Effect of Gestational Anemia on Haemoglobin Concentrations in Beninese Newborns at Birth
Received: 06-Aug-2019 / Accepted Date: 18-Jan-2020 / Published Date: 25-Jan-2020 DOI: 10.4172/2376-127X.1000426
Abstract
Although Gestational Anaemia (GA) is common in Benin, the period of gestation it is more harmful for the newborn is still unclear. In this study, the effect of timing of GA on newborn’s Haemoglobin (Hb) at birth has been assessed in southern Beninese pregnant women followed-up from the beginning of gestation until delivery. Women’s demographic, anthropometric, and biological data were collected. At birth, newborns were weighed, and cord blood samples collected for Hb concentrations determination. Multivariate logistic and linear regressions were used to assess the effect of GA on newborn anaemia. Gestational anaemia occurring between 25 and 28 weeks of gestation was associated with an important decrease in the newborn’s Hb concentrations (Adjusted coefficient=-11.2 g/L, 95% CI= [-20.9, -1.5], P=0.02) and an increased risk of newborn’s anaemia (adjusted odds ratio=4.5, 95% CI= [2.0, 11.0], P=0.001). Malaria during gestation was also associated with an increased risk for the newborn’s anaemia whereas primigravidity was associated with more than half reduction of the risk of newborn’s anaemia. This result argues in favor of strengthening the preventives measures against GA by promoting their implementation earlier during pregnancy.
Keywords: Gestational anaemia; Newborn’s haemoglobin; Anaemia; Malaria; Benin
Introduction
Gestational anaemia (GA) is common in developing countries, where it affects more than 57% of pregnant women [1,2]. In Benin, a previous study showed that over 68% of women experience GA [3,4]. Gestational anaemia is potentially associated with newborn's anaemia and low birth weight (LBW) which are high risk factors for perinatal morbidity and mortality [5-7].
In sub-Sahara Africa, preventive measures against GA recommended by World Health Organization (WHO) are based on the daily supplementation of iron and folic acid to all pregnant women, the prevention of malaria with Intermittent Preventive Treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine and the systematic administration of anti-helminthic drugs [8-10].
The effect of timing of anaemia during pregnancy, particularly in the first half of pregnancy, on perinatal outcomes remains poorly investigated in developing countries. Many studies that assessed the consequences of GA on newborn anaemia focused on the effect of GA at the end of gestation on the infant [5,11-15]. However, a unique measure of Haemoglobin (Hb) made at the time of delivery, cannot reflect maternal Hb changes during the whole gestation. Moreover, it’s also important to consider the effect of preventive measures administered to women during gestation. On the occasion of a multi-center trial of IPTp comparing sulfadoxine-pyrimethamine and mefloquine (MiPPAD study "Malaria in Pregnancy Preventive Alternative Drugs"), we had the opportunity to follow-up 1,005 Beninese pregnant women to investigate the influence of GA on newborns’ Hb [16].
Materials And Methods
Study methodology
We followed a cohort of 1,005 pregnant women included in MiPPAD trial, from early pregnancy until the time of delivery. The study site, population and procedure have been described elsewhere [3]. Briefly, the study was conducted in the district of Allada, a semirural area located in southern Benin. Malaria is perennial, and Plasmodium falciparum is the most common species. There are two high transmission peaks (April to July and October to November).
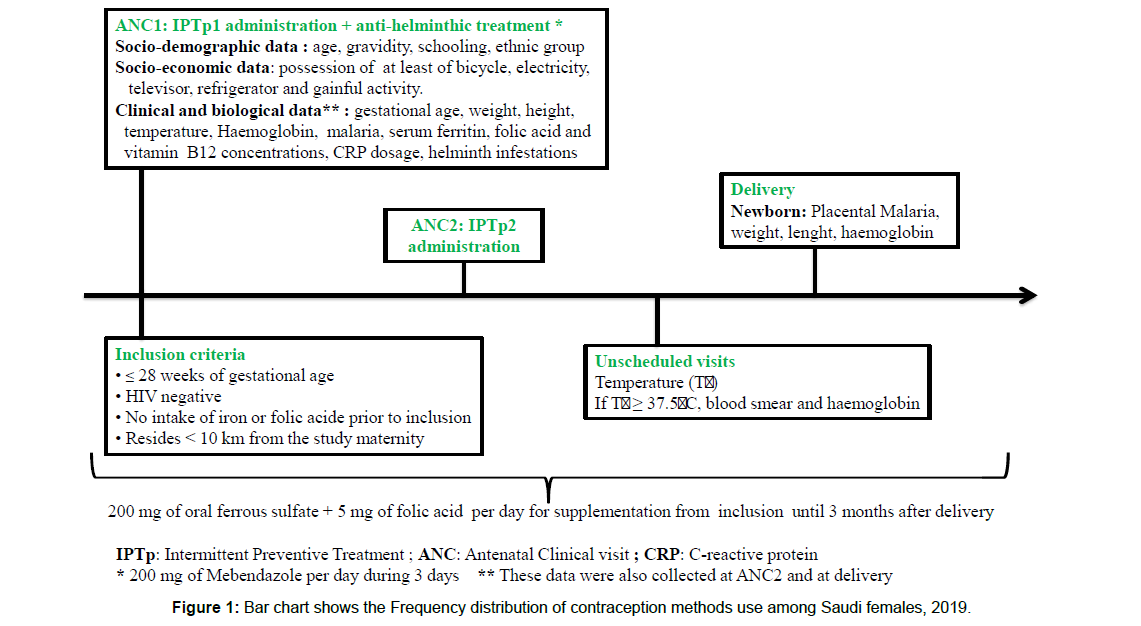
The study population was composed of human immunodeficiency virus (HIV)-negative pregnant women of less than or equal to 28 weeks of gestation. At inclusion (1st antenatal care visit “ANC1”), sociodemographic (age, parity, marital status, level of education) and clinical data (gestational age, weight, and height) were recorded. Gestational age and weight were also collected at the 2nd antenatal clinic visit (ANC2) and delivery. Mothers’ venous blood sample was collected at ANC1, ANC2 and delivery to assess Hb, CRP, serum ferritin, folic acid, and vitamin B12 concentrations and to perform thick blood smear (Figure 1). At birth, the newborn’s anthropometric characteristics (weight, length, head circumference) were measured. A placental blood smear was performed for assessing placental malaria and cord blood sample was collected for determining newborn’s Hb concentrations.
At inclusion, each woman received a long-lasting insecticidetreated net. A supplement of oral ferrous sulfate (200 mg per day) and folic acid (5 mg per day) were given to women for home treatment (Figure 1). Pregnant women with Hb below 110 g/L were treated according to the severity of anaemia. They were administered 200 mg of oral ferrous sulfate twice per day in case of mild and moderate anaemia (Hb between 70 and 110 g/L) or referred to the district tertiary hospital in case of severe anaemia (Hb<70 g/L).
Haemoglobin concentration was determined with a Hemo-Control photometer (EKF Diagnostics, Magdeburg, Germany) on 10 μL of blood. C-reactive protein (CRP) concentrations were determined with a rapid slide test (Cypress Diagnostics, Langdorp, Belgium). Serum ferritin and vitamin B12 concentrations were measured using a microparticle enzyme immunoassay method. A fluorescence polarization immunoassay technique was used to determine folic acid concentrations with an AxSym Immuno-Assay Analyzer (Abbott Diagnostics, Frankfurt, Germany). The Determine (HIV 1 and 2 kit, Alere Orgenics, Paris, France) and SD Bioline (HIV 1 and 23.0 package Umhlanga, South Africa) rapid tests were used to detect HIV infections with a serial testing algorithm. Lambaréné technique was used to assess malaria infection [17].
Definitions
GA and newborn anaemia were defined as Hb below 110 g/L and 150 g/L, respectively [6,18]. LBW was defined as a birth weight < 2,500 g. Iron Deficiency (ID) was defined as serum ferritin <12 μg / L or serum ferritin between 12 and 70 μg/L in a context of inflammation (CRP concentration ≥ 6 mg/mL). Folic acid and vitamin B12 deficiencies were respectively defined as a serum folic acid concentration <6 ng/ mL and serum vitamin B12 concentration <150 pg/mL. Gestational age at inclusion was transformed into categorical variable (≤ 15, 16-24 and 25-28 weeks of gestation) to assess the effect of timing of GA on newborn’s Hb.
Statistical analysis
Data were entered into Microsoft Access 2003 database and analyzed with STATA 12.0 Software for windows. Our primary outcome was the newborn’s Hb at birth (Hb concentration, anaemia). We first described the general characteristics of women and newborns. Afterwards, we studied the association between study outcomes and GA at inclusion and delivery. Finally, we assessed the effect of the timing of GA on newborn’s Hb concentrations and anaemia. Proportions and means were compared using the Chi square and the Student tests, respectively. All variables that were associated to the outcome at P ≤ 0.2 were considered for the multivariate analyses. Logistic and linear regressions were used for the multivariate analyses. The final models have been built using a backward strategy. The statistical significance was set to P ≤ 0.05.
Ethics clearance
This study was approved by the Ethics Committee of Faculty of Medicine of Cotonou in Benin. Before each inclusion, the study was explained in a local language to the participant, and her voluntary consent was obtained. In case a woman cannot read, an impartial witness was involved in the process. Mothers were free to interrupt their participation at any time of the course of the study.
Results
Study profile
As shown in Figure 2, 1,005 pregnant women were enrolled in the survey (Figure 2). They gave birth to 932 newborns. Eight hundred and sixty-two (862) mother-infant pairs were included in the analysis. Haemoglobin concentration was assessed in 100% (862 of 862) and 92.7% (799 of 862) of women at ANC1 and delivery. Newborns' Hb was measured in 86.7% (747 of 862).
Table 1 presents women’s general characteristics. On average women were 25.9 years old, 98.7%, 66.6% and 18.4% of them were married, illiterate and primigravidae, respectively. More than half of the women (55.1%) had a body mass index (BMI) over 20 kg/m².
| Characteristics | ANC1* | ANC2* | Delivery |
|---|---|---|---|
| Gestational age (weeks) | |||
| Mean | 22.1 (21.9-22.4) | 28.8 (28.5-29.0) | 39.7 (39.5-39.8) |
| Hb (g/L) | |||
| Mean | 103.1 (102.3-104.0) | 105.2 (104.5-105.9) | 111.9 (110.9-112.9) |
| Anaemia (Hb <110 g/L) (%) | 67.8 | 64.9 | 39.9 |
| Timing of gestational anaemia at inclusion (%) | |||
| Anaemia before 16 weeks of gestation | 5.5 | - | - |
| Anaemia between 16-24 weeks of gestation | 59.1 | - | - |
| Anaemia between 25-28 weeks of gestation | 35.4 | - | - |
| Serum ferritin† (µg/L) | |||
| Mean | 24.9 (23.7-26.3) | 36.8 (34.5-39.2) | 18.2 (17.4-19.1) |
| Iron deficiency (%) | 33.3 | 35.5 | 30.3 |
| Serum folic acid† (ng/L) | |||
| Mean | 8.2 (7.9-8.4) | 7.7 (7.3-8.1) | 9.4 (9.1-9.7) |
| Folic acid deficiency (%) | 31.2 | 17.1 | 38.9 |
| Serum Vitamin B12† (pg/L) | |||
| Mean | 359.3 (348.9-369.9) | 295.9 (285.9-306.3) | 335.1 (325.5-344.9) |
| Vitamin B12 deficiency (%) | 3.4 | 3.7 | 7.4 |
| Malaria infection (%) | 15.3 | 4 | 9.7 |
| Inflammation (%) | 21.2 | 11.6 | 33.6 |
| Hemoglobin genotypes (%) | |||
| AA | 72.2 | - | - |
| AS, AC | 26.5 | - | - |
| Others (SS, SC, CC, AF) | 1.3 | - | - |
| Illiterate (%) | 66.6 | - | - |
| Married (%) | 98.7 | - | - |
* First and second doses of IPTp administrations; † Geometric means; 95% CI values are in parentheses; Hb: Haemoglobin level
Table 1: Pregnant women general characteristics at the first and second antenatal clinic visits (ANC1 & ANC2) and delivery in Benin.
Gestational anaemia was frequent at inclusion (67.8%) and decreased progressively until delivery (39.9%). The proportions of P. falciparum infection at inclusion and delivery were 15.3% and 9.7%, respectively. Women’s mean gestational age at delivery was 39.7 weeks (95% Confidence Interval [CI] = [39.5-39.8]). Newborns' mean Hb was 139.3 g/L (95%CI= [137.5, 140.9]) and 63.5% of them were anaemic. On average, newborns weighed 3026.3 g (95%CI= [2996.6-3056.0]) and 11% were low birth weight. Placental malaria was prevalent in 9% (Table 2).
| Characteristics | Number | Mean or proportion |
|---|---|---|
| Gender (%) | ||
| Female | 441 | 52.3 |
| Male | 403 | 47.7 |
| Weight (g) | ||
| Mean | 856 | 3026.3 (2996.6-3056.0) |
| ≥ 2500 g | 762 | 89 |
| <2500 g | 94 | 11 |
| Preterm birth* (%) | 60 | 7.3 |
| Hb (g/L) | ||
| Mean | 741 | 139.2 (137.5-140.9) |
| Hb<150 g / L | 474 | 63.5 |
| Placental malaria (%) | 66 | 9.2 |
*Hb: Haemoglobin concentrations; 95% CI values are in parentheses * Preterm birth: Gestational age <37 weeks.
Table 2: Newborns’ clinical and biological characteristics at birth in Benin.
Relation between GA and newborn’s Hb
Table 3 shows the relation between GA at ANC1 and delivery and newborn’s Hb. After adjustment, GA at ANC1 was significantly associated with a decrease in newborn’s Hb concentrations (adjusted difference of mean [dm]=-4.0 g/L, P=0.03). Primigravidaes' newborns had a significantly higher Hb concentrations than multigravidaes’ offspring (dm=5.5 g/L, P=0.02). Gestational anaemia at ANC1 was not statistically associated with an increase in the risk of newborn’s anaemia (adjusted odds ratio [aOR]=1.2, P=0.2). Malaria infection was related to newborns' anaemia (aOR=1.7, P=0.004) whereas primigravidity was associated with a decreased risk in newborn’s anaemia (aOR=0.5, P=0.001) (Table 3).
| (Multivariate linear and logistic regressions) | ||||||
|---|---|---|---|---|---|---|
| Newborn haemoglobin at birth (g/L)* | At ANC1 (n=727) | At delivery (n=718) | ||||
| Mean difference | 95% CI | P value | Mean difference | 95% CI | P value | |
| Gestational anaemia | -4 | (-7.6, -0.4) | 0.03 | -3.1 | (-6.6, 0.4) | 0.09 |
| Primigravidae | 5.5 | (1.0, 10.0) | 0.02 | 5.4 | (0.9, 10.0) | 0.02 |
| Rainy season at delivery | -4.3 | (-7.8, -0.9) | 0.02 | -4.4 | (-7.9, -0.9) | 0.01 |
| Constant | 144 | (140.3, 147.6) | <0.001 | 142.6 | (139.5, 145.8) | <0.001 |
| Newborn Anaemia at birth (%) ** | Adjusted OR | 95% CI | P value | Adjusted OR | 95% CI | P value |
| Gestational anaemia | 1.2 | (0.9, 1.7) | 0.2 | 1.3 | (1.0, 1.8) | 0.06 |
| Primigravidae | 0.5 | (0.3, 0.7) | 0.001 | 0.5 | (0.3, 0.8) | 0.001 |
| At least 1 malaria episode | 1.7 | (1.2, 2.4) | 0.004 | 1.7 | (1.2, 2.4) | 0.004 |
* Adjusted on maternal age, gravidity, season of visit and newborn’s sex
** Adjusted on maternal age, gravidity, season of visit, iron deficiency and number of maternal malaria infection during pregnancy
Table 3: Relation between gestational anaemia at the first antenatal clinic visit, at delivery and newborn’s haemoglobin at birth (Multivariate linear and logistic regressions).
The association between GA at delivery and newborns' Hb was borderline significant (dm=-3.1 g/L, P=0.09). When the newborns' Hb is considered as binary variable, GA at delivery (aOR=1.3, P=0.06) and malaria during pregnancy (aOR=1.7, P=0.004) were associated with an increased risk in newborns' anaemia, whereas the primigravidity decreased the risk (aOR=0.5, P=0.001) (Table 3).
Effects of the timing of GA during the first half of pregnancy on newborn’s Hb
The univariate and multivariate analyses showed a constant association between GA and newborn’s Hb (Table 4). Hb concentration was lower among newborn of women presenting anaemia during the 2nd trimester than those of women presenting anaemia during the 1st trimester of pregnancy. The mean of difference was higher when GA occurred in late second trimester (between 25 and 28 weeks). When we analyzed the newborn’s Hb as dichotomous variable (anaemia or no anaemia), GA occurring late in the second trimester of pregnancy remained significantly associated with an increase in the risk of anaemia in newborns (aOR=4.5, 95% CI [2.0, 11.0], P=0.001) (Table 4).
| Timing of gestational anaemia* | Newborn’s haemoglobin level | Newborn’s anaemia | ||||
|---|---|---|---|---|---|---|
| Mean difference | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| ≤ 15 weeks | Reference | - | - | 1 | - | - |
| Between 16-24 weeks | -8.2 | (-17.7, 1.2) | 0.08 | 3 | (1.3, 6.9) | 0.009 |
| Between 25-28 weeks | -11.2 | (-20.9, -1.5) | 0.02 | 4.5 | (2.0, 11.0) | 0.001 |
* Adjusted on maternal age, gravidity, season of visit, newborn’s sex
Table 4: Relation between gestational anaemia at the first antenatal clinic visit, at delivery and newborn’s haemoglobin at birth (Multivariate linear and logistic regressions).
Discussion
This is one of few cohort studies that have investigated the influence of the timing of GA, especially in the first half of pregnancy, on newborn’s Hb in West Africa. In the study, pregnant women were followed up from the beginning of pregnancy until delivery. The results showed that GA occurring during the 2nd trimester of gestation was associated with an increased risk of newborn’s anaemia in Benin. The risk was more important when GA occurred late in the second trimester of pregnancy. Malaria infection during pregnancy was also associated with an increased risk for newborn’s anaemia whereas primigravidity was related to more than 50% reduction of the risk of newborn’s anaemia.
Gestational anemia was very common in the study population (more than 50%). This result is consistent with the findings of Bodeau- Livinec et al. (GA prevalent in 64.7%) in the same study area, but it is different to the Beninese’s Demographic Health Survey (DHS-IV) prevalence (42.2%) [19]. The difference could be partially explained by the fact that the DHS-IV is a cross-sectional survey with a potential risk of underestimation of anaemia during pregnancy. Otherwise, in the study, anemia at inclusion has been evaluated before any supplementation or antihelminthic treatment [3].
The lack of consensual threshold to define newborn’s anaemia makes complex the comparison of our findings with those found in the literature. For instance, Koura et al., using the same threshold of 150 g/L, showed that GA at delivery was also associated to newborn’s anaemia [6]. In the same trend, de Pee et al. have found in Indonesia that maternal Hb concentrations below 120 g/ L at delivery was related to an increased risk of anaemia in infants at age between 3 and 5 months. The risk of newborn’s anaemia was more important in low birth weight children [5]. It is known that maternal iron deficiency was associated with newborn’s iron deficiency and with an increased risk of anaemia in infants during the first year of life [13,20]. The only source of iron, a key component of hematopoiesis, for the child during intrauterine life derives from the mother to fetus transplacental iron-transfer [21]. For instance, primigravidae included in this study had a better iron stores than multigravidae and the risk for anaemia was almost half reduced in their newborns compared to multigravidae's offsprings [22].
It would have been more informative to consider the exact period of the occurrence of anaemia during pregnancy in the analyses because GA is a gradual syndrome. It is also important to notify that pregnant women were included in the study at different times of gestation and, most of them were attended the first ANC visit because of care-seeking rather than motivated by a systematic follow-up of pregnancy [3].
The proportion of pregnant women with anaemia has progressively decreased from beginning of pregnancy until delivery. This result could be explained partially by the hemodilution and hemoconcentration that occur during the second and 3rd trimesters of gestation, respectively [23,24]. After the enrolment in the study, pregnant women received GA preventive treatment including IPTp, helminth drug, iron and folic acid supplementation. Both the effect of GA preventive measures and the hemoconcentration occurring from the 3rd trimester might explain the decrease of Hb observed at delivery [4]. Newborns’ anaemia was also prevalent, affecting over 63% of children. This high proportion is consistent with WHO data on anaemia in Benin [2]. A previous study, conducted in Tori-Bossito, a neighboring area, showed also that more than 61% Beninese newborns were anaemic [6].
Malaria infection was also frequent, decreasing from beginning of pregnancy to delivery. Malaria was associated with a decrease in newborns’ Hb concentrations. Indeed, alterations of the placenta membranes due to malaria parasites disturb nutrients exchanges between the mother and fetus and to some extent, increase the risk for fetal anaemia [25,26].
Some factors such as sickle cell anaemia, use of cigarettes/tobacco products and altitude, which are known as important determinants of anemia in West Africa both in pregnant women and neonates, have not been evaluated in our study. First, the prevalence of sickle cell during pregnancy in our study was very low. Thirteen out of 1.005 pregnant women (1.3%) presented sickle cell disease during the study and less than 3% of their children were affected by sickle cell disease [3,27]. Secondly, the consumption of tobacco products is uncommon in Beninese women, especially in pregnant women. Indeed, according to DHS-IV, the proportion of women of reproductive age who declared to smoke cigarette or tobacco product was 0.2%. Therefore, it would be difficult to measure the influence of sickle cell disease and use of tobacco products on infant heamoglobin level in Beninese population. Finally, Benin is less than 1000 m above sea level thus no adjustment for altitude was made.
Limitations
The study was ancillary to a randomized clinical trial of prevention of malaria in pregnancy where all pregnant women received IPTp, antihelminth drugs and iron and folic supplementation since their enrolment. That would have underestimated the effect of anemia on adverse pregnancy outcomes. The assessment of timing of anemia was difficult because it is a gradual onset syndrome and the probability women are anemic before being pregnant, is strong in the context of Benin.
Conclusion
Gestational anaemia is common in Benin and we evidence that when it occurs between 25 and 28 weeks, it is associated with reduced newborn Hb concentrations. Malaria during gestation and primigravidae are also important factors contributing to reduce the newborn Hb concentrations. The current anaemia preventive measures (IPTp, anti-helminthic treatment, daily iron and folic acid supplement) should be reinforced by news strategies such as pre-gestational supplementations of iron and folic acid to all women of reproductive age.
Declarations
Acknowledgments
We thank the study staff for their dedication throughout the study and the authorities of the health district of Allada for their facilitations during the field stage of the study. We are also grateful to the European and Developing Countries Clinical Trial Partnership and the MiPPAD Executive Committee for the opportunity of this ancillary study.
Authors' contributions
As principal investigators SO and MC were involved in all aspects of the study; SO and MC conceived and designed the protocol; SO and MA implemented the study; SO, AO, MA, DAH analyzed the data; SO wrote the first draft of the manuscript; all authors contributed to the writing of the manuscript; all authors read and met ICMJE criteria for authorship and agree with manuscript results and conclusions.
Funding
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was supported by the Malaria in Pregnancy Consortium (grant OPP46099).
Competing interests
The authors do not have any conflicts of interest.
References
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, et al. (2013) Global, regional and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob Health 1: e16-25.
- WHO (2015) The global prevalence of anaemia in 2011. Geneva. World Health Organization.
- Ouédraogo S, Koura GK, Accrombessi MMK, Bodeau-Livinec F, Massougbodji A, et al. (2012) Maternal anemia at first antenatal visit: Prevalence and risk factors in a malaria-endemic area in Benin. Am J Trop Med Hyg 87: 418-424.
- Ouédraogo S, Koura GK, Bodeau-Livinec F, Accrombessi MMK, Massougbodji A, et al. (2013) Maternal anemia in pregnancy: Assessing the effect of routine preventive measures in a malaria-endemic area. Am J Trop Med Hyg 88: 292-300.
- De-Pee S, Bloem MW, Sari M, Kiess L, Yip R, et al. (2002) The high prevalence of low hemoglobin concentration among Indonesian infants aged 3-5 months is related to maternal anemia. J Nutr 132: 2215-2221.
- Koura GK, Ouedraogo S, Le Port A, Watier L, Cottrell G, et al. (2012) Anaemia during pregnancy: impact on birth outcome and infant haemoglobin level during the first 18Â months of life. Trop Med Int Health 17: 283-291.
- Vogel JP, Souza JP, Mori R, Morisaki N, Lumbiganon P, et al. (2014) Maternal complications and perinatal mortality: Findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 121: 76-88.
- WHO (2012) Archived: Daily iron and folic acid supplementation in pregnant women. Geneva, World Health Organization.
- WHO Malaria Policy Advisory Committee and Secretariat (2016) Malaria policy advisory committee to the WHO: Conclusions and recommendations of eighth biannual meeting (September 2015). Malar J 15: 117.
- WHO (2009) Weekly iron-folic acid supplementations (WIFS) in women of reproductive age: Its role in promoting optimal maternal and child health Position Statement. Geneva, WHO.
- Hercberg S, Galán P, Chauliac M, Masse-Raimbault AM, Devanlay M, et al. (1990) Nutritional anaemia in pregnant Beninese women: Consequences on the haematological profile of the newborn. Br J Nutr 57: 185-193.
- Brabin BJ, Ginny M, Sapau J, Galme K, Paino J (1990) Consequences of maternal anaemia on outcome of pregnancy in a malaria endemic area in Papua New Guinea. Ann Trop Med Parasitol 84: 11-24.
- Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, et al. (1990) Anaemia during pregnancy as a risk factor for infant iron deficiency: Report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol 4: 196-204.
- Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, et al. (1999) Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: A case-control study in Jordan. Int J Epidemiol 28: 461-468.
- Allen LH (2000) Anemia and iron deficiency: Effects on pregnancy outcome. Am J Clin Nutr 71: 1280S-4S.
- González R, Mombo-Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, et al. (2014) Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: A multicentre randomized controlled trial. PLoS Med 11: e1001733.
- Swysen C, Bruls M, Oyakhirome S, Drakeley C, Okech B, et al. (2010) Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J 10: 223.
- WHO (2011) Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization.
- Bodeau-Livinec F, Briand V, Berger J, Xiong X, Massougbodji A, et al. (2011) Maternal anemia in Benin: Prevalence, risk factors and association with low birth weight. Am J Trop Med Hyg 85: 414-420.
- Zhang Y, Jin L, Liu J-M, Ye R, Ren A (2016) Maternal hemoglobin concentration during gestation and risk of anemia in infancy: Secondary analysis of a randomized controlled trial. J Pediatr. 175:106-110.e2.
- Gambling L, Lang C, McArdle HJ (2011) Fetal regulation of iron transport during pregnancy. Am J Clin Nutr 94: 1903S-1907S.
- Ouédraogo S, Bodeau-Livinec F, Briand V, Huynh B-T, Koura GK, et al. (2012) Malaria and gravidity interact to modify maternal haemoglobin concentrations during pregnancy. Malar J 11: 348.
- Chesley LC (1972) Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 112: 440-450.
- Hytten F (1985) Blood volume changes in normal pregnancy. Clin Haematol 14: 601-612.
- Yamada M, Steketee R, Abramowsky C, Kida M, Wirima J, et al. (1989) Plasmodium falciparum associated placental pathology: A light and electron microscopic and immunohistologic study. Am J Trop Med Hyg 41: 161-168.
- Abrams ET, Kwiek JJ, Mwapasa V, Kamwendo DD, Tadesse E, et al. (2005) Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar J 4: 39.
- Accrombessi M, Ouédraogo S, Agbota GC, Gonzalez R, Massougbodji A, et al. (2015) Malaria in pregnancy is a predictor of infant haemoglobin concentrations during the first year of life in Benin, West Africa. PloS One 10: e0129510.
Citation: Ouédraogo S, Accrombessi M, Diallo AH, Ouattara A, Meda N, et al (2020) Effect of Gestational Anemia on Haemoglobin Concentrations in Beninese Newborns at Birth. J Preg Child Health 7:426. DOI: 10.4172/2376-127X.1000426
Copyright: © 2020 Ouédraogo S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1782
- [From(publication date): 0-2020 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1083
- PDF downloads: 699