Effect of Combined Treatment with Sodium Valproate and Methylprednisolone on Neurological Recovery after Experimental Spinal Cord Injury
Received: 06-Jan-2024 / Manuscript No. cmb-24-124688 / Editor assigned: 09-Jan-2024 / PreQC No. cmb-24-124688 / Reviewed: 16-Jan-2024 / QC No. cmb-24-124688 / Revised: 23-Jan-2024 / Manuscript No. cmb-24-124688 / Published Date: 30-Jan-2024
Abstract
We investigated the effect of Sodium valproate (VAP) combined with methylprednisolone (MP) on spinal cord injury (SCI), and its underlying mechanism. Following the establishment of the SCI model using SD mice, VPA treatment group: 8 hours after the establishment of SCI model, VAP (dissolved in normal saline) was injected intraperitoneal at a dose of 30 mg / kg for 30 days. MP treatment group: 30 min, 6 and 24 hours after successful establishment of SCI model, MP (dissolved in normal saline) was injected intraperitoneal at a dose of 30 mg/kg. Behavioral tests, Nissl staining, and hematoxylin-eosin staining were employed to assess motor function recovery and neuronal cell death. Western blot was used to assess the apoptosis-associated proteins (Bcl-2, caspase-3, Bax). VAP combined with MP can significantly improve the motor function of spinal cord and reduce neuronal death. Also, significant upregulation of Bcl-2 expressions, with the downregulation of Bax and caspase-3 expressions were found in the VAP combined with MP treated group. The protective effect of MP combined with MP on SCI may be mediated by inhibition of NF-κ B signal pathway. These results demonstrate the therapeutic potential of VAP combined with MP in SCI.
Keywords
Apoptosis; Spinal cord injury; Sodium valproate; Methylprednisolone
Introduction
With the development of society, spinal cord injury (SCI) is growing rapidly. How to treat SCI is a problem of common concern all over the world (Ahuja et al., 2017a). Primary spinal cord injury is caused by initial mechanical direct injury, while secondary injury includes activation of microglia, inflammatory response and abnormal activity of mitochondria, resulting in neuronal death and permanent neurological impairment (Fu et al., 2019). So far, no fully restorative treatment has been found, and the effect on the recovery of motor function in patients with severe SCI is limited. Therefore, using a variety of aspects to treat different targets, play a synergistic or superimposed effect, is a new treatment concept (Keles et al., 2019). Methylprednisolone (MP) administered with high dose within 8 hours after spinal cord injury is a common clinical treatment, but the clinical effect is not exact and may lead to other complications (Park et al., 2019) [1]. Although the treatment of high-dose MP is still controversial, MP has been shown to have neuroprotective effects in many SCI-related trials. Sodium valproate (VAP) is a short-chain fatty acid, which is widely used in the treatment of epilepsy and bipolar disorder (Chen et al., 2018b). In recent years, studies have confirmed that VAP has the effects of anti-inflammation, anti-apoptosis, nutrition and protection of nerve cells after SCI. It has attracted more and more attention because of its interrelated therapeutic effects in neuroprotection and neurogenesis (Chen et al., 2018a). In rat SCI model, sodium valproate can reduce the behavior of thermal pain and mechanical pain in rats, and has the effect of anti-neuritis and inhibiting neuronal apoptosis [2]. VPA also has neuroprotective effect on cerebral hemorrhage and improves functional recovery after SCI by inhibiting histone deacetylase (Lee et al., 2014). As the possibility of complete recovery of nerve function after SCI is generally low, any SCI treatment that can significantly restore function will be a great progress in clinical treatment. Because of the inexact curative effect and potential side effects of MP, the clinical use of VAP is still controversial, and the role of VAP in SCI has been confirmed by more and more experiments. Whether early use of MP combined with VAP can achieve a better effect has not been reported at home and abroad. Therefore, the purpose of this study is to explore the synergistic or superimposed effect of VAP combined with MP in rat SCI and its possible potential mechanism, so as to provide a new theoretical basis for the treatment of SCI [3].
Materials and Methods
Animals
All experiments were conducted with approval from the Animal Experimentation Ethics of Hebei North University Animal Center. Sixty SD mice (males, 8-10 weeks, 210 ± 20 g) purchased from the Institute of Medical Laboratory Animal, Chinese Academy of Medical Sciences were used in this study. The animals were housed at a constant temperature of 26°C on a day and night light cycle with free access to food and water. Sixty rats were randomly divided into sham operation group (n=12), SCI group (n=12), VPA treatment group (n=12), MP treatment group (n=12) and VPA+MP treatment group (n=12). Both sham and SCI mice were administered Saline at a dose of 0.3 mg/ kg/day for 30 days, and MP group mice were administered MP (at a dose of 30 mg/kg) at 30 min, 6 h, and 24 h after surgery. The VAPYang treated mice were administrated VAP (at a dose of 0.3 mg/kg/day for 30 days) by intraperitoneal injection (i.p.) 8 hours after SCI. VAP and MP were obtained from North China Pharmaceutical Company. All tissue samples were collected 7 days after surgery. Spinal cord tissues, with a diameter of about 1.0 cm around the lesion center, were used for subsequent experiments [4].
Surgery
The SCI mouse model was established as previously described (Basso et al., 1995). After abstaining from drinking water for 12 hours, 60 rats were anesthetized with 10% chloral hydrate (according to 30 mg/100 g) and fixed on the anatomical table in a prone position. The spinous process and lamina at T10 level were removed and the dorsal side of T9 spinal cord was exposed. In the sham operation group, only the spinal cord was exposed and no SCI treatment was performed. Modified Allen method was used to prepare rat SCI model in the other four groups. Taking the rat T9 segment as the center, the rat T9 segment SCI model was established by using a self-made spinal cord percussion device and a 3G flat head impactor to hit the dorsal side of the spinal cord at 3 cm. Bladders were massaged twice daily after the SCI till tissue samples were collected [5].
Behavioral testing
The Basso Beattie Bresnahan (BBB) and Rivlin score was used to assess functional recovery after SCI. Hind limb motor functions were assessed on days 0, 1, 3, 7, 14, 30, and 60 after surgery in an open field experiment. The BBB score ranged from 0 to 21 (Zheng et al., 2016), the Rivlin ranged from 0 to 56° with an assessment of locomotion, plantar stepping, coordination, and paw position. All experiments were performed in a double-blind manner [6].
Hematoxylin-eosin (HE) staining
Take paraffin sections of spinal cord tissue of each group, after deparaffin and rehydration, stain for 6 minutes with hematoxylin solution under dark conditions, soak in 1% acid ethanol for a few seconds, rinse in distilled water for 10 minutes, add eosin solution for 5 minutes, and then use alcohol to separate dehydration. The slides were sealed with neutral balsam and photographed under a microscope. Three tissue sections were randomly selected from each specimen for analysis [7].
Nissl staining
The spinal cord specimens near the injury center were fixed with formaldehyde, dewaxed with xylene, then rehydrated in graded ethanol, treated with Nissl staining for 5 minutes, and fixed with neutral balsam (Zhu et al., 2020). When the motoneurons were observed under the microscope, the apoptotic neurons shrank or had vacuoles, and the normal neurons had relatively large cell bodies, complete cell bodies and round and large nuclei. Count the number of Nissl substances arranged in polygons and count only the cells visible in the nucleus. Six sections of each specimen were randomly selected for observation and counting, and the results were averaged [8].
Immunohistochemical staining
After routine dewaxing and hydration of tissue sections of rats in each group, immunohistochemical staining was performed by SABC method, and the operation was carried out strictly according to the instructions of SABC kit. Six non-repetitive visual fields were randomly selected from each section, and the expression of TNF-α and IL-1 β was observed. The cytoplasm containing TNF-α and IL-1 β proteins showed brown granules. Using Image-Pro Plus image analysis, the staining intensity score criteria: negative or weak staining was 1 point, moderate staining was 2 points, strong positive staining was 3 points; the scoring standard of positive cell percentage: positive percentage < 10% was 1 point, 10% to 50% was 2 points, and more than 50% was 3 points. Immunohistochemical staining score=staining intensity score × positive cell percentage score. Each slice was scored 3 times repeatedly, and the results were averaged [9].
Western blot analysis
The spinal cord tissue of the injured area of rats in each group was cut into powder and cracked with RIPA. After centrifugation of 14000 rpm and 10 min, a small amount of supernatant was taken for protein quantification by BCA method, SDS-PAGE gel electrophoresis and membrane transfer were completed, and 5% skimmed milk and 0.1%TBS were used to seal the membrane for 3 hours at 20°C. B Lymphoma-2 (Bcl-2) (1Rau 400), Bcl-2-related X factor (Bax) (1RV (200) and caspase-3 (1RV 200) were added at 4°C and incubated overnight at 4°C. Goat anti-mouse IgG-HRP (1RV 3500) labeled with horseradish peroxidase was added. After incubation at room temperature for 1 hour, the development was completed with ECL solution. The ChemiDoc touch imaging system (Bio-Rad) was used to prepare the protein for exposure. With β-actin as the internal reference, the ratio of target protein to β-actin represents the relative expression of the target protein. The intensity of the strip was analyzed by image laboratory software (Zheng, Ye, 2016) [10].
Statistical analysis
Statistical analysis was performed using 22.0 SPSS software. Data are presented as mean ± standard deviation. Two or multiple groups were compared using repeated measure analysis of variance followed by Fisher’s least significant difference. Significant differences were defined at P < 0.05 [11].
Results
VAP combined with MP promotes motor function after SCI
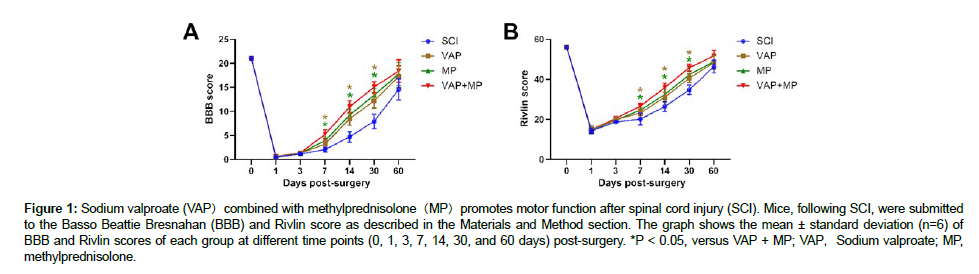
In assessing neurological function after SCI, the BBB and Rivlin score was used to assess functional improvement at different time points (0, 1, 3, 7, 14, 30, and 60 days) after surgery (Figure 1). Mice in the SCI, VAP, MP, and VAP+MP groups showed complete immobility after spinal cord injury (BBB score of 0, Rivlin score of 56°). Slight ankle movement on day 3 post-injury was discerned in SCI, VAP, MP, and VAP+MP mice, albeit, no significant difference in BBB and Rivlin score (P > 0.05). The BBB scores for the VAP-treated mice at days 7, 14, and 30 post-surgery were 1.29326 ± 0.21152, 3.21826 ± 0.6968, and 8.23561 ± 1.42523,respectively (n=6);that for the MP-treated mice were 1.23532 ± 0.1546, 3.8789 ± 0.63126, and 9.16865 ± 1.14662, respectively (n=6); that for the VAP+MP-treated mice were 1.33512 ± 0.22846, 5.17619 ± 0.83156, and 10.76865 ± 1.24332, respectively (n=6); and that for the SCI group mice were 1.12231 ± 0.22172, 2.0313 ± 0.45271, and 4.56221 ± 1.43146, respectively (n=6) [12]. The Rivlin scores for the VAP-treated mice at days 7, 14, and 30 post-surgery were 1.91341 ± 1.21478°, 23.11826 ± 1.2968°, and 30.93136 ± 1.42523°, respectively (n=6); that for the MP-treated mice were 19.54662 ± 1.1516°, 24.5649 ± 1.66726°, and 32.16261 ± 2.44612, respectively (n=6); that for the VAP+MP-treated mice were 20.13212 ± 0.71623°, 26.12612 ± 1.23157°, and 35.76825 ± 2.32332°, respectively (n=6); and that for the SCI group mice were 18.13212 ± 0.71239°, 20.12612 ± 2.23327°, and 26.76825 ± 2.32782° respectively (n=6). The BBB and Rivlin score was significantly higher in VAP+MP mice than the VAP and MP group at days 7, 14, and 30 post-surgery (P < 0.05). However, there was no significant difference between VAP-treated mice and MP-treated mice [13].
Figure 1: Sodium valproate (VAP)combined with methylprednisolone(MP)promotes motor function after spinal cord injury (SCI). Mice, following SCI, were submitted to the Basso Beattie Bresnahan (BBB) and Rivlin score as described in the Materials and Method section. The graph shows the mean ± standard deviation (n=6) of BBB and Rivlin scores of each group at different time points (0, 1, 3, 7, 14, 30, and 60 days) post-surgery. *P < 0.05, versus VAP + MP; VAP,Sodium valproate; MP, methylprednisolone.
VAP combined with MP enhances tissue recovery after SCI
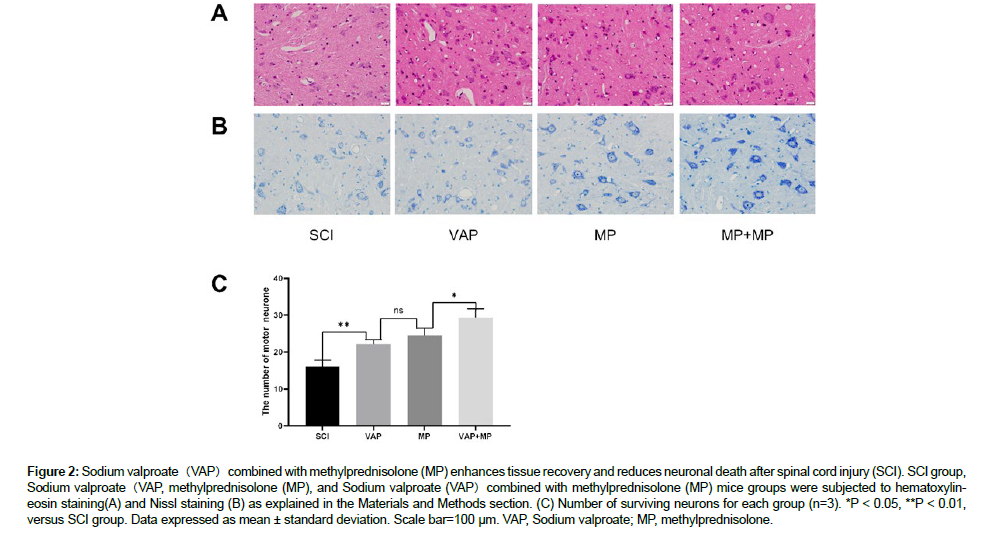
On day 7 post-injury, HE staining was used to detect the histological characterization. Changes in the SCI group mice were more severe compared with both VAP, MP and VAP+MP mice, with enlarged intercellular space and disordered arrangement of the neurons, which correlated with a significant decline in locomotors function (Figure 2A). In addition, sections of the spinal cord were submitted to Nissl staining for neuronal counting following the lesion. The number of neurons in the spinal cord was considerably lower in the SCI group mice when compared with the sham mice (P < 0.001). Also, the neuronal numbers were increased in VAP, MP and VAP+MP mice when compared with the SCI group mice (P < 0.01 and (P < 0.01) (Figure 2B and 2C) [14].
Figure 2: Sodium valproate(VAP)combined with methylprednisolone (MP) enhances tissue recovery and reduces neuronal death after spinal cord injury (SCI). SCI group, Sodium valproate(VAP, methylprednisolone (MP), and Sodium valproate (VAP)combined with methylprednisolone (MP) mice groups were subjected to hematoxylineosin staining(A) and Nissl staining (B) as explained in the Materials and Methods section. (C) Number of surviving neurons for each group (n=3). *P < 0.05, **P < 0.01, versus SCI group. Data expressed as mean ± standard deviation. Scale bar=100 µm. VAP, Sodium valproate; MP, methylprednisolone.
VAP combined with MP inhibits inflammatory response
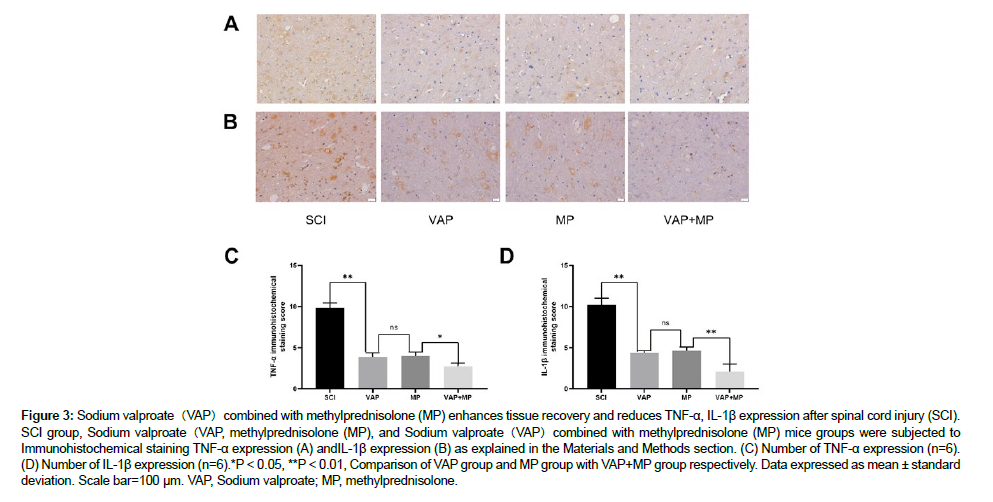
Seven days after injury, the samples were taken and the expressions of TNF-α and IL-1 β were detected by immunohistochemical staining. Compared with VAP, MP and VAP+MP mice, the expression of TNF-α and IL-1 β in SCI group was higher, which was related to the degree of inflammatory reaction after SCI (Figure 3A). In addition, the expression of TNF-α and IL-1 β was counted in spinal cord sections after injury. The expression of TNF-α and IL-1 β in spinal cord injury group was significantly higher than that in VAP group, MP group and VAP+MP group (P < 0.001). The number of TNF-α and IL-1 β expression in VAP+MP group was significantly lower than that in VAP group and MP group (P < 0 01 and P < 0 01) (Figure 3B and 3C) [15].
Figure 3: Sodium valproate(VAP)combined with methylprednisolone (MP) enhances tissue recovery and reduces TNF-a, IL-1ß expression after spinal cord injury (SCI). SCI group, Sodium valproate(VAP, methylprednisolone (MP), and Sodium valproate(VAP)combined with methylprednisolone (MP) mice groups were subjected to Immunohistochemical staining TNF-a expression (A) andIL-1ß expression (B) as explained in the Materials and Methods section. (C) Number of TNF-a expression (n=6). (D) Number of IL-1ß expression (n=6).*P < 0.05, **P < 0.01, Comparison of VAP group and MP group with VAP+MP group respectively. Data expressed as mean ± standard deviation. Scale bar=100 µm. VAP, Sodium valproate; MP, methylprednisolone.
VAP combined with MP reduces apoptosis after SCI
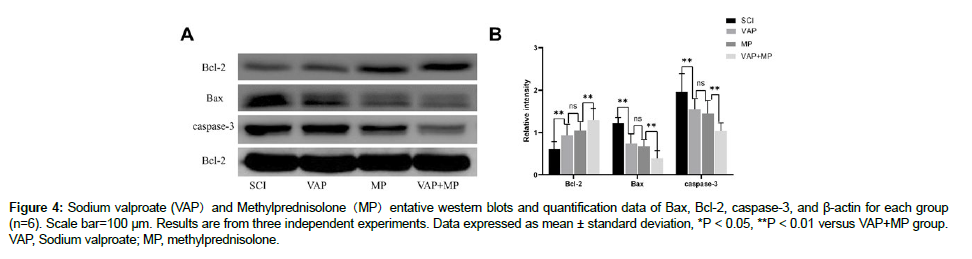
The expression of apoptotic related proteins, Bcl-2, caspase-3, and Bax, were increased when compared with the SCI group following Spinal cord injury. VAP, MP and VAP+ MP treatment significantly decreased the expression of Bax and caspase-3, while increasing the Bcl-2 expression (P < 0.05) (Figure 4). Caspase-3 expression was significantly lower in VAP, MP and VAP+MP mice than the SCI mice and VAP+MP is more effective than VAP and MP groups (P < 0.05 and P < 0.01) in the spinal cord sections Immunoreactivity staining [16].
Figure 4: Sodium valproate (VAP)and Methylprednisolone(MP)entative western blots and quantification data of Bax, Bcl-2, caspase-3, and ß-actin for each group (n=6). Scale bar=100 µm. Results are from three independent experiments. Data expressed as mean ± standard deviation, *P < 0.05, **P < 0.01 versus VAP+MP group. VAP, Sodium valproate; MP, methylprednisolone.
Discussion
SCI is a life-changing catastrophic event that can lead to permanent neurological dysfunction and other serious complications (Freund et al., 2019). Unfortunately, so far, no fully restorative treatment has been found. The difficulty in the treatment of spinal cord injury is closely related to the timing of secondary injury, including destruction of tissue integrity, vascular and axon injury, cell edema and cell membrane damage, and the establishment of an adverse environment that includes a wide range of inflammatory cells, glial scars and molecular inhibitors (Courtine and Sofroniew, 2019). The current treatment strategy is to inhibit inflammatory response after SCI, reduce neuronal apoptosis and protect residual spinal cord function (Hutson and Di Giovanni, 2019). Because the primary SCI is directly damaged, and the secondary SCI causes continuous destruction of nerve tissue gradually, and can be intervened by medical means [17]. Inflammatory response after SCI plays a key role in secondary injury. The control of early inflammatory response can inhibit neuronal apoptosis and facilitate the recovery of spinal cord function (Pukos et al., 2019). In this experiment, the expression of TNF-α and IL-1 β in SCI tissue was measured 7 days after SCI, and the degree of inflammatory response after SCI was measured. TNF-α is a key inflammatory initiator and plays a pivotal role in inflammation. IL-1 β, as an important proinflammatory substance, is involved in inducing apoptosis of neurons and glial cells. Previous experiments have confirmed that the protein expression of Bcl-2, Bax and Caspase-3 is related to the apoptosis of rat spinal cord neurons, and the proportion of Bcl-2/Bax protein expression in spinal cord cells can indicate the degree of apoptosis (Lu et al., 2019). Therefore, in this experiment, in addition to selecting the expression of TNF-α and IL-1 β, evaluating the effect on the inflammatory response after SCI, selecting the expression of related apoptotic proteins Bcl-2, Bax and caspase-3 to evaluate the apoptosis, while selecting BBB and Rivlin score, HE and Nissl staining to supplement, comprehensively evaluate the improvement of neurological function [18].
Previous experimental studies have confirmed that early use of MP in SCI can significantly inhibit inflammatory response, while VPA can not only reduce inflammatory reaction, but also has neurotropic and protective effects, but whether the combined use of the two has the superposition or synergistic effect of SCI recovery has not been reported in the literature (Ahuja et al., 2017b). VPA is a short-chain fatty acid, which can exert its effect through the blood-brain barrier. It is widely used in clinical treatment as an emotional stability and antiepileptic drug (Chen, Ye, 2018b) [19]. It has been found that VAP can resist inflammation, resist apoptosis, reduce lipid peroxidation and nourish nerve cells (Pandamooz et al., 2019). Experiments have confirmed that VPA can reduce the activation of NF-κ B and reduce inflammatory responses, such as TNF-α, IL-1 β and so on (Warner et al., 2019). At the same time, it reduces the activation of JAK2/STAT3 signal pathway, reduces the production of downstream caspase-3 and other proteases, and reduces the apoptosis of nerve cells [20]. VPA can enhance the expression of heat shock protein 70 (heat stress protein-70, HSP70) in the brain. The HSP70 family, which has a strong protective effect on cells, can inhibit the formation of apoptotic bodies and the activation of downstream Caspase-3 through a variety of ways, and block nerve cell apoptosis and reduce scar formation by activating the expression of B lymphocyte tumor-2 (Yoshizumi et al., 2013). At the same time, VPA inhibits the expression of pro-apoptotic factors caspase-3 and Bax, promotes the production of anti-apoptotic factor Bcl-2, protects neurons and thus inhibits neuronal apoptosis (Lee et al., 2012). MP is a glucocorticoid, which can exert powerful anti-inflammatory and antioxidant effects, and can effectively reduce secondary injury after SCI. It is a common method of clinical drug treatment at present (Liu et al., 2019) [21]. The infiltration and aggregation of inflammatory cells caused by the release of a large number of inflammatory factors after SCI is the main cause of nerve cell necrosis, apoptosis and autophagy (Liu et al., 2020). Studies have shown that early use of MP can not only effectively reduce NF-κB protein expression, thereby preventing NF- κB from binding to the inflammatory response control gene κB site, reduce TNF-α, IL-1β gene transcription, and reduce JAK2/STAT3 activation (Vidal et al., 2018). Reduce all downstream tyrosine residues, such as caspase-3, etc., so as to reduce the protease cascade reaction, inhibit the production of Bax and caspase-3, increase the expression of anti-apoptotic factor Bcl-2, reduce neuronal apoptosis, and produce oxygen-containing compounds with anti-apoptotic effects, which can improve spinal cord function and reduce apoptosis at the same time. Celik (Celik et al., 2016) confirmed that the use of MP in SCI in the early stage can significantly relieve spinal cord edema and maintain the morphological structure and contour integrity of the injured spinal cord [22].
To sum up, the production of TNF-α and IL-1 β increased significantly after SCI, while the expression of TNF-α and IL-1 β in VPA+MP group was significantly lower than that in SCI group, VAP group and MP group at each time point, indicating that the effect of VPA and MP on spinal cord function recovery may be related to TNF-α and IL-1 β. HE and Nissl staining results also showed that VPA+MP treatment group was better than VAP treatment group and MP treatment group in inhibiting neuronal apoptosis. The same results can be obtained by BBB and Nissl scores and inhibition of apoptosis-related protein Bax and caspase-3 expression. After the early treatment of SCI with MP and daily continuous administration of VAP, the significant effect of combined treatment may be related to the maintenance of continuous anti-inflammatory effect [23].
Therefore, compared with the single use of VAP or MP, combined with VAP and MP, it has a stronger inhibitory effect on some inflammatory factors and neuronal apoptosis after SCI in rats, and the two may have synergistic or superimposed effects. Due to the small number of samples in this experiment, the relevant parameters are not comprehensive, in the future experiments, to make up for these deficiencies, the specific molecular mechanism and signal transduction pathway need to be further explored [24].
Acknowledgments and Funding
We are grateful to Hebei North University for their technical assistance. This work was supported by Hebei Provincial Science and Technology Plan-Science and Technology Winter Olympics Special (20477707D); 2023 Zhangjiakou Science and Technology Plan Project (2322032D); 2024 Hebei Province Medical Applicable Technology tracking item (GZ2024093).
Conflict of Interest
All authors declare no conflicts of interest.
References
- Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, et al. (2017) Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 80: 9-22.
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, et al. (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3: 17-18.
- Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotors rating scale for open field testing in rats. J Neurotrauma 12: 1-21.
- Celik H, Karatay M, Erdem Y, Yildirim AE, Sertbas I, et al. (2016) The Biochemical, Histopathological and Clinical Comparison of the Neuroprotective Effects of Subcutaneous Adalimumab and Intravenous Methylprednisolone in an Experimental Compressive Spinal Cord Trauma Model. Turk Neurosurg 26: 622-31.
- Chen JY, Chu LW, Cheng KI, Hsieh SL, Juan YS, et al. (2018) Valproate reduces neuroinflammation and neuronal death in a rat chronic constriction injury model. Scientific reports 8: 16-57.
- Chen S, Ye J, Chen X, Shi J, Wu W, et al. (2018) Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflammation. 15: 150.
- Courtine G, Sofroniew MV (2019) Spinal cord repair: advances in biology and technology. Nat Med 25: 898-908.
- Freund P, Seif M, Weiskopf N, Friston K, Fehlings MG, et al. (2019) MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol 18: 1123-35.
- Fu XM, Wang Y, Fu WL, Liu DH, Zhang CY, et al. (2019) The Combination of Adipose-derived Schwann-like Cells and Acellular Nerve Allografts Promotes Sciatic Nerve Regeneration and Repair through the JAK2/STAT3 Signaling Pathway in Rats. Neuroscience 422: 134-45.
- Hutson TH, Di Giovanni S (2019) The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat Rev Neurol 15: 732-45.
- Keles I, Bozkurt MF, Aglamis E, Fidan AF, Ceylan C, et al. (2019 ) Protective effects of dantrolene and methylprednisolone against spinal cord injury-induced early oxidative damage in rabbit bladder: A comparative experimental study. Adv Clin Exp Med 28: 1697-704.
- Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, et al. (2012) Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121: 818-29.
- Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, et al. (2014) Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. J Neurotrauma 31: 582-94.
- Liu LJW, Rosner J, Cragg JJ (2020) Journal Club: High-dose methylprednisolone for acute traumatic spinal cord injury: A meta-analysis. Neurology 2020.
- Liu Z, Yang Y, He L, Pang M, Luo C, et al. (2019) High-dose methylprednisolone for acute traumatic spinal cord injury: A meta-analysis. Neurology 93: 41-50.
- Lu P, Gomes-Leal W, Anil S, Dobkins G, Huie JR, et al. (2019) Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Reports 13: 105-14.
- Pandamooz S, Salehi MS, Zibaii MI, Safari A, Nabiuni M, et al. (2019) Modeling traumatic injury in organotypic spinal cord slice culture obtained from adult rat. Tissue Cell 56: 90-7.
- Park J, Zhang Y, Saito E, Gurczynski SJ, Moore BB, et al. (2019) intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc Natl Acad Sci 116: 47-54.
- Pukos N, Goodus MT, Sahinkaya FR, McTigue DM (2019) Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 67: 2178-202.
- Vidal PM, Ulndreaj A, Badner A, Hong J, Fehlings MG (2018) Methylprednisolone treatment enhances early recovery following surgical decompression for degenerative cervical myelopathy without compromise to the systemic immune system. J Neuroinflammation 15: 222.
- Warner FM, Jutzeler CR, Cragg JJ, Tong B, Grassner L, et al. (2019) The Effect of Non-Gabapentinoid Anticonvulsants on Sensorimotor Recovery After Human Spinal Cord Injury. CNS Drugs 33: 503-11.
- Yoshizumi M, Eisenach JC, Hayashida K (2013) Valproate prevents dysregulation of spinal glutamate and reduces the development of hypersensitivity in rats after peripheral nerve injury. J Pain14: 85-91.
- Zheng B, Ye L, Zhou Y, Zhu S, Wang Q, et al. (2016) Epidermal growth factor attenuates blood‐spinal cord barrier disruption via PI3K/Akt/Rac1 pathway after acute spinal cord injury. J Cell Mol Med 20: 62-75.
- Zhu N, Ruan J, Yang X, Huang Y, Jiang Y, et al. (2020) Triptolide improves spinal cord injury by promoting autophagy and inhibiting apoptosis. Cell Biol Int 44: 85-94.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Yang X, Du Y, Sun J, Zhai X (2024) Effect of Combined Treatment with Sodium Valproate and Methylprednisolone on Neurological Recovery after Experimental Spinal Cord Injury. Cell Mol Biol, 69: 304
Copyright: © 2024 Yang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2436
- [From(publication date): 0-2024 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 2087
- PDF downloads: 349