Research Article Open Access
Effect of Heavy Metal Uptake by E. coli and Bacillus sps
| Vijayadeep C1* and Sastry PS2 | |
| 1Research Scholar, Department of Microbiology, Maharani Lakshmi Ammanni College for Women, Science PO, Malleswaram, Bangalore, Bangalore university, India | |
| 2HOD, Department of Botany & Microbiology, Vijaya College, Bangalore, India | |
| Corresponding Author : | Vijayadeep C Research Scholar, Department of Microbiology Maharani Lakshmi Ammanni College for Women Science PO, Malleswaram, Bangalore Bangalore University, India Tel: 9886755403 E-mail: vijayadeep1979@gmail.com |
| Received May 19, 2014; Accepted July 14, 2014; Published July 18, 2014 | |
| Citation: Vijayadeep C, Sastry PS (2014) Effect of Heavy Metal Uptake by E. coli and Bacillus sps. J Bioremed Biodeg 5:238. doi:10.4172/2155-6199.1000238 | |
| Copyright: © 2014 Vijayadeep C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Over the past century, unrestricted mining, extensive industrialization, modern agricultural practices and faulty waste disposal methods have resulted in the release of unprecedented levels of toxic heavy metals like Cd, Hg, Ag, Sn, Pb, Cu, Co, Mn, Zn, etc into the environment. Many metals are essential for microbial growth in less concentration, yet are toxic in higher concentrations. Biosorption is an attractive alternative approach which involves the binding or adsorption of heavy metals to living or dead cells. Many microbes have the ability to selectively accumulate metals.
The present study is intended to analyze the uptake systems of Bacillus and E. coli against different conc. of heavy metals like Zn, Cu, Cd, and Hg in their salt form incorporated into nutrient broth medium observed over a regular interval of time. Analysis was based on how much of the metal from the original conc. Used was left behind in the media after the rest being up taken by the organism. This was done using AAS which was indirectly the representation of percent uptake of heavy metal by the respective organism.
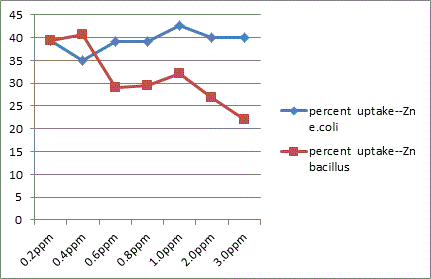
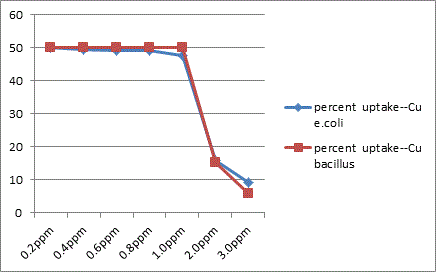
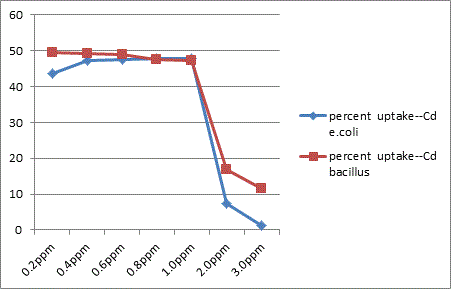
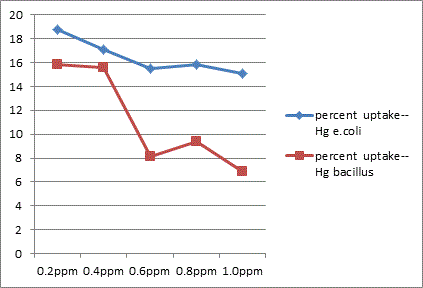
The study showed that Gram –ve organisms like E. coli exhibited more resistance to metals like Zn, Cu and Hg in relative comparison with Gram +ve organisms like Bacillus. Bacillus sps was less sensitive to effect of Cd than in E. coli.
The present study is intended to analyze the uptake systems of Bacillus and E. coli against different conc. of heavy metals like Zn, Cu, Cd, and Hg in their salt form incorporated into nutrient broth medium observed over a regular interval of time. Analysis was based on how much of the metal from the original conc. Used was left behind in the media after the rest being up taken by the organism. This was done using AAS which was indirectly the representation of percent uptake of heavy metal by the respective organism.
The study showed that Gram –ve organisms like E. coli exhibited more resistance to metals like Zn, Cu and Hg in relative comparison with Gram +ve organisms like Bacillus. Bacillus sps was less sensitive to effect of Cd than in E. coli.
Looking into the increased environmental awareness, removal of toxic heavy metals is of prime importance and relevance for a healthy environment. Removal strategies are both in terms of conventional and biosorption methods. A wide variety of microbes have good potentials of metal absorption/adsorption. Metal transport systems in microbes are of varying specificity. Rates of uptake can depend on the physiological state of cells, as well as the nature and composition of the environment or growth medium. With toxic heavy metals, permeabilization of cell membranes can result in further exposure of intracellular metal-binding sites and increase passive accumulation. Intracellular uptake may ultimately result in death of sensitive organisms unless a means of detoxification is induced or already possessed [2]. Different microbes have varied capacity of metal uptake in differing concentrations based on their relative tolerance levels. The present work is oriented with the following objectives as to study growth kinetics of bacterial cells under heavy metal environment and to help the formulation of new possibilities in bacterial biosorbents of heavy metals. Hence an attempt was made in the present study to see the biosorption potentials of two selected organisms Bacillus sps and E. coli
Four heavy metals in their salt form Zinc sulphate (ZnSO4.7H2O), Copper sulphate (CuSO4.5H2O), Cadmium chloride (CdCl2) and Mercuric chloride (HgCl2) were used in ppm concentrations.
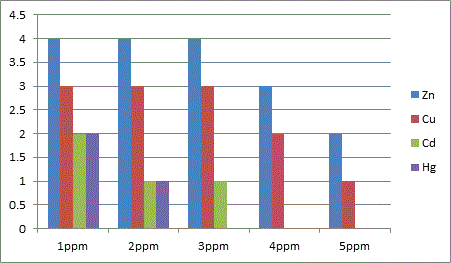
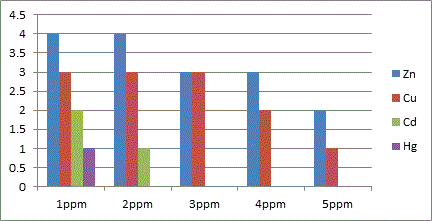
Initial screening with relatively high concentration of each of the heavy metallic salts (1-5 ppm) was carried out to assess the broad range effect of the heavy metal and its tolerance by the organisms with incubation at 37°C for 2, 4, 6 and 8 days period. After every 48 hour incubation, the media was subjected for turbidometric analysis to study the growth of the organism in the presence of the heavy metal under a particular concentration. A set of flasks were also maintained as control with no heavy metal for all the days. Nutrient agar medium was also employed to substantiate the effect of heavy metal on the growth pattern of the organisms. A direct comparison for the effect of a particular concentration of a metal on both the organisms was available from this technique. Equally this method also supported the selection of conc. of heavy metal for its sub lethal dosage. Sub lethal dosages were fixed as 0.2, 0.4, 0.6, 0.8, 1.0, 2.0 and 3.0 ppm for each metal except for Hg where the concentration was limited to 1 ppm. After incubation and turbidometric analysis, the samples were centrifuged at 4000-5000 rpm for 10 minutes to separate out the cell mass and the broth medium. The cell mass was discarded and the supernatant was used for the heavy metal analysis. The analysis was based on how much of the metal from the original concentration used was left behind in the media after the rest being absorbed/adsorbed by the organism. This was done using Atomic Absorption Spectroscopy (AAS) which was indirectly the representation of percent uptake of heavy metal by the respective organism. The results of percent uptake of heavy metal was calculated and tabulated.
Based on these results of broad range concentration of the selected heavy metals, further analysis was done with sub lethal dosages and effect of percent uptake of heavy metal was compared between E. coli and Bacillus.
In conclusion the present study highlights the microbes possess a high potential for metal remediation strategies and can minimize the bioavailability and biotoxicity of heavy metals. Gram negative bacteria like E. coli have proved to be good biosorbents for metals like Zn, Cu and Hg, while Bacillus can be a biosorbent of choice against Cd toxicity. These organisms can be exploited as ecological indicators for bioremedial purposes.
References
- Gadd GM (1990)Heavy metal accumulation by bacteria andother microorganisms.Experientia46: 834-840.
- Gourdon R, Bhende S, Rus E, Sofer SS (1990)Comparison of cadmium biosorption by Gram-positive and Gram-negative bacteria from activated sludge. Biotechnology Letters 12: 839-842.
- Gelmi M, Apostoli P, Cabibbo E, Porru S, Alessio L, et al. (1994) Resistance to cadmium salts and metal absorption by different microbial species. Curr Microbiol 29: 335-341.
- Hoyle BD, Beveridge TJ (1984) Metal binding by the peptidoglycan sacculus of Escherichia coli K-12. Canadian Journal of Microbiology30:204-211.
- Sastry PS, Choudhary BR (1988)Uptake of heavy metals by unicellalar green alga Closterium moniliferum (Bory) Ehrenb. Phykos 27:140-145.
- Ross IS (1993) Stress Tolerance in fungi, Marcel Dekker, New York, 1993(b) 237-257.
- Volesky B, Phillips MHA (1995)Biosorption of heavy metals by Saccharomyces cerevisiae. Applied Microbiology Biotechnology 42:797-806.
- Rajendran P, Gunashekaran P (2006) Microbial Bioremediation, MJP Publishers, Chennai, India, 282.
Figures at a glance
 |
 |
 |
| Graph 1 | Graph 2 | Graph 3 |
 |
 |
 |
| Graph 4 | Graph 5 | Graph 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 19699
- [From(publication date):
September-2014 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 14680
- PDF downloads : 5019
