) after the operation in opioid-dependent patients undergoing orthopedic lower-extremity surgeries.
Keywords: Postoperative pain; Opioid-dependency; Gabapentin
Introduction
Postoperative pain management is one of the important challenges that the anesthesiologists can face with. Improper pain control after surgery may lead to undesirable acute or chronic complications. Painful perioperative stimuli can cause reactions that may ultimately increase the risk of morbidity and mortality. Therefore, reducing postoperative pain can alleviate the frequency and severity of untoward side effects. Among the surgical procedures, orthopedic interventions, especially on lower extremities, are considered severe painful procedures as reported by patients (NRS ≥ 7). A multidimensional approach toward the postoperative pain control can facilitate rehabilitation and recovery, as well as reducing the duration of the hospital stay. The effect of opioid compounds on moderate to severe postoperative pain through influencing μ-receptors of the central nervous system has been previously proven. Theoretically, one of the pain-alleviating advantages of opioids is that they do not have a dose limit; however, the level of tolerance is limited because of complications such as nausea, vomiting, sedation, and reduced respiratory system function [1,2].
The issue of postoperative pain control in opioid-dependent patients could be more complicated and often requires a multidimensional approach. These patients have some obstacles to perfect pain control due to opioid induced hyperalgesia and pain sensitivity, tolerance and decreased opioid effectiveness and finally increased sympathetic activity due to opioid withdrawal [3]. It has been shown that the amount of pain-killer drug requirement is about 2-3 times higher than in opioid-naïve patients [4]. Therefore, the risk of opioid-related side effects may also be higher in this population. In recent decades, researchers have focused on non-opioid drugs for pain control such as local anesthetics and analgesics, acetaminophen, non-steroidal antiinflammatory drugs, anti-epileptics, N-methyl-D-aspartate receptor antagonists, alpha-2 adrenergic receptor agonists and corticosteroids [5].
Gabapentin is an antiepileptic drug considered as the structural analog of the neurotransmitter gamma-amino butyric acid. While some studies suggest that gabapentin does not reduce postoperative pain score and opioid analgesic consumption, in multiple others research it has been shown that preemptive gabapentin was effective in controlling postoperative pain and nausea and vomiting [6]. So it is used for treating chronic painful conditions such as those seen in neuropathies [7]. Also different studies have shown its effectiveness in reducing postoperative pain in wide range of surgical procedures (8-16). We aimed to assess the effect of a single oral dose of gabapentin; 1200 mg administered an hour before surgery on postoperative pain in opioid-dependent patients undergoing orthopedic surgeries of the lower extremity.
Methods
This trial has been registered in IRCT by the registration number of IRCT2014122019470N4 at April 1, 2015 by Saeed Khademi). In this randomized double-blind clinical trial, 64 opioid-dependent patients aged 20-70 with ASA Class I or II, scheduled for elective surgical fixation of lower-extremity fractures at Chamran Hospital, Shiraz, southern Iran, were recruited. Patients screened for opioid dependency according to universally accepted Diagnostic and Statistical Manual for Mental Disorders Fourth Edition, Text Revision (DSM-IV, TR). Current opium user were those who inhaled the smoke of ignited opium and/or ate it in crude form (both types are common among the addicts) for at least three times a week and had been used opium within 4 weeks of surgery.
The protocol of the study was approved by the Ethics Committee of Shiraz University of Medical Sciences. Moreover, written informed consent was obtained from each participant.
Patients with known allergy to gabapentinoids, liver or kidney function insufficiency, history of advanced peripheral nerve disease, or those who were unable to cooperate were excluded from study.
Patients were taught about the method of pain assessment- the Numerical Rating Scale (NRS)-as well as how to use the Patient- Controlled Analgesia (PCA) pumps. They were randomly assigned to gabapentin (G) or Placebo (P) groups using the block randomization method. Gabapentin and placebo capsules, similar in shape and color, were placed in covered packages labeled with the patients’ consecutive numbers according to their participation in the study, by the only person aware of the study groups. Other colleagues as well as the patients were blinded to the type of the capsules prescribed. Considering a type I error of 5% and a power of 80%, thirty two participants in each group were calculated.
After recording the patients’ personal characteristics and the other basic data, such as age, sex, weight, type of fracture, type of opioids used and route of consumption, an hour before surgery the patients in group G received 4 capsules of gabapentin (Biopentine 300 mg, Bakhtar Bioshimi, Kermanshah, Iran) while the other group patients (P) received the same number of placebo capsules.
At the operating theater first the standard monitoring devices (electrocardiogram, pulse oximeter, and non-invasive blood pressure manometer) were established. Then midazolam 50 μg.kg-1 and fentanyl 2 μg.kg-1 were administered and anesthesia was induced by thiopental sodium 5 mg.kg-1 and muscle relaxation by atracurium 0.6 mg.kg-1 for tracheal intubation. Anesthesia was maintained by isoflurane and a 50:50 mixture of oxygen and nitrous oxide. At the beginning of surgery all patients received 0.1 mg.kg-1 intravenous morphine. Each time during the maintenance phase of anesthesia the patient’s heart rate and systolic arterial blood pressure increased to more than 20% of the baseline values, an intravenous bolus of fentanyl, 1 μg.kg-1, was injected and the total amount was recorded. At the end of the surgical procedure muscle relaxation was reversed by neostigmine and atropine and the patient was transferred to the recovery room.
The patient’s pain was evaluated every 15 minutes by NRS during his/her stay in recovery room. Time interval to the first request for analgesic medication (measured from the patient's arrival to recovery room) was recorded. Whenever in recovery room or surgical ward (despite the self-administration of morphine through the PCA pump) the patient reported a pain intensity of more than 7, two milligrams of morphine sulfate were injected intravenously and this amount was repeated every 5 minutes while monitoring vital signs and the level of consciousness until pain intensity dropped to less than 7. If the patient’s pain severity was 4 to 7, 1 mg morphine was injected every 5 minutes until the pain score value declined to less than 4. These amounts of morphine were also recorded. After discharge from recovery room and in the surgical ward a PCA pump loaded by a syringe contained morphine sulfate was prepared for intravenous selfinjection by the patient with the following setup: bolus dose of 2 mg, lockout interval of 6 minutes without background infusion. The patient was asked to call acute pain service personnel each time he/she felt a pain with intensity of more than 4 despite using the PCA pump. In such condition the above mentioned pain control protocol would be followed and the extra amount of administered morphine was recorded.
In the ward, the patient's pain intensity was evaluated 2, 4, 6, 12, 18, and 24 hours after operation by NRS as the primary outcome of the study. The side effects of gabapentin and morphine were also recorded at these junctures. Sedation score was assessed using Wilson’s criteria and nausea and vomiting was measured by a three point scale: 1 (none), 2 (nausea), and 3 (nausea and vomiting). Urinary retention and pruritus were reported as: 1=negative, 2=mild and 3=severe). The frequency of other complications such as diplopia, chills, and respiratory depression were also recorded.
The total amount of morphine both self-administered by the pump and the extra amount which injected by the personnel were recorded. At last, the patient’s satisfaction of the method of pain control was assessed: 1=completely dissatisfied, 2=partially dissatisfied, 3=partially satisfied and 4=completely satisfied. Any serious complication of the medication used in the study was treated according to the local protocols of the Chamran Hospital.
Statistical analysis
The collected data was analyzed by SPSS software version 19. To assess the normality of gathered data, Kolmogorov-Smirnov test was used. Also, to compare the variables between two groups, chi-square, independent sample t-test, Mann-Whitney U tests were applied. A two sided p-value of less than 0.05 was considered statistically significant.
Results
Two of 64 patients both from gabapentin group were declined to finish the study since they used opioids at their own will (Figure 1).
Table 1 shows the demographic data and information about addiction and type of fracture in the two groups of the study. As can be seen no significant differences were observed between the demographic data.
| Group P (n=32) | Group G (n=30) | Variable |
|---|---|---|
| 32/0 | 30/0 | Male/female ratio |
| 41.4 ± 12.7 | 39.4 ± 11.8 | Age, years (mean ± SD) |
| 63.8 ± 8.7 | 67.3 ± 12.4 | Weight, kg (mean ± SD) |
| Type of fractures | ||
| 4 | 2 | Hip |
| 5 | 6 | Femur |
| 1 | 2 | Knee |
| 19 | 17 | Tibia |
| 3 | 3 | Ankle |
| Type of drug abused | ||
| 78.12% | 73.33% | Opium |
| 21.88% | 26.67% | Other drugs |
| Route of consumption | ||
| 68.80% | 66.70% | Inhalational |
| 28.10% | 33.40% | Oral |
| 3.10% | 0 | Intravenous |
Table 1: Demographic data and other baseline values of the patients in two groups.
Mean (± SD) fentanyl dosage administered during the surgery in response to hemodynamic changes was 50 μg (± 61.59) in group G and 42.19 μg (± 63.64) in group P (p=0.625, t test). In the first hour after surgery, 11 (36.7%) patients in group G and 23 (71.9%) patients in group P requested analgesics (p=0.005, Chi-square test). Mean (± SD) time to the first request for analgesic medication was 66.17 minutes (± 32.69) in group G and 40.13 minutes (± 33.31) in group P (p=0.003, Mann-Whitney test). Total amount of morphine administered in the first hour after surgery in the recovery room for groups G and P were 3.87 mg (± 8.25) and 5.03 mg (± 5.36), respectively (p=0.038, Mann- Whitney test).
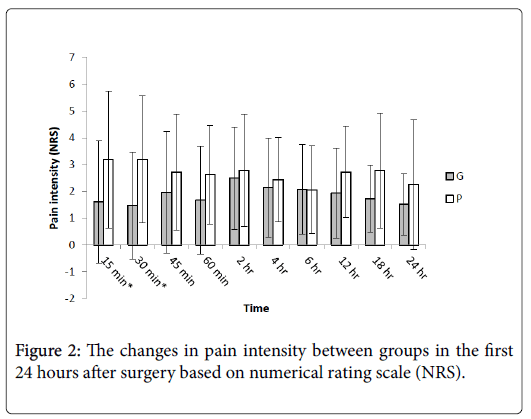
Figure 2 reveals the changes in pain intensity in the two groups during the first 24 hour after surgery measured by NRS. Comparing each pair of data related to the mentioned time intervals by independent t-test showed that the only significant differences were related to 15 and 30 minutes after surgery (p=0.013 and 0.003, respectively).
The total amount of morphine (mean ± SD values) administered within 24 hours, including the medication administered by the recovery room personnel, PCA pumps, and additional doses injected by acute pain service staff members according the mentioned protocol was 40.62 mg (± 30.04) in group G and 49.43 mg (± 28.02) in group P (p=0.24, independent t-test).
Table 2 shows the level of patient’s satisfaction from pain control methods in the two groups. No significant difference was observed in this regard. It should be noted that none of the patients of both groups reported any adverse drug reaction considered in this study.
| P value | Group P | Group G | |
|---|---|---|---|
| completely dissatisfied | |||
| 3 (9.5%) | 0 | ||
| 4 (12.5%) | 4 (13%) | partially dissatisfied | |
| 0.258 | 8 (25%) | 12 (40%) | partially satisfied |
| 17 (53%) | 14 (47%) | completely satisfied |
Table 2: The level of patient’s satisfaction from pain control methods in two groups.
Discussion
Pain control after surgery has always been an important issue for anesthesiologists, which has remained a serious problem despite recent advances in pain control methods. So far, many studies have been conducted to find out the effect of adjuvant medications prescribed before, during and after surgery on postsurgical pain control.
The present study shows that fewer patients in the gabapentin group (received 1200 mg orally before surgery) requested morphine in the first hour after surgery and longer mean time to the first request for this drug in the gabapentin group and also lower amount of morphine administered in the first hour after surgery in the recovery room. There was also a significant difference in pain severity between the two groups only at 15 and 30 minutes after surgery. Otherwise, during the first 24 hours the two groups had the same amount of morphine consumption and pain intensity.
The effect of oral gabapentin administered pre-operatively on postsurgical pain control has been the subject of numerous studies, using this compound in different surgical procedures and according to different protocols.
Turan and colleagues compared 1200 mg oral gabapentin administered pre-operatively with placebo in lower extremity surgeries and have found that in the gabapentin group, pain intensity was lower 1, 4, 8, 12, 16 hours after surgery with less paracetamol consumption and less PCA requirement [8-10]. Similarly have Montazeri et al. reported less postoperative pain 2, 4, 12, and 24 hours after surgery. On the other hand, some studies have established no effect for preoperationally administered gabapentin on postoperative pain management. Adam and colleagues administered 800 mg gabapentin 2 hours before shoulder arthroscopy and concluded no difference in postoperative pain scores and analgesic requirement, compared to placebo. Radhakrishnan have also demonstrated no effect for 800 mg gabapentin on postoperative pain of lumbar laminectomy and discectomy, although the result of the later studies may denote the inefficiency of lower doses of gabapentin [11-17].
In the present study gabapentin had only a short term effect on relieving postoperative pain, only during the first hour after the surgical procedures. The almost same results obtained by Panah akhahi et al. in a research on open fixation of tibia fracture in healthy and non-drug dependent patients [18]. This finding can be related to the limited duration of the action of a single dose gabapentin used in this pain intolerant population of patients and the painful nature of orthopedic procedure; per se. Maximum plasma level of the drug is achieved 3-3.2 hours after ingestion [19]. May be more steady plasma levels resulted from multiple dose administration of the drug are needed to better show the real effect of gabapentin on postoperative pain relieve in this population of patients. Salehi et al. showed that long term use of gabapentin (1600 mg/d for three weeks) could effectively reduce withdrawal symptoms of opiate addicted patients during methadone-assisted detoxification [20]. The growing evidence reveals that continuing gabapentinoids to postoperative period could be more effective than just a single preoperative dose [21]. Using more prolonged and multi-dose drug regimens extended to postoperative period such as those used in Turan (2006) or Gilron (2005) studies in opiate addicted patients could be the subject of future studies [22,23].
Recent studies have shown some antiemetic activity for gabapentin during postoperative period [24,25] but the present study cannot address the issue since the participants are among the low risk population for PONV (smokers and male gender) and also we know that prolonged opioid consumption can gradually lead to tolerance to the adverse effects of these compounds [26,27].
We concluded that 1200 mg oral gabapentin reduces opioid requirement and postoperative pain intensity during the first hour after lower extremity surgeries in opioid-dependent patients. Yet, according to the special characteristics of substance-dependent patients, more studies considering the different protocols of gabapentin administration are seem to be required.
References
- Gerbershagen HJ, Aduckathil S, Wijck AJ, Peelen LM, Kalkman CJ, et al. (2013) Pain intensity on the first day after surgery a prospective cohort study comparing 179 surgical procedures. Anesthesiology 118: 934-944.
- Quinlan J, Cox F (2017) Acute pain management in patients with drug dependence syndrome. Pain Rep 2: 611.
- Marshall S, Jackson M (2011) Acute pain management for opioid tolerant patients. WFSA 2011: 35-39.
- Gilron I, Orr E, Tu D, Mercer CD, Bond D (2009) A randomized, double-blind, controlled trial of perioperative administration of gabapentin, meloxicam and their combination for spontaneous and movement-evoked pain after ambulatory laparoscopic cholecystectomy. Anesth Analg 108: 623-630.
- Chang CY, Challa CK, Shah J, Eloy JD (2014) Gabapentin in acute postoperative pain management. Biomed Res Int 2014: 1-7.
- Kong V, Irwin M (2007) Gabapentin: A multimodal perioperative drug? Br J Anaesth 99: 775-786.
- Turan A, Kaya G, KaramanlioÄŸlu B, Pamukcu Z, Apfel C (2006) Effect of oral gabapentin on postoperative epidural analgesia. Br J Anaesth 96: 242-246.
- Al-Mujadi H, A-Refai AR, Katzarov MG, Dehrab NA, Batra YK, et al. (2006) Preemptive gabapentin reduces postoperative pain and opioid demand following thyroid surgery. Can J Anaesth 53: 268-273.
- Mikkelsen S, Hilsted KL, Andersen PJ, Hjortso NC, Enggaard TP, et al. (2006) The effect of gabapentin on post-operative pain following tonsillectomy in adults. Acta Anaesthesiol Scand 50: 809-815.
- Adam F, Ménigaux C, Sessler DI, Chauvin M (2006) A single preoperative dose of gabapentin (800 milligrams) does not augment postoperative analgesia in patients given interscalene brachial plexus blocks for arthroscopic shoulder surgery. Anesth Analg 103: 1278-82.
- Turan A, White PF, Karamanlioglu B, Pamukçu Z (2007) Premedication with gabapentin: the effect on tourniquet pain and quality of intravenous regional anesthesia. Anesth Analg 104: 97-101.
- Montazeri K, Kashefi P, Honarmand A (2007) Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singapore Med J 48: 748.
- Mohammadi SS, Seyedi M (2008) Comparing oral gabapentin versus clonidine as premedication on early postoperative pain, nausea and vomiting after general anesthesia. Int J Pharmacol 4: 153-156.
- Mohammadi SS, Seyedic M (2008) Effects of gabapentin on early postoperative pain, nausea and vomiting in laparoscopic surgery for assisted reproductive technologies. Pak J Biol Sci 11: 1878.
- Pandey C, Priye S, Ambesh S, Singh S, Singh U, et al. (2006) Prophylactic gabapentin for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. J Postgrad Med 52: 97.
- Radhakrishnan M, Bithal PK, Chaturvedi A (2005) Effect of preemptive gabapentin on postoperative pain relief and morphine consumption following lumbar laminectomy and discectomy: a randomized, double-blinded, placebo-controlled study. J Neurosurg Anesthesiol 17: 125-128.
- Panah Khahi MYAA, Marashi SH (2011) Effect of pre-emptive gabapentin on postoperative pain following lower extremity orthopaedic surgery under spinal anaesthesia. Singapore Med J 52: 879-882.
- Rose MA, Kam PC (2002) Gabapentin: pharmacology and its use in pain management. Anaesthesia 57: 451-462.
- Salehi M, Kheirabadi GR, Maracy MR, Ranjkesh M (2011) Importance of gabapentin dose in treatment of opioid withdrawal. J Clin Psychopharmacol 31: 593-596.
- Schmidt PC, Ruchelli G, Mackey SC, Carroll IR (2013) Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology 119: 1215-1221.
- Turan A, White PF, Karamanlioglu B, Memis D, Tasdogan M, et al. (2006) Gabapentin: an alternative to the cyclooxygenase-2 inhibitors for perioperative pain management. Anesth Analg 102: 175-181.
- Gilron I, Orr E, Tu D, O'Neill JP, Zamora JE, et al. (2005) A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain 113: 191-200.
- Achuthan S, Singh I, Varthya SB, Srinivasan A, Chakrabarti A, et al. (2015) Gabapentin prophylaxis for postoperative nausea and vomiting in abdominal surgeries: a quantitative analysis of evidence from randomized controlled clinical trials. Br J Anaesth 114: 588-597.
- Heidari M, Honarmand A, Safavi M, Chitsazi M, Khalighinejad F (2015) Geranisetron versus gabapentin in preventing postoperative nausea and vomiting after middle ear surgery in adults: A double-blinded randomized clinical trial study. Adv Biomed Res 4: 22.
- Collett BJ (1998) Opioid tolerance: the clinical perspective. Br J Anaesth 81: 58-68.
- Dumas EO, Pollack GM (2008) Opioid tolerance development: a pharmacokinetic/ pharmacodynamic perspective. AAPS J 10: 537-551.
Citation: Khademi S, Farbood A, Sabokseir E, Ghani M (2018) Effect of Pre-operative Oral Gabapentin on Postoperative Pain in Opioiddependent Patients Undergoing Orthopedic Surgeries of the Lower Extremity: A Randomized Double-blind Placebo-controlled Trial. J Pain Relief 7:336. DOI: 10.4172/2167-0846.1000336
Copyright: © 2019 Khademi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4249
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3421
- PDF downloads: 828