Effect of Food Intake on Intravoxel Incoherent Motion and T2* in the Healthy Liver
Received: 26-Nov-2017 / Accepted Date: 04-Dec-2017 / Published Date: 11-Dec-2017 DOI: 10.4172/2167-7964.1000285
Abstract
Introduction: To evaluate the effect of increasing portal flow due to food intake on the parameters of Intravoxel Incoherent Motion (IVIM) and T2* relaxation time in healthy liver.
Materials and methods: The subjects consisted of 15 healthy volunteers. We used a 1.5 T MRI system. All subjects received MRI three times as follows: after overnight fasting but before food intake, at 30 min, and at 4 h after food intake. MRI was repeated at a greater than 1 week interval. All subjects had 800 kcal of Calorie Mate. The echoplanar diffusion-weighted imaging was performed under free breathing and 10 b-values (0, 10, 20, 30, 50, 80, 100, 200, 400, 800 s/mm2) were obtained. T2*-weighted imaging with multi-echo gradient-echo sequence was performed with Siemens MapIt software under breath-holding. The portal flow measurement was performed with phase contrast sequence. The parameters of IVIM including ADC, DC, D*, and PF were calculated and T2* relaxation time was obtained using MapIt.
Results: The portal blood flow increased significantly 30 min after food intake (P<0.0001). The ADC, DC, D*, PF and T2* values after overnight fasting, at 30 min, and at 4 h after food intake showed no significant differences after food intake.
Conclusion: Increasing portal venous flow after food intake does not affect IVIM parameters and T2* of liver parenchyma in healthy volunteers.
Keywords: Liver; Intravoxel incoherent motion; Food intake; Diffusion weighted imaging
Introduction
MRI has some advantages, such as high contrast resolution, no radiation exposure, and enabling objective evaluation irrespective of the operator’s skill. Recently, evaluation with MRI of hepatic functional reserve and hepatic fibrosis has been reported. The quantitative analysis using the contrast enhancement ratio between liver parenchyma and other organs or Diffusion-Weighted Imaging (DWI) was proposed for the grading of liver fibrosis. MR elastography was also proposed. Contrast enhancement ratio cannot be used in patients with renal dysfunction and with allergic history. High cost is also a drawback. MR elastography has high sensitivity in diagnosing liver fibrosis; however, a special purpose instrument is necessary and executable MRI machines are also limited. DWI satisfies these considerations. DWI can perform stably due to the advance of MRI systems. Le Bihan et al. proposed the concepts of intravoxel incoherent motion (IVIM) that can evaluate true diffusion and microcirculation separately by DWI in 1986 [1,2]. Recent advances in equipment brought the feasibility of IVIM to clinical use. Luciani et al. reported that IVIM was useful for diagnosis of liver cirrhosis [3]. They clarified using IVIM that decreasing microcirculation led to the diffusion restriction in liver cirrhosis.
On the other hand, as the fibrosis of liver progresses, the oxygenation decreases. Therefore, evaluating the oxygenation in liver has the potential to understand the extent of liver fibrosis. The blood oxygen level dependent (BOLD) effect currently used in functional MRI can evaluate the variation in oxygenation. If the arterial blood flows into a certain area and it increases the blood flow in that area, consequently the blood flow in the capillary bed is diluted. The arterial blood contains oxy-hemoglobin (oxy-Hb) and the blood in the capillary bed and vein mainly contains deoxy-hemoglobin (deoxy-Hb). As deoxy-Hb has paramagnetic characteristics, it disturbs the local magnetic field. Therefore, after the dilution of deoxy-Hb, the local magnetic field becomes homogenous and the MR signal increases. The MR signal intensity on T2*-weighted imaging decreases in the presence of large amounts of deoxy-Hb, but otherwise it increases. T2* value is usually dependent on T2 value and blood flow [4,5].
The blood flow of liver is supplied by both the hepatic artery and portal vein. Portal blood flow is usually dominant at about 75% and the remaining 25% is by arterial flow. The portal flow increases markedly with diet compared to arterial flow [6]. Currently there are only a few reports on whether the increase in portal flow affects IVIM parameters (including the concept of microcirculation) and whether T2* value is dependent on blood flow [7]. It is important to know the necessity of fasting before examination. Therefore, the purpose of this study is to investigate whether food intake affects IVIM and T2* relaxation time.
Materials And Methods
Our institutional review board approved this study and informed consent was obtained from all candidates.
Subjects
Fifteen healthy volunteers (thirteen men, two women; age range 27-51 years) were enrolled in this study. All subjects had no history of liver disease and no abnormalities in blood chemistry during the regular medical check. All subjects had been fasting for at least 8 hours before the examination. The food intake was 800 kcal of Calorie Mate (Otsuka Pharmaceutical Co., Tokyo), and 500 mL of water (Volvic, Kirin Co., Ltd., Tokyo). The ingredients of Calorie Mate are as follows: energy 800 kcal, protein 16 g, lipid 44 g, carbohydrate 80 g, and dietary fiber 4 g, iron 5.0 mg.
MRI protocol
All MRI examinations were performed using a 1.5 T superconductive MRI scanner (Avanto; Siemens, Erlangen, Germany) with a 32-channel body array coil. The maximum gradient of the system was 45 mT/m and the maximum slew rate was 200 T/m/s. All subjects received MRI three times as follows: after overnight fasting but before food intake, at 30 min, and at 4 h after food intake. To confirm reproducibility, we performed the same examination on a different day more than one week later.
DWI was acquired spin echo based single shot echo-planar imaging. The acquisition parameters of DWI were as follows: TR/ TE 3000/60 ms; average 4; matrix 128 × 78; FOV 40 × 27.5 cm; slice thickness, 5 mm; bandwidth 2056 Hz/pixel; scan time 5:49; b-values: 0, 10, 20, 30, 50, 80, 100, 200, 400, 800 s/mm2; free breathing (work in progress). We acquired T2*-weighted images with MapIt (Siemens, Erlangen, Germany), and the acquisition parameters were as follows: TR/TE 239 / 2.4, 3.81, 5.22, 6.63, 8.04, 9.45, 10.86, 12.27, 13.68, 15.09, 16.5, 17.91 ms; average 1; matrix 192 × 1192; FOV 38 × 29.6 cm; slice thickness 6 mm; bandwidth 960 Hz; FA 20°; GRAPPA 2; scan time 23 s; breath holding. In addition, we measured the portal flow using phase contrast sequence. The acquisition parameters were as follows: TR/TE 12.65/3.35 ms; average 1; matrix 192 × 192; FOV 40 × 27.5 cm; slice thickness 6 mm; bandwidth 395 Hz; flow mode single direction; velocity 50 cm/s; scan time 2 s; 6 times acquisition consecutively; breath holding.
Analysis
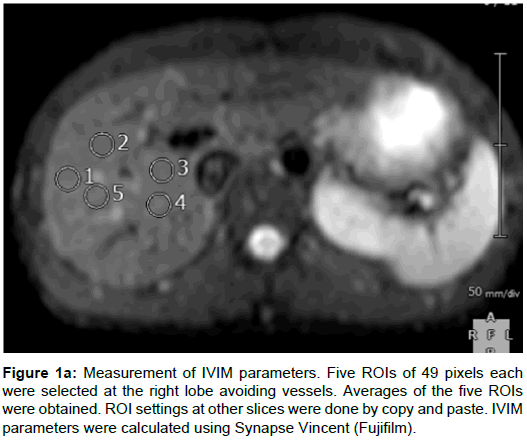
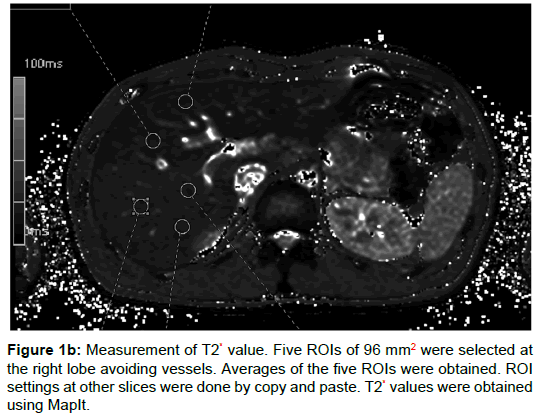
One radiologist with 4 years of experience and one radiology technician with 20 years of experience set five regions of interest (ROIs) on parenchyma of the right lobe of the liver avoiding vessels, and took the average value. The size of ROIs for IVIM was 49 pixels (Figure 1A), and for T2* 96 mm2 (Figure 1B), and we copied and pasted these ROIs on the other remaining slices. We used Synapse Vincent (Fujifilm) to measure IVIM parameters and MapIt to measure T2* values. To measure portal blood flow, the slice was set vertically to the portal vein. The IVIM model is considered to provide the pure molecular diffusion (DC) separately from the blood microcirculation (proportion of blood microcirculation [PF] and pseudo-diffusion coefficient [D*]), when multiple b-values are obtained, from low b-values (2) to high b-values (>200 s/mm2) [1]. IVIM parameters were calculated using the following formula [1]:
Sb/S0 = f · exp [–b(DC+D*)] + (1– f) · exp [–DC × b]
Sb, S0: signal intensity with and without the application of the diffusion gradient, respectively. The 2-step fitting procedure was adopted to determine PF, DC, and D*. Parameters of IVIM (ADC, DC, D*, PF), T2* values, and portal blood flow were evaluated in 15 healthy volunteers (30 examinations). Data are represented as the mean ± standard deviation.
Statistical analysis was performed as follows. Portal blood flow, IVIM parameters (ADC, DC, D*, PF), and T2* values in a total of 30 examinations were evaluated using repeated measure ANOVA. If significant, pairwise comparison was performed using Tukey’s test. Relative reliability in the identical subjects on a different day was evaluated using Bland-Altman plot analysis. To discriminate systematic bias, fixed bias was evaluated by the range of confidence intervals of the differences in the data. P<0.05 was considered significant.
Results
Portal blood flow
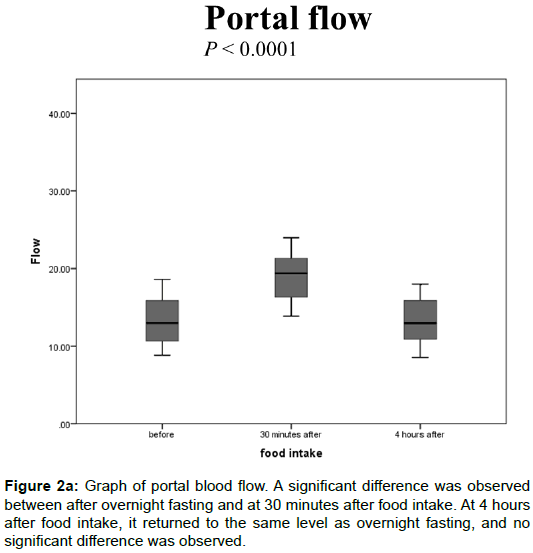
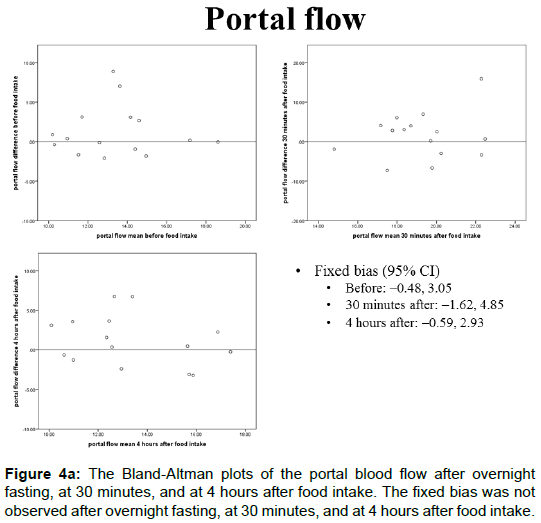
The portal blood flow values after overnight fasting but before food intake, at 30 minutes, and at 4 hours after food intake were 13.4 ± 2.90, 19.2 ± 3.64, and 13.4 ± 2.87 (mL/s), respectively. A significant difference was observed between after overnight fasting and at 30 min after food intake (Figure 2A, P<0.0001). At 4 h after food intake, it returned to the same level as overnight fasting, and no significant difference was observed.
IMIM parameters
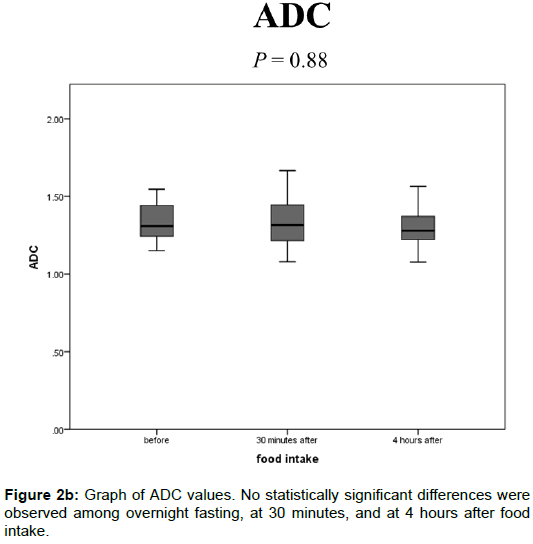
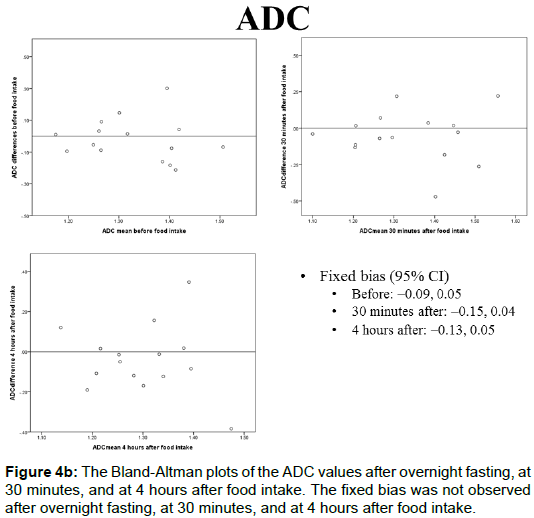
The ADC values after overnight fasting, at 30 min, and at 4 h after food intake were 1.33 ± 0.11, 1.34 ± 0.16, and 1.30 ± 0.12 (× 10-3 mm2/s), respectively, and no statistically significant differences were observed among them (Figure 2B).
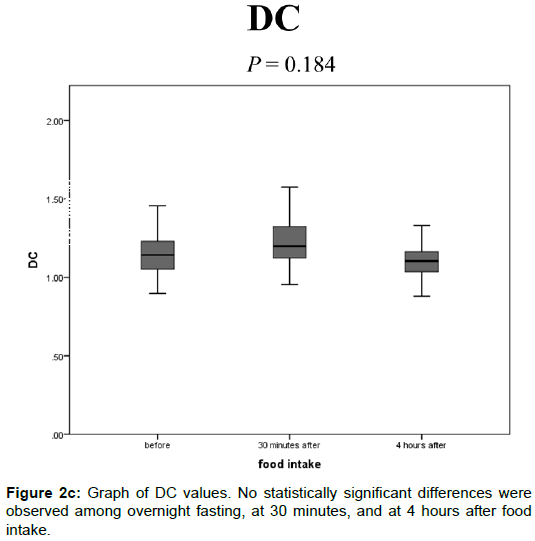
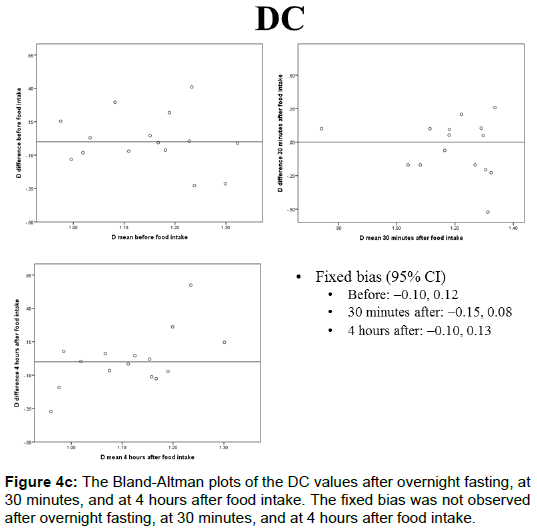
The DC values after overnight fasting, at 30 min, and at 4 h after food intake were 1.15 ± 0.15, 1.19 ± 0.18, and 1.11 ± 0.14 (× 10-3 mm2/s), respectively, and no statistically significant differences were observed among them (Figure 2C).
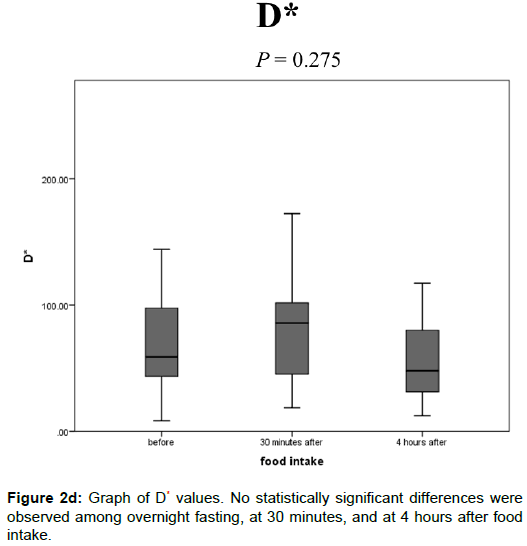
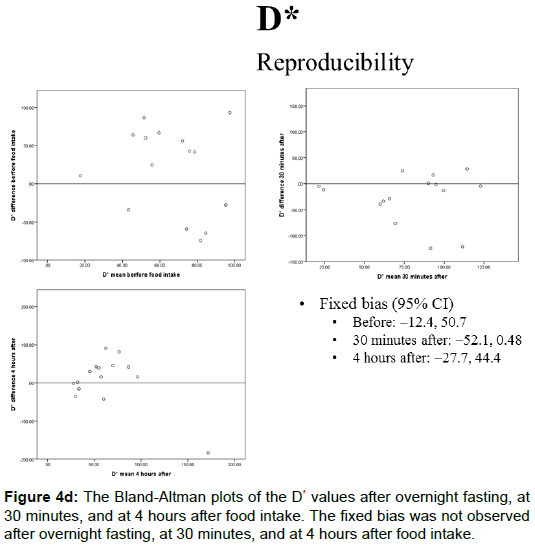
The D* values after overnight fasting, at 30 min, and at 4 h after food intake were 65.7 ± 36.5, 79.6 ± 39.9, and 63.8 ± 48.0 (× 10-3 mm2/s), respectively, and no statistically significant differences were observed among them (Figure 2D).
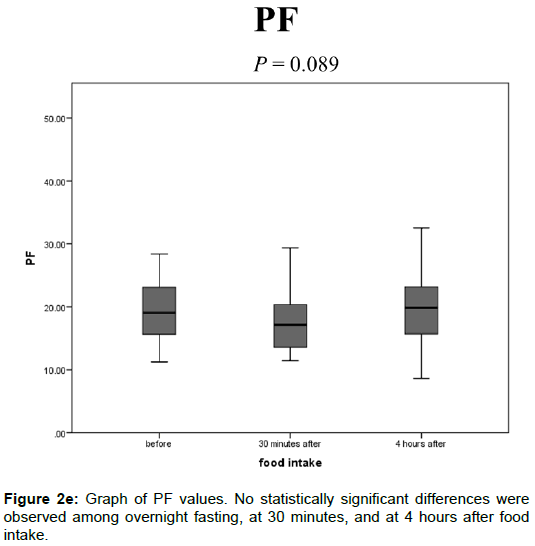
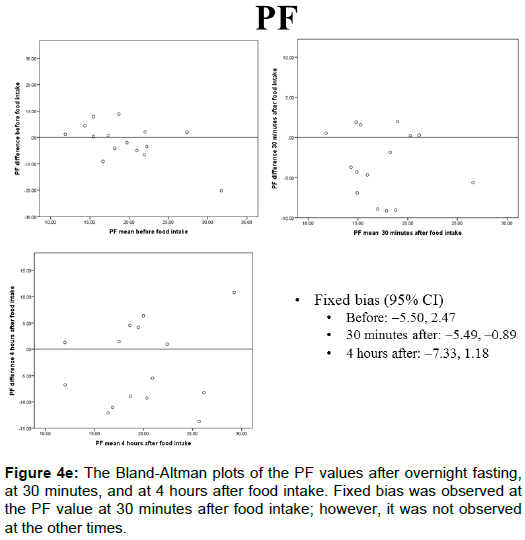
The PF values after overnight fasting, at 30 min, and at 4 h after food intake were 19.6 ± 6.20, 17.4 ± 4.37, and 19.7 ± 6.24 (%), respectively, and no statistically significant differences were observed among them (Figure 2E).
T2* value
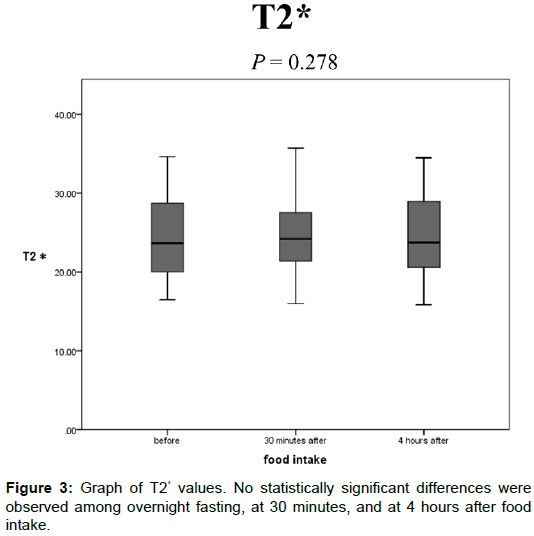
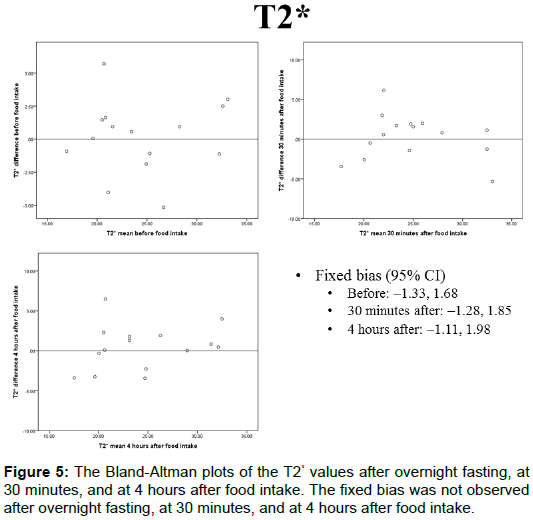
The T2* values after overnight fasting, at 30 min, and at 4 h after food intake were 24.5 ± 5.20, 24.9 ± 4.85, and 24.4 ± 4.98 (ms), respectively, and no statistically significant differences were observed among them (Figure 3).
Reproducibility
The portal blood flow, IVIM parameters, and T2* values of the Bland-Altman plots after overnight fasting, at 30 min, and at 4 h after food intake are shown in Figures 4A-4E and 5. The fixed bias was observed only at the PF value at 30 min after food intake; however, it was not observed at other times for PF or in other parameters.
Discussion
Our study showed that increased portal blood flow in healthy volunteers after food intake does not affect IVIM parameters. As reported previously, our study also showed that portal blood flow increases significantly 30 min after food intake [6]. However, there were no significant changes in parameters related to perfusion, such as D* and PF, after food intake. Unlike our study, Regini et al. reported that D* increased after food intake [8]. We supposed that two factors might contribute to the different results. Large values of standard deviation of D* was the one of the reasons. Some reports showed D* and PF are not reproducible [9-11]. In addition, some reports showed that the standard deviation and its coefficient of variation in D* and PF are large [7,11]. Another reason was settings of b-values. The number of b-values in our study was 10 in total, namely, 0, 10, 20, 30, 50, 80, 100, 200, 400, and 800 s/mm2, and that of the report from Regini et al. was 14 (0, 25, 50, 75, 100, 150, 200, 250, 300, 400, 500, 600, 700, and 800 s/ mm2) [8]. They obtained more b-values than in our study; however, we obtained lower b-values that affected the perfusion more than in their study. Many acquired b-values less than 200 s/mm2 were suitable to evaluate microperfusion [1,12]. Therefore, we believe that the present study is more adequate for evaluation of perfusion than their study.
Pazahr et al. reported that portal blood flow did not affect ADC value [13], and the result of the present study is consistent with previous reports. However, Hollingsworth et al. reported that ADC of the right anterior segment showed a significant difference between fasting and after food intake; on the other hand, that of the right posterior segment did not show a significant difference, and therefore they recommended fasting in case of using lower b-values [14]. In spite of using flowsensitive lower b-values in the present study, the present study did not show the effect of increasing portal blood flow, whereas their study showed an effect. We supposed the difference of ROI setting was a major factor. The number of ROIs in their study was two and those ROIs were larger than ours. Furthermore, in the present study, we set five small ROIs and took the average. This method can be minimized to include large vessels, and even if included, the effect may be small.
Increase in portal blood flow after food intake did not affect T2* values of liver parenchyma in healthy volunteers. Food intake increases not only portal blood flow, but also oxygen consumption [15]. These changes act opposite to the T2* and R2*. As blood flow increases, the deoxy-Hb decreases, the magnetic field becomes homogeneous, and its resultant R2* decreases. In the case of increasing oxygen consumption, the deoxy-Hb increases and R2* increases. Haque et al. reported that the increase in blood flow and oxygen consumption after oral ingestion of glucose led the R2* to decrease significantly [16]. This meant the effect of decreasing R2* due to increasing blood flow was stronger than that of increasing R2* due to oxygen consumption. However, no significant change in T2* values was observed in the present study. We supposed that the differences in liver blood flow and oxygen consumption were due to differences in ingested substance (only oral intake of glucose in Haque et al.) and imaging acquisition timing [16]. Also, different acquisition parameters of the T2*-weighted imaging might affect the sensitivity of the T2* value.
Because subjects were limited to healthy volunteers, it is uncertain whether patients with chronic liver disease such as cirrhosis are affected by food intake. There are reports that D* and ADC values of patients with cirrhosis are significantly lower than in healthy individuals [3]. The increase in portal blood flow due to food intake is larger in healthy people, so it is expected that there will be little effect in patients with cirrhosis. For this matter, future research is necessary. In addition, Haque et al. examined Blood Oxygen Level-Dependent (BOLD) contrast imaging before and after food intake with oxygen, and they reported conflicting results in healthy subjects and patients with liver disease [16]. Therefore, different results about T2* may occur in patients with liver disease, but in this study T2* relaxation time also did not show a significant difference before and after food intake, so it is desirable to study imaging conditions in the future.
In conclusion, increasing portal venous flow after food intake does not affect either IVIM parameters or T2* values of liver parenchyma in healthy volunteers. In addition, reproducibility, except for PF, was obtained. From the above it is considered that fasting is not necessary for the measurement of IVIM parameters or T2* values in the evaluation of the liver of healthy subjects.
References
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, et al (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497-505.
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161: 401-407.
- Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, et al (2008) Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology 249: 891-899.
- Christen T, Lemasson B, Pannetier N, Farion R, Remy C, et al. (2012) Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology 262: 495-502.
- Padhani AR, Krohn KA, Lewis JS, Alber M (2007) Imaging oxygenation of human tumours. Eur Radiol 17: 861-872.
- Iwao T, Toyonaga A, Oho K, Sakai T, Tayama C, et al. (1996) Postprandial splanchnic hemodynamic response in patients with cirrhosis of the liver: evaluation with "triple-vessel" duplex US. Radiology 201: 711-715.
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B (2014) Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PloS one 9: e97355.
- Regini F, Colagrande S, Mazzoni LN, Busoni S, Matteuzzi B, et al. (2015) Assessment of liver perfusion by IntraVoxel Incoherent Motion (IVIM) Magnetic Resonance-Diffusion-Weighted Imaging: correlation with Phase-Contrast portal venous flow measurements. J Comput Assist Tomogr 39: 365-372.
- Koh DM, Blackledge M, Collins DJ, Padhani AR, Wallace T, et al. (2009) Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol 19: 2728-2738.
- Andreou A, Koh DM, Collins DJ, Blackledge M, Wallace T, et al. (2013) Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 23: 428-434.
- Dyvorne HA, Galea N, Nevers T, Fiel MI, Carpenter D, et al. (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters-a pilot study. Radiology 266: 920-929.
- Le Bihan D, Turner R, MacFall JR (1989) Effects of intravoxel incoherent motions (IVIM) in steady-state free precession (SSFP) imaging: application to molecular diffusion imaging. Magn Reson Med 10: 324-337.
- Pazahr S, Nanz D, Rossi C, Chuck N, Stenger I, et al. (2014) Magnetic resonance imaging of the liver: apparent diffusion coefficients from multiexponential analysis of b values greater than 50 s/mm2 do not respond to caloric intake despite increased portal-venous blood flow. Invest Radiol 49: 138-146.
- Hollingsworth KG, Lomas DJ (2006) Influence of perfusion on hepatic MR diffusion measurement. NMR Biomed 19: 231-235.
- Brundin T, Branstrom R, Wahren J (1996) Effects of oral vs. I.V. glucose administration on splanchnic and extrasplanchnic O2 uptake and blood flow. Am J Physiol 271: E496-504.
- Haque M, Koktzoglou I, Li W, Carbray J, Prasad P (2010) Functional MRI of liver using BOLD MRI: effect of glucose. J Magn Reson Imaging 32: 988-991.
Citation: Shimizu T, Saito K, Shirota N, Harada TL, Tajima Y, et al. (2017) Effect of Food Intake on Intravoxel Incoherent Motion and T2* in the Healthy Liver. OMICS J Radiol 6: 285. DOI: 10.4172/2167-7964.1000285
Copyright: ©2017 Shimizu T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4241
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3442
- PDF downloads: 799