Research Article Open Access
Effect of Donepezil on Sleep and Activity in Alzheimer's Disease: Actigraphic and Polysomnographic Assessment
Tsuyoshi Miyaoka1*, Junya Tsukada2, Soichi Mizuno1, Ryoji Nishimura2, Yasushi Inami3 and Jun Horiguchi1
1Department of Psychiatry, Shimane University School of Medicine, Izumo, Japan
2Department of Psychiatry, Fukuoka University School of Medicine, Fukuoka University, FukuoKa, Japan
3Ehime Rosai Hospital, Imabari, Japan
- Corresponding Author:
- Tsuyoshi Miyaoka
Department of Psychiatry
Shimane University School of Medicine
89-1 Enyacho, Izumo 693-8501, Japan
Tel: 81-853-20-2260
E-mail: miyanyan@med.shimane-u.ac.jp
Received date: January 16, 2014; Accepted date: September 25, 2014; Published date: October 03, 2014
Citation: Miyaoka T, Tsukada J, Mizuno S, Nishimura R, Inami Y et al. (2014) Effect of Donepezil on Sleep and Activity in Alzheimer’s Disease: Actigraphic and Polysomnographic Assessment. J Alzheimers Dis Parkinsonism 4:157. doi: 10.4172/2161-0460.1000157
Copyright: © 2014 Miyaoka T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Aim: To examine the effect of donepezil on sleep and daily activity in patients with Alzheimer-type dementia (ATD) using polysomnography and actigraphy. Methods: Ten patients with mild to moderate ATD (mean age: 76 ± 6.2 years) were studied. The Alzheimer’s disease Assessment Scale-cognitive component-Japanese version (ADAS-Jcog),polysomnography, and 7-day recording of actigraphy data were performed. Following this baseline assessment, donepezil (5 mg daily) was administered every morning for 6 weeks, after which the ADAS-Jcog, polysomnography, and actigraphy were repeated. Results: After 6 weeks of treatment with donepezil, daily activity was significantly increased (276.2 ± 89.2 vs. 326.1 ± 98.6; p < 0.05). Similarly, rapid eye movement (REM) sleep (11.3 ± 4.1 vs. 17.1 ± 4.4; p<0.01) and sleep efficiency (76.2 ± 16.3 vs. 82.8 ± 10.6; p<0.05) were significantly increased compared with baseline. Although the ADAS-Jcog score did not decrease significantly (18.5 ± 6.8 vs. 15.9 ± 7.3; P= 0.054), there was a significant positive correlation between the decrease of this score and the increase of daily activity (r= 0.2137, p= 0.4809). Conclusions: An increase of daily activity and cognition were induced by donepezil treatment in patients with ATD, possibly based on improvement of the sleep structure. Trial Registration: Controlled-trials. Com Identifier: UMIN000005018
Keywords
Donepezil; Sleep; Daily activity; Alzheimer-type dementia
Introduction
In general, elderly persons show changes of their sleep structure due to aging and such changes are more distinct in patients with Alzheimer-type dementia (ATD). An increased duration and frequency of waking after sleep onset, a decreased percentage of slow wave sleep and REM sleep, decreased REM density, and sleep-waking cycle disorder that features daytime drowsiness are often seen in ATD patients [1]. There have been a number of studies on the correlations between sleep and memory. For example, it has been reported that REM sleep plays a major role in memory consolidation [2]. It has also been suggested that sleep disorder is closely connected to cognitive impairment in patients with Alzheimer’s disease.
Donepezil hydrochloride is an acetylcholinesterase inhibitor, which is designed to improve cognitive function by inhibiting the enzyme acetylcholinesterase and thus activating cholinergic neurons via an increase of acetylcholine [3]. There have been no studies assessing the effect of donepezil on sleep and changes of daytime activity associated with modification of sleep.
Our hypothesis is that the effect of donepezil treatment on cognition might be associated with recovery of sleep quality and day time activity.
Therefore, we conducted the present study to assess the effects of donepezil on sleep, cognition, and daytime activity in ATD patients.
Materials and Methods
Study cohort
This study was approved by the Ethics Committee of Shimane University School of Medicine. Among the patients receiving treatment at the Department of Psychiatry of Shimane University School of Medicine, 10 patients with mild to moderate ATD who met the following criteria were enrolled in this study. They fulfilled the NINCDS-ADRDA clinical diagnostic criteria [4] for “probable Alzheimer’s disease (AD)†and had a Stage 1 or 2 score on the Clinical Dementia Rating [5]. Other eligibility criteria were no co-morbid psychiatric disorders or somatic disorders and no prior treatment with psychotropic medications including donepezil. Before enrollment, candidate subjects and their main family members (caregivers) were given an explanation about the potential risks of this study, and written informed consent was obtained from each of the families.
Methods
Baseline assessment: Before donepezil administration, the patients underwent baseline assessment of cognition, sleep structure, and daily activity using the Alzheimer’s disease Assessment Scale-Cognitive Subscale Japanese version (ADAS-Jcog) [6], polysomnography (PSG), and actigraphy, respectively.
Assessment of cognition with the ADAS-Jcog: The ADAS-cog score can range from 0 to 70 points, with higher scores indicating more severe cognitive impairment. To avoid any influence of the time of testing, the ADAS-Jcog test was done at 15:00 on the days of PSG recording, which was scheduled twice during the study.
Assessment of sleep by PSG: PSG was performed from 21:00 till 7:30 on the next morning on the scheduled test days. The electroencephalogram (EEG, leads C3-A2 and 01-A2), electro-oculogram (EOG), electromyogram of the chin (chin EMG), and electrocardiogram (ECG) were recorded, while the abdominal and thoracic respiratory movements, oral and nasal airflows, and arterial oxygen saturation were also measured to exclude sleep apnea syndrome. Data were processed by standard analytical procedures [7] to determine the awake time after sleep onset, sleep efficiency, percentage of non-REM sleep in Stages 1 to 4 of sleep, percentage of REM sleep, and REM sleep latency.
Actigraphy: A wrist-type ambulatory actigraph (Actiwatch) manufactured by Mini Mitter was used to measure the level of activity [8]. Subjects wore Actiwatch on the wrist of the non-dominant hand continuously for 24 hours apart from bath time. The interval length was set at 1 minute and activity during each 1-minute period was recorded. Actigraphic counts from 6:00 to 21:00 were used as daytime activity data, 6:00 to 16:00 were used as diurnal activity, 16:00 to 21:00 were used as evening activity, and 21:00 to 6:00 were used nocturnal activity.
Donepezil treatment: Subjects took 3 mg of donepezil hydrochloride once daily after breakfast for the first week, and then the dose was increased to 5 mg once daily after breakfast.
Assessment during donepezil treatment
ADAS-Jcog: The ADAS-Jcog test was carried out just before the first dose of donepezil to obtain the baseline cognition score. The test was performed again after 6 weeks of treatment with donepezil to obtain the post-treatment cognition score.
PSG:PSG was recorded just before and after 6 weeks of donepezil treatment. Changes of sleep parameters after treatment were evaluated by comparison with the baseline data.
Actigraphy: Subjects wore the Actiwatch continuously for 7 days before the start of donepezil treatment. Daytime and night time activity counts during the 5 days from Day 2 through Day 6 were used as baseline activity data. The daytime and night time activity counts for 5 days from Day 2 through Day 6 during Week 6 of donepezil treatment were used as the post-treatment activity data and were compared with the baseline data.
Statistical analysis
Changes of the ADAS-Jcog score and changes of sleep parameters due to donepezil treatment were assessed by comparison with the baseline data using the paired t-test. Correlations between changes of activity and changes of the ADAS-Jcog score or between changes of sleep parameters and change of the ADAS-Jcog score were evaluated by using Spearman’s correlation coefficient analysis.
Results
ADAS-Jcog score
The baseline ADAS-Jcog score was 18.5 ± 6.8. It decreased to 15.9 ± 7.3 after donepezil treatment, although the difference was not statistically significant (p=0.054) (Table 1).
| Sleep parameters | Baseline | After treatment with donepezil |
|---|---|---|
| Total sleep time (min) | 428.0 ± 113.6 | 435.0 ± 57.3 |
| Sleep period time (min) | 482.0 ± 83.6 | 492.4 ± 44.9 |
| Sleep latency (min) | 65.6 ± 82.8 | 33.6 ± 40.1 |
| Sleep efficiency* | 76.2 ± 16.3 | 82.8 ± 10.6 |
| Time of wake after sleep onset %SPT† | 15.7 ± 10.0 | 11.7 ± 7.7 |
| Stage 1, %TST | 22.4 ± 13.6 | 17.5 ± 6.0 |
| Stage 2, %TST | 63.6 ± 12.4 | 63.8 ± 7.7 |
| Stage 3, %TST | 2.4 ± 3.0 | 1.3 ± 1.1 |
| Stage 4, %TST | 0.3 ± 0.7 | 0.1 ± 0.3 |
| Stage REM %TST** | 11.3 ± 4.1 | 17.1 ± 4.4 |
| REM sleep latency (min) | 103.7 ± 55.9 | 70.4 ± 28.5 |
Mean ± SD †p<0.1; *p<0.05; **p<0.01; TST: total sleep time; SPT: sleep period time; REM: rapid eye movement.
Table 1: Changes of sleep parameters after treatment.
Sleep parameters
Table 1 shows changes from baseline of various sleep parameters after 6 weeks of treatment with donepezil. The percentage of REM sleep to total sleep time was significantly increased by donepezil treatment (p<0.01). Sleep efficiency was also significantly increased by donepezil treatment compared with baseline (p<0.05). However, the other sleep parameters showed no significant changes after treatment with donepezil.
Daytime activity
The daytime activity count (activity count/min) was 276.2 ± 89.2 at baseline. After treatment with donepezil, it was significantly increased to 326.1 ± 98.6 (p<0.05).The diurnal activity count was 130.0 ± 66.3 at baseline. After treatment, it was significantly increased to 196.4 ± 74.3 (p<0.05).The evening activity count was 54.9 ± 26.2 at baseline. After treatment, it was not significantly decreased to 48.1 ± 25.3.The noctual activity count was 45.2± 31.4 at baseline. After treatment, it was significantly decreased to 31.3 ± 30.4 (p<0.05).
Correlations between the ADAS-Jcog score and activity or sleep parameters
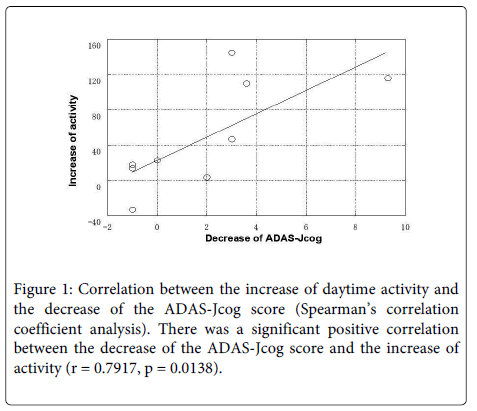
Figure 1 shows the correlation between the decrease of the ADAS-Jcog score and the increase of daytime activity. The decrease of the ADAS-Jcog score showed a significant positive correlation with the increase of daytime activity (r=0.7917, p=0.0138). However, the decrease of the ADAS-Jcog score showed no significant correlation with the increase of REM sleep time (r=-0.2137, p=0.4809). There was also no correlation between the decrease of the ADAS-Jcog score and the increase of sleep efficiency after donepezil treatment.
Discussion
As a result of treating ATD patients with donepezil, the percentage of REM sleep and sleep efficiency were increased and daytime activity and diurnal activitywere also increased, and nocturnal activity is decreased. Furthermore, our study demonstrated a correlation between the increase of daytime activity and improvement of cognition.
A previous study regarding the effect of donepezil on sleep structure showed an increase of REM sleep with donepezil treatment in healthy non-elderly subjects [9]. There have been few studies of sleep parameters (determined by PSG) in AD patients before and after treatment with donepezil. Moraes and colleagues conducted such a study, and they reported that REM sleep was increased but other sleep parameters were not significantly changed by donepezil in AD patients [10].
A trend toward daytime drowsiness has been reported in AD patients [11]. These findings and the present results suggest that administration of donepezil to AD patients increases the percentage of REM sleep and improves sleep efficiency, leading to improvement of daytime drowsiness and an increase in daytime activity.
Bonanni and colleagues reported that there was a negative correlation between daytime drowsiness and cognitive capacity in AD patients [12]. It has also been shown that treatment with the muscarinic agonist scopolamine causes cognitive impairment [13]. Our study demonstrated a significant correlation between the increase of daytime activity and the decrease of the ADAS-Jcog score (i.e., improvement of cognition). It is speculated that an increase of daytime activity due to treatment with donepezil may have improved daytime attention/concentration and this augmentation of mental capacity led to improvement of cognitive test scores involving apprehension and judgment.
Karni et al. assessed visual discrimination tasks in non-elderly healthy individuals, and reported that the task achievement rate was increased in subjects who had normal nighttime sleep while there was no such increase in subjects selectively deprived of REM sleep (REM sleep deprivation) [14]. Subsequently, it was reported that learning efficiency was decreased by REM sleep deprivation in animals [15] and that there was a positive correlation between REM sleep and memory in healthy elderly individuals [16]. It has been reported that AD patients have a lower percentage of REM sleep than healthy elderly people [11]. As discussed above, the cholinergic nervous system plays a key role in the onset of REM sleep. Our study showed an increase in the percentage of REM sleep as a result of donepezil treatment, but no correlation was found between the significant increase of REM sleep and improvement of the ADAS-Jcog score. These results do not support the hypothesis that daytime activity increased secondary to improvement of cognition after the increase of REM sleep.
The sample size was limited in the present study. Increase the number of subjects to a level that will determine whether the non-significant trends seen in the changes in sleep are real. We will continue to monitor the clinical course of more AD patients in order to evaluate the long-term effects of donepezil therapy.
Acknowledgements
Part of this work was supported by Grant-in-Aid for Scientific Research on Priority Areas No. 13770544 and 50284047 from the Ministry of Education, Science, Sports and Culture of Japan. There is no conflict of interest.
Authors’Contribution
Tsuyoshi Miyaoka, MD, PhD: Contributions to conception and design, or acquisition of data; JunyaTsukada, MD, Contributions to acquisition of data; Soichi Mizuno, MD, PhD, Contributions to conception and design, or acquisition of data; Ryoji Nishimura, MD, PhD, Contributions to conception and design, or acquisition of data; Yasushi Inami, MD, PhD Contributions to acquisition of data; Jun Horiguchi, MD, PhD Contributions to analysis and interpretation of data
References
- Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, et al. (1982) Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am GeriatrSoc 30: 86-93.
- Wagner U, Gais S, Born J (2001) Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Mem 8: 112-119.
- Takeda A, Loveman E, Clegg A, Kirby J, Picot J, et al. (2006) A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry 21: 17-28.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140: 566-572.
- Honma A, Fukuzawa K, Tsukada Y, Ishii T, Hasegawa K, et al. (1992) Development of Japanese version of Alzheimer’s disease Assessment Scale (ADAS). Jpn J Psychiatry 3: 647-655.
- Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques, and scoreing system for sleep states of human subjects. USPHS Publication No. 204, U.S Government Pringting Office, Washington, DC.
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, et al. (2003) The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26: 342-392.
- Kanbayashi T, Sugiyama T, Aizawa R, Saito Y, Ogawa Y, et al. (2002) Effect of donepezil on the rapid eye movement sleep of normal subjects. Psychiatry ClinNeurosci 56: 307-308.
- MoraesWdos S, Poyares DR, Guilleminault C, Ramos LR, Bertolucci PH, et al. (2006) The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep 29: 199-205.
- Vitiello MV, Borson S (2001) Sleep disturbances in patients with Alzheimer's disease: epidemiology, pathophysiology and treatment. CNS Drugs 15: 777-796.
- Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, et al. (2005) Daytime sleepiness in mild and moderate Alzheimer's disease and its relationship with cognitive impairment. J Sleep Res 14: 311-317.
- Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, et al. (2006) Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 332: 455-459.
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D (1994) Dependence on REM sleep of overnight improvement of perceptual skill. Science 265: 679-682.
- Smith C, Rose GM (1996) Evidence for a paradoxical sleep window for place learning in the Morris water maze. PhysiolBehav 59: 93-97.
- Schredl M, Weber B, Leins ML, Heuser I (2001) Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. ExpGerontol 36: 353-361.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15998
- [From(publication date):
November-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11398
- PDF downloads : 4600