Research Article Open Access
Effect of Metal Stress due to Strontium and The Mechanisms of Tolerating it by Amaranthus caudatus L.
Ramasubramanian Venkatachalam*
Department of Plant Biology and Plant Biotechnology, Nadar Janaki Ammal College, Sivakasi-626124, Tamil Nadu, India
- *Corresponding Author:
- Venkatachalam R
Department of Plant Biology and Plant Biotechnology
Nadar Janaki Ammal College, Sivakasi-626124
Tamil Nadu, India
Tel: 91-948756899
E-mail: drvrams@gmail.com
Received date May 29, 2016; Accepted date July 12, 2016; Published date July 19, 2016
Citation: Venkatachalam R (2016) Effect of Metal Stress due to Strontium and The Mechanisms of Tolerating it by Amaranthus caudatus L. Biochem Physiol 5:207. doi: 10.4172/2168-9652.1000207
Copyright: © 2016 Venkatachalam R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Heavy metals are major environmental pollutants when present in high concentration in soil and have toxic effects on growth and development of plants. Experiment were carried out to find out the effect of different levels of strontium element commonly used in the fireworks – cottage industries of this area and the predominant pollutant on growth, biochemical and enzymatic characteristics of Amaranthus caudatus L. widely cultivated in this area. This study is aimed at assessing the stress tolerant ability of Amaranthus caudatus L. The seedlings of Amanranthus caudatus L. were treated with various concentration of strontium (2 mM, 4 mM, 6 mM, 8 mM, 10 mM) for ten days and its effect on the morphometric, biochemical and enzymatic characters were analyzed. After ten days of treatment, the growth parameters such as leaf area, fresh weight, dry weight, shoot and root length were found decreased than in the control. Biochemical characteristics such as the content of chlorophyll, carotenoid, soluble sugar and protein also decreased with the increase in the concentration of strontium. In contrary, the content of free amino acid, proline, leaf nitrate and the activities of anti-oxidative enzymes such as catalase and peroxidase were found increased with the increase in the concentration of strontium while the activity of nitrate reductase was found decreased. The result suggest that Amaranthus caudatus L. has been affected adversely by metal stress due to strontium but at the same time the plant adopts mechanisms such as accumulation of anthocyanin and enhanced activities of antioxidant enzymes to overcome the ill effects of the metal ions.
Keywords
Strontium; Amanranthus caudatus L.; Free amino acid; Proline; Catalase; Peroxidase
Introduction
Heavy metals are defined as group of elements that have specific weights higher than about 5 g/cm3. Iron, Mn, Mo, Ni, Zn and Cu are essential micronutrients required for normal growth and metabolic processes in plants [1]. Cd, Pb, Cr and Hg are nonessential and highly toxic to plants [2]. Heavy metals inhibit physiological processes such as respiration, photosynthesis, cell elongation and affect plant water relationship as well as mineral nutrition [3]. The most common heavy metal contaminants are Cd, Cr, Cu, As, Hg, Pb and Ni. Other naturally occurring metallic elements with high molecular weight are also considered as important pollutants. These elements occur either naturally in soil or get deposited through the use of agricultural chemicals, urban waste and polluted water [4]. As metals cannot be broken down, when concentrations within the plant exceed optimal levels, they adversely affect the plant both directly and indirectly. Some of the direct toxic effects caused by high metal concentration include inhibition of cytoplasmic enzymes and damage to cell structures due to oxidative stress [5]. Heavy metal toxicity is known to injure cell membranes, reduces transpiration, causes the breakdown of the protein synthesis, damages the photosynthesis apparatus, inhibits the photosynthetic rate, and affects the activity of several enzymes [6]. Heavy metals are known to interfere with chlorophyll synthesis either through direct inhibition of an enzymatic step or by inducing deficiency of an essential nutrient [7]. Reactive oxygen species (ROS) are continuously produced at low level during normal metabolic processes [8]. But in biological systems, increasing the synthesis of ROS is one of the initial responses to different stress factors [9]. ROS induce damage to the biomolecules through peroxidation of membrane lipids, alteration of protein functions, DNA mutation, damage to chlorophyll and disruption of metabolic pathways (electron transport chain and ATP production) [10]. Therefore the tolerance of plants to stress conditions depends on their ability to make balance between the production of toxic oxygen derivatives and capacity of antioxidative defense systems. Therefore, plants have complex ROS scavenging mechanisms at the molecular and cellular levels. These mechanisms with inhibition or slowing the oxidation of biomolecules and oxidative chain reactions [11] decrease the cellular oxidative damage and increase resistance to heavy metals. Pb induces toxicity in plants in terms of their growth, development, and biochemical attributes. Primary effects of Pb toxicity in plants include stunted root growth, probably due to inhibition of cell division in root tips. Secondarily, it induces oxidative stress via reactive oxygen species generation and results in cellular damage [12]. Farrag et al. [13] noticed the stress of heavy metals on some physiological parameters of Amaranthus hybridus, Chenopodium ambrosioides, Mentha longifolia and Typha domingensis. The expression of proteins in stressed plant was significantly decreased at all the levels compared to unstressed plant samples. Heavy metals increase the activities of catalase, glutathione peroxidase and glutathione reductase. Recently Muradoglu et al. [14] reported that the excessive Cd reduced chlorophyll contents, increased antioxidant enzyme activities and change plant nutrition concentrations in both roots and leaves. The higher concentration of Cd has negative effect on chlorophyll content and nearly decreased 30% of plant growth in strawberry. The present experiment was undertaken to investigate the changes in the level of growth, biochemical aspects, and enzymatic characteristics in Amaranthus caudatus L. treated with strontium and to assess its stress tolerating efficacy. This crop may further be useful in soil reclamation through the process of phytoremediation. In the present day context our study is very crucial because the soil in our area remains highly polluted due to strontium indiscriminately used in fireworks industries which effects most commonly cultivated Amaranthus caudatus L. - the chief crop of vegetable source of the locals. Thus there is an urgent necessity to understand the ill effects of strontium on the crop mentioned and the necessary steps to taken to overcome the problem.
Materials and Methods
Plant material
Amaranthus caudatus L. was selected for present study for its economic importance since it is cultivated in large scale in Virudhunagar district, Tamil Nadu, India.
Experimental design
Healthy viable and uniform sized seeds of Amaranthus caudatus L. were selected and were surface sterilized for 20 min with 1% (v/v) sodium hypochlorite, and then washed several times with distilled water. Both control and experimental plants were allowed to grow in the soil. After ten days of growth, they were treated with various concentration of strontium (2 mM, 4 mM, 6 mM, 8 mM,10 mM v/v) for seven days and the growth parameters such as root length, shoot length, leaf area, fresh weight and dry were analyzed. The various biochemical and enzyme activities were estimated following the methods proposed by those mentioned within the bracket. Chlorophyll and carotenoids [15], Anthocyanin [16], Total soluble sugar [17], Protein content [18], amino acid content [17], Proline [19], leaf nitrate [20] in vivo nitrate reductase activity [21], peroxidase and catalase activity [22].
Statistics Analysis
The Growth parameters were determined with ten independent replicates. Biochemical characters and enzymatic assay were carried out for five times. The data reported as mean ± SE and within parentheses represent the per cent activity. Statistical analysis (One way ANOVA – Tukey test) was applied using the statistical package, SPSS 16.0.
Results and Discussion
The results obtained have been summarized and discussed as follows.
Effect of strontium on plant growth
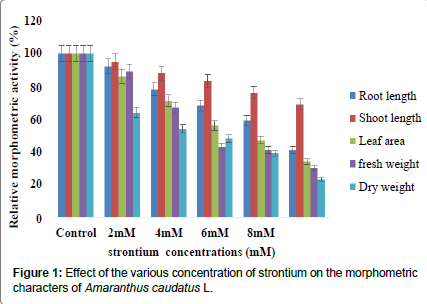
Amaranthus caudatus L. seedling exposed to different concentration of strontium exhibited inhibition of morphometric characters such as root length, shoot length, leaf area, fresh weight and dry weight compared to the control. The reduction of seedling growth was 41%, 69%, 35%, 30% and 23% respectively at 10mM concentration compared to the control (Figure 1 and Table 1). Reduced root length is due to reductions in both new cell formation and cell elongation in the extension region of the root [23]. The reduced root length of plants on metal treatments could be due to the reduction in mitotic cell division in meristematic zone [24]. This is in agreement with the findings of Soleimani et al. [25] who stated that the amount of accumulated Pb in tall fescue and Bermude grass (Cynodon dactylon) were higher in roots compared to the shoots. Likewise, similar response to lead treatment was previously noticed in water hyacinths. The plant growth was significantly inhibited (50%) at 1000 mg/L Pb concentration. Similar observations were also recorded under Mentha arvensis [26]. The same trend was observed earlier by other workers [27-30]. Accumulation of Pb was high in root than in shoot tissues [31]. Similar changes in the content by various metal treatments were recorded by Muradoglu et al. [14] with cadmium.
Effect of strontium on photosynthetic pigments
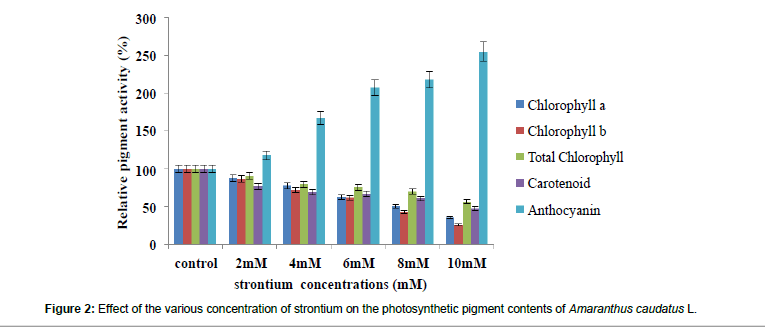
Chlorophyll is the important photosynthetic pigment which plays a vital role in the photosynthetic process. A significant decrease in Chlorophyll a, b and total chlorophyll contents was observed in the range of 64%, 74%, 43%, respectively at the maximum concentration (Figure 2 and Table 2). Similarly, carotenoids content also got significantly decreased to the range of 62% (Figure 2 and Table 2). But in case of anthocyanin it was significantly increased. This reduction in the growth and photosynthetic pigments could be due to the disturbance in photosystem I and induced activity of chlorophyllase enzyme [32]. The protective function of anthocyanin against the stress conations is fairly clears [33]. Similarly, observations have been made in many plants species [34-36]. Other results that support what has been shown here, are those by Kapoor et al. [37] with his study on Brassica juncea L. under Cd stress, Rastgoo et al. [38] with their study on Aeluropus littoralis under copper, nickel and zinc. It has been proposed that Cu at toxic concentration interferes with enzymes associated with chlorophyll biosynthesis and protein composition of photosynthetic membranes [39]. Many studies have shown the decrease of photosynthetic pigments under high concentration of metals [40-47].
Effect of strontium on biochemical characters
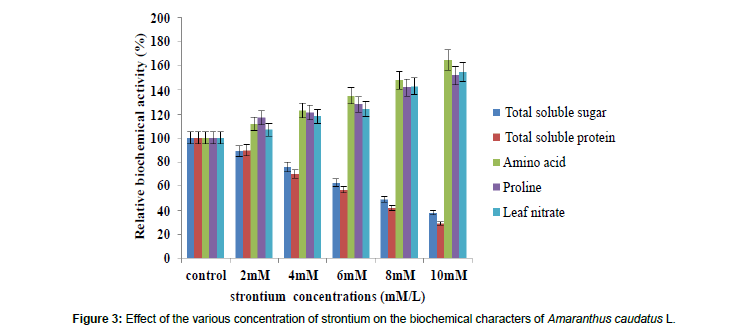
The total soluble sugar and protein contents got significantly decreased with increasing concentration of strontium. At 10 mM concentration the levels of reduction was 63% and 71% respectively while leaf nitrate, free amino acids, proline (Figure 3 and Table 3) contents was significantly increased with the increasing metal concentration. Under the heavy metal treatment, there was a considerable reduction in the growth and photosynthetic pigments, which could be due to the disturbance in photosystem I and induced activity of chlorophyllase enzyme. This disturbance paralleled with the reduction in sugar content that could be attributed to reduction in chlorophyll contents of the leaf and also a decline in protein. This change might have already affected the photosynthetic activity in the plant and hence the reduction in carbohydrate contents [32]. Similar observation was reported by Somashekaraiah et al. [48] in Phaseolus vulgaris and Neelu et al. (2000) in Vicia faba treated with Cd. A reduction in leaf protein indicated the reduction in RUBACase, which caused a reduction in photosynthetic activity, which in turn, affects the total soluble sugar level [49]. As a result of protein degradation during stress condition, the availability of free amino acid is significantly high. It may be due to destruction of protein or due to the biosynthesis of amino acid from the nitrate sources which were not utilized in the protein synthesis [50]. Accumulation of proline has been frequently used as biochemical marker for water stress in plants [51]. The free proline has been shown to protect plants against free radicle induced damage by quenching of singlet oxygen [52]. The results of the present study were confirmed by Vinod et al. [29] who noticed similar reduction in protein due to Cu and Zn. Gautam et al. [36] with their study on Spinacea oleracea L. under Zinc treatment, Rastgoo et al. [38] by their study on Aeluropus littoralis under copper, nickel and zinc, Muradoglu et al. [14] by their study on strawberry under Cd. Bhupendra et al. [53] also observed the ill effect of arsenic, and chromium on in vitro seed germination of black gram (Vigna mungo L.) and green gram (Vigna radiata L.) reducing protein content. The results of the present study was confirmed by Ravikumar and Thamizhiniyzn [54] who observed proline changes in black gram seedlings by under in Pb stress. Moreover, Sharma [55] in his study on Brachythecium populeum proved the impact of heavy metals on some physiological parameters like total chlorophyll, sugar, protein content and confirmed the inhibitory effect of heavy metal on biochemical contents.
| Growth Parameters | Control | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM |
|---|---|---|---|---|---|---|
| Root length (cm) | 11.33 ± 0.176 (100) |
10.43 ±0.088a* (92) |
9.56 ± 0.084a* (78) |
8.46 ± 0.066a* (69) |
7.26 ± 0.054a* (59) |
5.06 ± 0.145a* (41) |
| Shoot length(cm) | 15.46 ± 0.240 (100) |
14.70 ± 0.057a* (95) |
13.66 ± 0.066a* (88) |
12.76 ±0.088a* (83) |
11.76 ± 0.088a* (76.) |
10.60 ± 0.057a* (69) |
| Leaf Area (cm2) | 6.88 ± 0.026 (100) |
5.89 ± 0.038a* (86) |
4.93 ± 0.026a* (72) |
3.84 ± 0.023a* (56) |
�? 3.23 ± 0.003a* (47) |
2.38 ± 0.293a* (35) |
| Fresh Weight (gm) | 1.99 ± 0.052 (100) |
1.77 ± 0.098a* (89) |
1.33 ± 0.294a* (70) |
0.87 ± 0.005a* (44) |
0.81 ± 0.046a* (41) |
0.59 ± 0.008a* (30) |
| Dry Weight (gm) | 0.38 ± 0.014 (100) |
0.24 ± 0.008a* (64) |
0.20 ± 0.008a* (54) |
0.18 ± 0.003a* (48) |
0.15 ± 0.005a* (39) |
0.086 ± 0.003a* (23) |
Values in parameters indicate percent activity; values are represents means of five observation. Values in parentheses are activity with respect to control. Mean (±) SE
a - refers to values compared with control in various concentrations of metals, a* - refers to significant (P = 0.05 ��? Tukey test)
a# - refers to non ��? significant
Table 1: Impact of various concentration of strontium on the morphometric characteristics of Amaranthus caudatus L.
Effect of strontium exposure on antioxidant enzyme activities
| Growth Parameters | Control | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM |
|---|---|---|---|---|---|---|
| Chlorophyll .a (mg/gLFW) |
15.28 �?± 0.199 (100) |
13.38 �?± 0.122 a* (88) |
11.97 �?± 0.063a* (78) |
9.69 �?± 0.053a* (63) |
7.81 �?± 0.143a* (51) |
5.55 �?± 0.048a* (36) |
| Chlorophyll.b (mg/gLFW) |
10.23 �?± 0.104 (100) |
8.81 �?± 0.010 a* (87) |
�? 7.35 �?± 0.121 a* (72) |
6.30 �?± 0.122a* (61) |
4.42 �?± 0.072a* (43) |
2.67 �?± 0.257 a* (26) |
| Total.Chlorophyll (mg/gLFW) |
25.51 �?± 0.271 (100) |
23.03 �?± 0.132 a* (91) |
20.34 �?± 0.074 * (79) |
19.3 �?± 0.254a* (76) |
17.76 �?± 0.056 a* (64) |
14.56 �?± 0.070 a* (57) |
| Carotenoids (mg/gLFW) |
7.85 �?± 1.722 (100) |
6.04 �?± 0.276 a* (77) |
5.39 �?± 0.289 a* (68) |
5.27 �?± 0.037a* (54) |
4.82 �?± 0.003a* (46) |
3.75 �?± 0.003 a* (38) |
| Anthocyanin (�?µg/gLFW) |
2.22 �?± 0.058 (100) |
2.62 �?± 0.045 a* (118) |
3.71 �?± 0.213a* (167) |
4.63 �?± 0.029 a* (208) |
4.86 �?± 0.016 a* (219) |
5.68 �?± 0.023 a* (256) |
Values in parameters indicate percent activity; values are represents means of five observation. Values in parentheses are activity with respect to control. Mean (�?±) SE
a - refers to values compared with control in various concentrations of metals, a* - refers to significant (P = 0.05 ��? Tukey test)
a# - refers to non ��? significant
Table 2: Impact of various concentration of strontium on the pigments characteristics of Amaranthus caudatus L.
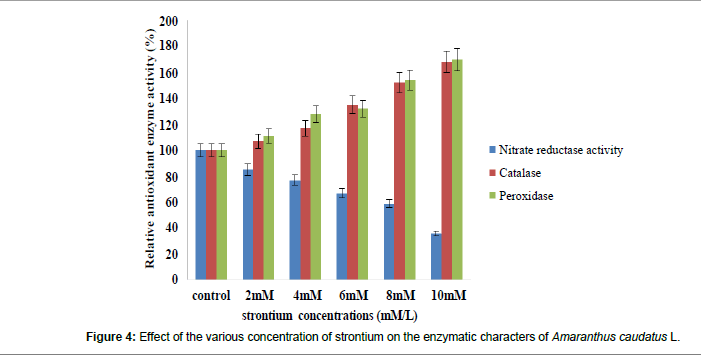
The notable decrease in in vivo nitrate reductase activity of 63% at 10 mM concentration of strontium was noticed. There is a dramatic rise in catalase and peroxidase activities after exposure to metal stress (Figure 4 and Table 4). Catalase activity was found significantly increased in all the experiment plants than the control plants. The increase was about 35% at 6 mM. Peroxidase activity was significant increased in all treatments. The increase was about 33% at 6 mM. Plant cells are equipped with a protective system including antioxidant enzymes like catalase and peroxidase which can flush free radicals [56]. Reduction in in vivo NR activity with increased concentration of cadmium on Vigna radiata has been reported earlier [57]. Catalase is an antioxidant and scavenging enzyme and it is activity was found increased with the increasing concentration of strontium. Catalase is special type of peroixdase enzyme which catalase the degradation of H2O2, which is a natural metabolite toxic to plants [58]. Sharma [55] in his study on Brachythecium populeum, proved that the under the heavy metal stress, catalase and peroxidase enzymes activities were significantly high. In addition, certain changes was observed earlier under cadmium on strawberry [14]. Results of the study by Farrag et al. [13] on heavy metal analysis with an increase in catalase, peroxidase enzyme activity with increasing heavy metal concentration supporting our findings. Recently Malar et al. [31] reported that catalase, peroxidase enzyme activities were increased under high concentration of Pd treatment.
| Growth Parameters | Control | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM |
|---|---|---|---|---|---|---|
| Total Soluble sugar (mg/gLFW) |
7.77 ±0.030 (100) |
6.90 ±0.032a* (89) |
5.92 ±0.017a* (76) |
4.92 ±0.020 a* (63) |
3.83 ±0.085 a* (49) |
2.92 ±0.017 a* (37) |
| Total Soluble Protein (mg/gLFW) |
4.85 ±0.049 (100) |
4.35 ±0.033a* (90) |
3.41 ±0.088a* (70) | �? 2.75±0.047a* (57) |
2.02 ±0.030 a* (42) |
1.41 ±0.012 a* (29) |
| Amino acid (�?µ mole/g LFW) |
2.37 ±0.204 a* (100) |
2.68 ±0.191 a* (112) |
3.07 ±0.057a* (123) |
3.32 ±0.128a* (135) |
4.31 ±0.147a* (148) |
4.75 ±0.017 (165) |
| Proline (�?µ mole/g LFW) |
3.27 ±0.025 (100) |
3.84 ±0.008a* (117) |
4.505 ±0.035a* (121) |
5.43 ±0.001a* (128) |
5.83 ±0.003 a* (142) |
7.44 ±0.005 a* (152) |
| Leaf Nitrate (�?µg/gLFW) |
5.34 ±2.100 (100) |
5.84 ±0.026a* (107) |
6.44 ±0.057a* (118) |
6.75 ±4.150a* (124) |
7.77 ±0.033 a* (143) |
8.44 ±0.103 a* (155) |
Values in parameters indicate percent activity; values are represents means of five observation. Values in parentheses are activity with respect to control. Mean (±) SE a - refers to values compared with control in various concentrations of metals, a* - refers to significant (P = 0.05 â�?�? Tukey test). a# - refers to nonâ�?�?significant.
Table 3: Impact of various concentration of strontium on the biochemical characteristics of Amaranthus caudatus (L).
Conclusion
The present study provides data on the toxicity of strontium on morphological, biochemical parameters and antioxidant enzymes of Amaranthus caudatus L. Our data suggest that the toxic effects of strontium invokes a strong inhibition of root length, shoot length, leaf area, fresh weight, dry weight, photosynthetic pigments (Chlorophyll a, b, total chlorophyll and carotenoids), protein but a significant enhancement of anthocyanin, proline, free amino acids, leaf nitrate. Likewise the activity of nitrate reductase got significantly reduced while activity of catalase and peroxidase were increased [59]. Our result suggest that strontium in high concentration makes the soil toxic to the plants and results in growth inhibition, structural damage, decline in physiological and biochemical activities of plants. As the same time plants adopt certain damage controlling mechanisms such as increasing the activity of antioxidant enzymes and accumulation of anthocyanin as a protective measure helping them to overcome the ill effect of the metal stress.
| Parameters | Control | 2 mM | 4 mM | 6 mM | 8 mM | 10 mM |
|---|---|---|---|---|---|---|
| Nitrate Reductase (�?µ mole/g LFW) |
9.60 ± 0.274 (100) |
8.16 ± 0.059 a* (85) |
7.46 ± 0.148a* (78) |
6.49 ± 0.105 a* (68) |
5.69 ± 0.016a* (60) |
3.51 ± 0.038 a* (37) |
| Catalase activity (�?µ mole/g LFW) |
3.80 ± 0.005 (100) |
4.08 ± 0.003 a* (107) |
4.46 ± 0.035a* (117) | �? 5.14 ± 0.033 a* (135) |
6.91 ± 0.020a* (152) |
7.76 ± 0.041 a* (166) |
| Peroxidase activity (�?µ mole/g LFW) |
2.57 ± 0.023 (100) |
2.88 ± 0.039 a* (111) |
3.16 ± 0.009a* (128) |
3.406 ± 0.018a* (132) |
4.38 ± 0.023a* (154) |
5.23 ± 0.021 a* (170) |
Values in parameters indicate percent activity; values are represents means of five observation. Values in parentheses are activity with respect to control. Mean (±) SE a - refers to values compared with control in various concentrations of metals, a* - refers to significant (P = 0.05 â�?�? Tukey test). a# - refers to non â�?�? significant
Table 4: Impact of various concentration of strontium on the enzyme activity characteristics of Amaranthus caudatus (L.)
Acknowledgement
The author is very much thankful to Dr. V. Ramasubramanian, Associate Professor and Head Department of Plant Biology and Plant Biotechnology, ANJA College, Sivakasi. Dr. R. Sevugaperumal, Associate Professor of Botany, ANJA College, Sivakasi for their guidance and The management of ANJA College, Sivakasi for providing necessary facilities to carry out the present study.
References
- Vangronsveld J, Clijsters H (1994) Toxic effects of metals, plants and the chemical elements.Biochemistry, uptake, tolerance and toxicity. Verlagsgesellschaft, Weinheim 150-177.
- Sebastiani I, Scebba F, Tongttti R (2004) Heavy metal accumulation and growth responses in poplar clones Eridano (Populusdeltoidesmaximowiczii) and I-214 (P. euramericana) exposed to industrial waste. Environmental and Experimental Botany 52: 79-86.
- Zornoza P, Vazquez S, Esteban E, Fernandez-Pascual M, Carpena R (2002) Cadmium-stress in nodulated white lupin: Strategies to avoid toxicity. Plant Physiology Biochemistry 40: 103-107.
- Yadav SK (2010) Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76: 167-179.
- Jadia CD, Fulekar MH (2009) Phytoremediation of heavy metals: recent techniques. African Journal of Biotechnology 8: 921-928.
- John P, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod 3: 65-76.
- Van Assche, Clijsters H (1990) Effect of metals on enzyme activity in plants. Plant Cell Environment 13: 195-206.
- Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. CurrSci 82: 1227-1238.
- Singh S, Sinha S (2005) Accumulation of metals and its effects in Brassica juncea (L.) Czern (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicol Environ Saf 62: 118-127.
- Semane B, Dupae J, Cuypers A, Noben JP, Tuomainen M, et al. (2010) Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J Plant Physiol 167: 247-254.
- Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15: 523-530.
- Kaur G (2014) Pb-Induced toxicity in plants: effect on growth, development and biochemical attributes. Journal of Global Biosciences 3:881-889.
- Farrag HF, Yasin M, Al-Sodany, Otiby FG (2014) Effect of heavy metal pollution on protein expression, enzyme activity, pigments and phytohormones in some plants growing in WadiAlargy wetlands, Taif, Saudi Arabia. Life Sci J 11:148-155.
- Muradoglu F, Gundogdu M,Ercisli S, Encu T, Balta F, et al. (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biological Research 48:11.
- Wellburn AR, Lichtenthaler H (1984) Advances in photosynthesis Research. MartinusNijhoff Co, The Hague.
- Mancinelli AL, Lunguist PHY, Anderson DR, Rabino I (1973) Photocontrol of anthocyanin synthesis. The action of streptomycin on the synthesis of chlorophyll. Plant Physiol 55: 251��?257.
- Jeyaraman J (1981) Laboratory manual in Biochemistry. Willey-Eastern Company Limited, Madras 1-65.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of the proline in water stress studies. Plant and Soil 39: 205��?208.
- Cataldo DA, Garland TR, Wildung RE (1978) Nickel in plants. I. Uptake kinetics using intact soybean seedlings. Plant Physiology and Biochemistry 62: 563-565.
- Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochemistry Biophysics Research Communication. 43: 1274-1279.
- Kar M, Mishra D (1976) Catalase, Peroxidase and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol 57: 315-319.
- Shafiq M, Kabir M, Iqbal MZ, Faroozi ZR (2008) Reduction in germination and seedling growth of Thespesiapopulina, caused by lead and cadmium treatment. Pak J Bot 40: 2419-2426.
- Godbold DL, Kettner C (1991) Lead influences root growth and mineral nutrition of Piceaabies seedlings. J Plant Physiol 139: 95-99.
- Soleimani M, Hajabbasi MA, Afyuni M, Charkhabi AH, Shariatmadari H (2009) ��?Bioaccumulation of nickel and lead by Bermuda grass (Cynodondactylon) and tall fescue (Festucaarundinacea) from two contaminated soils�. Caspian Journal of Environmental Science 7: 59-70.
- Manikandan R, Venkatachalam P (2011) Risk assessment of mercury ion heavy metal exposure on physiological and biochemical changes and DNA damage using RAPD analysis in Menthaarvensis seedlings. Plant Cell Biotech MolBiol 12: 41��?50.
- Islam E, Liu D, Li T, Yang X, Jin X, et al. (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtziaargyi. J Hazard Mater 154: 914-926.
- Akinci IE, Akinci S (2010) Effect of chromium toxicity on germination and early seedling growth in melon (Cucumismelo L.). African Journal of Biotechnology 9: 4589-4594.
- Vinod K, Awasthi G, Chauhan PK (2012) Cu and Zn tolerance and responses of the Biochemical and Physiochemical system of Wheat. Journal of Stress Physiology and Biochemistry 8: 203-213.
- Tandon PK, Vikram A (2014) Toxic effects of chromium on growth and metabolism of Oryzasativa (Rice) plants. J BiolChem Research 31: 970-985.
- Malar S, Vikram SS, Favas PJC, Perumal V (2014) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhorniacrassipes (Mart.)]. Botanical Studies 55: 54.
- Swaminathan K, Arjunan J, Gurusamy R (1998) Effect of glucose factory effluents on seed germination and seedling development of groundnut (Arachishypogaea L.). The Academy of Environmental Biology 81-87.
- MishraAgarwal (2006) Interactive effects between supplemental ultraviolet ��? B radiation and heavy metals on the growth and biochemical characteristics of Spinaciaoleracea. Braz J Plant Physiol 18: 742-748.
- Piotrowska A, Bajguz A, Godlewska B, Czerpak R, Kaminska M (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffiaarrhiza (Lamnaceae). Environ Exp Bot 66: 507��?513.
- Singh RP, Agrawal M (2010) Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol Environ Saf 73: 63��?641.
- Gautam S, Kannaujiya P, Srivastava MN (2015) Growth and biochemical responses of spinach (Spinaceaoleracea L.) grown in Zn contaminated soils. Int J Rec Biotech 3: 7-12.
- Kapoor D, Kaur S, Bhardwaj R (2014) Physiological and biochemical changes in Brassicajunceaplants under Cd-induced stress. BioMed Research International 1-13.
- Rastgoo L, Alemzadeh A, Tale AM, Tazangi SE, Eslamzadeh T (2014) Effects of copper, nickel and zinc on biochemical parameters and metal accumulation in gouan, Aeluropuslittoralis. Plant Knowledge Journal 3: 31-38.
- Singh D, Nath K, Sharma YK (2007) Response of wheat seed germination and seedling growth under copper stress. Environ Biol 28: 409-414.
- Thapar R, Srivastava AK, Bhargava P, Mishra Y, Rai LC (2008) Impact of different abiotic stress on growth, photosynthetic electron transport chain, nutrient uptake and enzyme activities of Cu-acclimated Anabaena doliolum. J Plant Physiol 165: 306-316.
- Sharma S, Sharma P, Datta SP, Gupta V (2009) Morphological and biochemical response of Cicerarietinum (PUSA-256) towards an excess zinc concentration. Afric J Basic ApplSci 1: 105-109.
- Ghani A (2010) Effect of Lead Toxicity on growth, chlorophyll and Lead (Pb+) contents of two varieties of maize (Zea mays L.). Pak JNutr 9: 887-891.
- Revathi K, Harbabu TE, Sudha PN (2011) ��?Phytoremediation of chromium contaminated soil using sorghum plant�. International Journal of Environmental Sciences 2: 417-428.
- Sinha J, Shrivastava S (2012) Pot experiment study showed the effect of Pb and Cd in Brassica juncea L. by Chlorophyll and Ascorbic acid content estimation. Journal of Current Pharmaceutical Research 9: 33-36.
- Dey S, Mazumder PB, Paul SB (2014) Effect of copper on growth and chlorophyll content in tea plants (Camellia sinensis (L.) O. Kuntze). International Journal of Research in Applied 2: 223-230.
- Kalaikandhan R, Vijayarengan P, Sivasankar R, Mathivanan S (2014) The pigment content of Sesuviumportulacastrum L. under copper and zinc stress. Int J CurrMicrobiol App Sci 3: 1056-1066.
- Bahri NB, Laribi B, Soufi S, Rezgui S, Bettaieb T (2015) Growth performan ce, photosynthetic status and bioaccumulation of heavy metals by Paulownia tomentosa (Thunb.) Steud growing on contaminated soils. International Journal of Agronomy and Agricultural Research 6: 32-43.
- Somashekaraiah BV, Padmaja K, Prasad A (1992) Phototoxicity of cadmium ions on germinating seedlings of Mung bean (Phaseolus vulgaris): Involvement of lipid peroxoides in chlorophyll degradation, Plant Physiol 85: 85-89.
- Goodwin FW, Mercer FT (2005) Introduction to plant biochemistry. Pergamon Press, New York.
- Sharma SS, Schat HA, Vooijs R (1997) Effect of heavy metals on the amino acid composition of Phaesolus vulgaris. Plant Soil 78: 139-148.
- Schat H, Sharma SS, Voojis R (1997) Heavy metal induces accumulation of proline in metal tolerant and non-tolerant ecotypes of Silenevulgaris. Physiol Plant 101: 477-482.
- Matysik J, Bhalu BA, Mohanty P (2002) Molecular mechanisms of quencing of reactive oxygen species by proline under stress in plants. CurrSci 82: 525-532.
- Bhupendra, Kiran, Rizvi G (2014) Effect of arsenic, manganese and chromium on in vitro seed germination of black gram (Vignamungo L.) and green gram (Vigna radiate L.). J Chem Pharm Res 6: 1072-1075.
- Ravikumar S, Thamizhiniyzn P (2014) Impact of lead on growth, bio chemical and enzymatic changes in black gram. Int Res J Pharm App Sci 4:1-3.
- Sharma S (2009) Study on impact of heavy metal accumulation in Brachytheciumpopuleum. BSGecological indicators 9: 807��?811.
- Cho VH, Park JO (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156: 1-9.
- Jayakumar S, Ramasubramanian V (2009) Bioremoval of chromium using seaweeds as biosorbents. J Basic and App Biol 3: 121��?128.
- Balasimha D (1982) Regulation of peroxidase in higher plants a review. Plant Physiology and Biochemistry 9: 130-143.
- Suganya M, Karthi S, Shivakumar MS (2016) Effect of cadmium and lead exposure on tissue specific antioxidant response in Spodopteralitura. Free Radicals and Antioxidants 6: 90-100
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 11272
- [From(publication date):
October-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10496
- PDF downloads : 776