Research Article Open Access
Effect of Arsenite and Arsenate on Lipid Peroxidation, Enzymatic and Non-Enzymatic Antioxidants in Zea mays Linn
| Ranjeet Kumar Yadav* and Shailendra Kumar Srivastava | |
| Department of Biochemistry and Biochemical Engineering, Sam Higginbottom Institute of Agriculture, Technology and Sciences, Allahabad- 211007 (U.P) India | |
| Corresponding Author : | Ranjeet Kumar Yadav Department of biochemistry and Biochemical Engineering Sam Higginbottom Institute of Agriculture, Technology and Sciences Allahabad- 211008, U.P., India Tel: +91-9454989762 Fax: 91-532-2684394 E-mail: ranjeetmsbc@gmail.com |
| Received: September 23, 2015; Accepted: October 16, 2015; Published: October 23, 2015 | |
| Citation: Yadav RK, Srivastava SK (2015) Effect of Arsenite and Arsenate on Lipid Peroxidation, Enzymatic and Non-Enzymatic Antioxidants in Zea mays Linn. Biochem Physiol 4:186. doi: 10.4172/2168-9652.1000186 | |
| Copyright: © 2015 Yadav RK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The effect of As (III) and As (V) was investigated in nutritionally important plant, Zea mays L. and the response on malondialdehyde (MDA) content, Glutathione, Protein, Chlorophyll A, Chlorophyll B, Carotenoids and the enzymatic antioxidants viz., superoxide dismutase (SOD), peroxidase (POD) and ascorbate peroxidase (APX) was found in leaves of the metal treated plants. The effect of As (III) and As (V) were subjected to 5 ppm and 10 ppm treatments for 7 days and 14 days as respect to controls. The level of lipid peroxidation was significantly increased at higher exposure treatments (10 ppm). Arsenite (III) induced stress was observed as indicated by high level of lipid peroxidation, than arsenate (V). The level of Protein content decrease in the leaves at all the As [III] and As [V] concentrations at 7 days and 14 days as compared to their respective controls and with maximum significant increase of 28.8% (7 d As [V] at 5 ppm), significant (p<0.05) decrease in protein content was observed with maximum decrease of 43.3% (14 d As [III] at 10 ppm), compared to control. The chlorophyll and carotenoid content are also decreased in part of the overall expression of Arsenic exposures. The level of SOD, APX and POD was at high in Arsenite (III) however low in arsenate (V). Arsenite (III) induced stress was observed as indicated by high level of lipid peroxidation, than arsenate (V). The oxidative stress may increase the production of free radicals and subsequently resulted in peroxidation of lipids. Further, addition of arsenic increased the activity of superoxide dismutase (SOD) which may be due to enhanced production of oxygen free radical and related tissue damage. Correlation coefficient was evaluated between different antioxidant parameters with lipid peroxidation.
| Keywords |
| Arsenite; Arsenate; Antioxidant; Antioxidant enzyme; Lipid peroxidation; Zea mays |
| Introduction |
| It is well documented that Arsenic (As) is a semi-metal element in the periodic table with number 33. It is widely distributed in the Earth’s crust, and has a steel grey metal-like colour. Arsenic is a naturally occurring metalloid element that is found in soil, air and water [1,2]. It has been reported that in West Bengal and Bangladesh, high level of arsenic occur in ground water, and recently reports are emerging from India’s states like Uttar Pradesh, Assam, Bihar and Jharkhand. It has been reported that irrigation with As-contaminated water causes accumulation of as in the soil and eventually this is reflected in higher levels of as in the edible parts of the crops [3,4]. Nowadays, Arsenic is a big issue of environmental pollution. In recent years, arsenic has received great attention as a global problem [5]. It is highly toxic for plants and animals, at elevated concentrations. In plants, arsenic is accumulated mainly in the root system, to a lesser degree in the above ground organs, and causes physiological changes and damages, and reduction in the crop productivity [6,7]. Environmental arsenic exists in both organic and inorganic states. Organic arsenicals are generally considered nontoxic [8], whereas inorganic forms are toxic. Inorganic arsenic exists predominantly in trivalent As (III) and pentavalent As (V) forms, where trivalent compounds are more toxic than pentavalent ones [2,9]. Under aerobic conditions, pentavalent arsenic is more stable and predominates, whereas trivalent species predominate under anaerobic conditions [2]. Arsenic concentrations in uncontaminated soil are generally in the range 0.2–40 mg/kg [10]. However, levels of 100–2500 mg/kg have been found in the vicinity of copper smelters [10,11]. In the past, numerous arsenical pesticides were used widely and, as a result, arsenic concentrations of 200–2500 mg/kg occurred in the soil of orchards [12]. Arsenic is an active component of antifungal wood preservatives (e.g., Wolman’s salt, which contains 25% sodium arsenite). In the United States, 74% of arsenic is contained in products used for wood preservation [13]. More common drinking-water sources generally contain arsenic at concentrations of less than 10 μg/litre. With the exception of some kinds of seafood, most foods contain low levels of arsenic, normally less than 0.25 mg/kg. Marine organisms may contain large amounts of organo-arsenicals (e.g., arsenobetaine). These arsenic derivatives are not acutely toxic because of their low biological reactivity and their rapid excretion in urine. Concentrations in seafood amount to 2.4–16.7 mg/kg in marine fish, 3.5 mg/kg in mussels [14] and more than 100 mg/kg in certain crustaceans [15]. Wine made from grapes sprayed with arsenic pesticides may contain appreciable levels of arsenic (up to 0.5 mg/litre) in the trivalent inorganic form [16], however, excess concentrations are believed to generate oxidative stress as understood by an increase in the steady state concentration of reactive oxygen species (ROS) within subcellular compartments of the plant cell which are derived from the metabolism of oxygen. ROS can directly damage proteins, amino acids and nucleic acids and cause peroxidation of membrane lipids [17]. To combat these effects, enzymatic and nonenzymatic antioxidants are mobilized to quench ROS. It has been demonstrated that in Zea mays, catalase and SOD were all stimulated after exposure to arsenic [18]. Arsenic accumulated in the plant tissue stimulates peroxidase synthesis during the early phases of plant development, long before the appearance of visible changes [19,20]. Reactive oxygen species which include superoxide anion radical (OÍÂ?2•), hydrogen peroxide (H2O2), hydroxyl radical (OH •) and singlet oxygen (1O2), are amongst the most reactive compounds known to be produced during heavy metal stress [21]. These protective enzymes include antioxidant enzymes such as superoxide dismutase (SOD), peroxidases like ascorbate (APX) and guiacol (POD) peroxidase. SOD is located in various cell compartments and catalyzes the disproportionation of two O2 radicals to H2O2 and O2. Ascorbate peroxidase is primarily located in chloroplast and cytosol and it is the key enzymes of the ascorbate– glutathione cycle that uses ascorbate as reducing substrate for H2O2 detoxification. However, hydrogen peroxide is also toxic to cells. In plant cells, the most important reducing substrate for H2O2 detoxification is ascorbate [22]. Inorganic As was the major species in plants, and mycorrhizal inoculation generally decreased concentrations of arsenite [As (III) in maize roots and concentrations of As (III) and arsenate As (V)] in the shoots. However, attention has not been given to understand the mechanism of tolerance in the plant. Thus, it was thought to be of more relevance to study the effect of Arsenic on the antioxidant levels in the plant of Zea mays. It is obvious that these results are more reliable to understand the mechanism of action in living plant under laboratory conditions. |
| Materials and Methods |
| Experimental setup and design |
| Seeds of Zea mays L. var. Ruchi (cv. SRHM 445) were procured from Sri Rama Agri- Genetics (India) Pvt. Ltd., Kurnool Andhra Pradesh. The disinfected seeds of maize were germinated in plastic trays, placed between blotting sheets moistened by modified 30% Hoagland’s nutrient solution supplementing with 5 μg ml-1 of Fe-EDTA [23]. After 7 d of growth, three uniform size seedlings (6-8 cm) were transferred to glass beaker (250 ml) containing 100 ml of 30% Hoagland’s nutrient solution. The plants were placed in the beaker allowing only the roots to remain submerged in the nutrient solution and the aerial parts of the plants were erect and exposed to light. Under such conditions, the plants were allowed to grow for another 7 d for normalization. The glass beakers were covered with black coating in order to restrict the roots from exposure to light and this condition was maintained throughout the experiment. The different concentrations (5 ppm and 10 ppm) of Sodium arsenite and Sodium arsenate were prepared in 10% Hoagland’s solution using ferric chloride (FeCl3). Three sets of each concentration (three separate beaker for each concentrations served as three replicates) were kept in 100 ml beaker (100 ml solution) containing different concentrations (5 ppm and 10 ppm) of As (III) and As (V) in 10% Hoagland’s solution. The pH of the Hoagland’s nutrient solution and treatment solutions ranged between 6.8 and 7.0. The treatments were applied after 7 d of normalization after transferring the plants to the glass beakers. During the entire experiment the nutrient solution were replaced after every 2nd day. Plants were harvested for analysis after 7 d (1st harvest) and 14 d (2nd harvest) of treatment. After harvesting, these plants were used for the determination of various biochemical parameters. |
| Malondialdehyde (MDA) content |
| The lipid peroxidation in the plant tissue was measured in terms of malondialdehyde (MDA) content determined by the thiobarbituric acid (TBA) reaction following the method of Heath and Packer, 1968 [24]. The plant samples (leaves, 300 mg each) were homogenized in 3 ml of 0.2%, trichloroacetic acid. |
| Estimation of antioxidants |
| Plant tissues, leaves (200 mg each) were homogenized in 2 ml of 100 mM potassium phosphate buffer, pH 7.5 containing 1 mM of EDTA in presence of pinch of polyvinyl polypyrrolidone (PVP). The homogenate was centrifuged at 12000 g for 15 min at 4ºC. All steps in the preparation of enzyme extract were carried out at 0–4ºC. This supernatant was used to measure the activities of superoxide dismutase, ascorbate peroxidase and guiacol peroxidase. |
| Superoxide dismutase (EC 1.15.1.1): The assay system for superoxide dismutase was adopted from the method of Nishikimi and Rao, 1972 [25] and activity was expressed as units per g fw. Assay mixture contained 1.2 ml sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml 186 μM phenazine methosulphate, 0.3 ml 300 μM nitroblue tetrazolium, 0.2 ml NADH (780 μM), 50 μl plant extract and water (1.15 ml) in a total volume of 3 ml. |
| Ascorbate peroxidase (EC 1.11.1.11): The activity of ascorbate peroxidase was measured of the method of Nakano and Asada, 1981 [26] by estimating the rate of ascorbate oxidation at 290 nm. Enzyme activity was calculated in terms of μ mol of ascorbate oxidized min-1 g-1 fresh weight at 25 ± 2ºC. |
| Guiacol peroxidase (EC 1.11.1.7): Guiacol peroxidase was measured in plant parts, following the method of Curtis, 1971[27], modified by Kato and Shimizu, 1987 [28]. Activity was calculated using the extinction coefficient of 26.6 mM-1 cm-1 at 470 nm for oxidized tetraguiacol polymer. One unit of peroxidase activity was defined as the calculated consumption of 1 μmol of H2O2 min-1 g-1 fresh weight. |
| Chlorophyll and carotenoid content: Plant tissues, leaves (200 mg) were crushed in 5 ml of (80%, v/v) chilled acetone by the method of Arnon, 1949; Duxbury and Yentsch, 1956 [29,30]. Extract was centrifuged at 10,000 g for 10 min and absorbance of the supernatant was read at 510 and 480 nm using GBC Cintra 10e UV-VIS Spectrophotometer. Carotenoid content was calculated in mg g-1 fw by the formula as given below (Duxbury and Yentsch, 1956) [31]. |
| Carotenoid (mg g-1fw) = [[7.6 (A480) – 2.63 (A510)] x V] / 1000 x W |
| Where: |
| A510 and A480 = Absorption at these wavelengths |
| V=Final extract volume (ml) |
| W=Weight of sample (g) |
| Protein content: Protein was estimated by the method of Lowry et al. [32], using BSA as a standard protein, is a globular protein of molecular weight 68,000. Leaves (100 mg) of treated and control plants were crushed in 3 ml of 10% chilled tricholoracetic acid (TCA) and centrifuged at 10,000 g for 10 min and then last, the absorbance was recorded at 660 nm using bovine serum albumin (BSA) as a standard. |
| Glutathione (GSH): GSH also participates in the detoxification of xenobiotics as a substrate for the enzyme glutathione-S-transferase. The well documented tolerance of maize to the triazine herbicides is the result of conjugation of GSH to the herbicide [33]. The measurement of total GSH assay was estimated by the method of Gomez and Gomez, 1984 [34], plant tissue (300 mg) was homogenized in 0.1 M sodium phosphate −0.005 M EDTA buffer (pH, 8.0, 6 ml) and 25% HPO3 (1 ml) which was used as a protein precipitant. The total homogenate was centrifuged at 10,000 g for 20 min at 4°C and then we prepared reaction mixture as following, NADPH- 1.4 ml, DTNB- 0.2 ml and plant extract- 0.4 ml mix gently and transfer the cubit to record absorbance at 30ºC for 15 minutes of these solution at 412 nm. |
| Statistical analysis |
| The whole experiment was set up in the randomized block design. All the data were subjected to an analysis of variance (ANOVA) using Microsoft Excel 2010 followed by least significant difference (LSD) between treatments to test the significance as compared to control. The correlation coefficient (r) significant at 5% level (p<0.05) was evaluated between various physiological parameters of the plants. Similarly, the correlation coefficient (r) was also calculated between various antioxidant parameters and MDA content in both parts of the plants [35]. |
| Results |
| Effect on malondialdehyde (MDA) content |
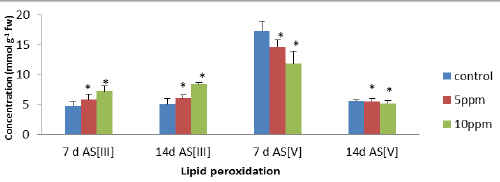
| The level of peroxidation was measured as MDA content which was considered as a general indicator of lipid peroxidation. In leaves, the level of lipid peroxidation was significantly increased at higher exposure treatments (10 ppm). The maximum significant (p<0.05) increases of 51.69% (7 d AsIII) and 66.47% (14 d AsIII) were observed in leaves, respectively as compared to controls. The results were compared between different exposure treatments, which showed decrease in MDA content of 31% and 6.9% in the leaves after 7 d As [V] and 14d As [V] at all the metalloid concentration as compared to controls. Malondialdehyde (MDA) is often used as an indicator of peroxidation of membrane lipids in plants (Figure 1). |
| Effect on the activities of some antioxidant enzymes |
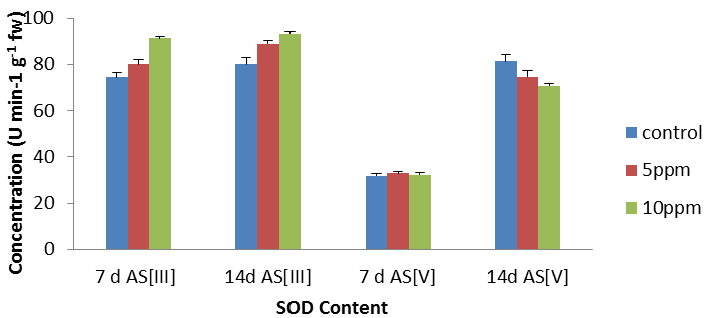
| The activities of these enzymes in leaves of the plant subjected to metal stress are presented in Figure 2. In leaves (Figure 2), an increase in SOD activity was found with increase in metal concentrations except 14 d As [V] as compared to their respective controls. In leaves, SOD activity increased with increase in metal concentrations with maximum stimulation of 22.56% (p<0.05). The maximum decrease of 13% was recorded respectively at 14 d As [V] at 10 ppm as per graphical analysis (Figure 2). In the present experiment, an increase in the SOD activity was found in the leaves, [36] also reported an increase in SOD activity in Fe treated plants of Hydrilla verticillata. |
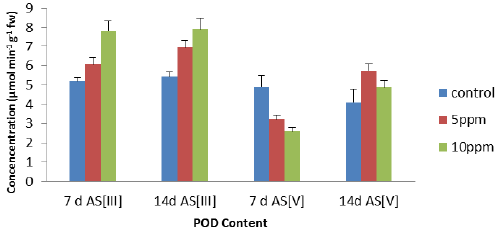
| The peroxidase activity has been assayed with guiacol as universal substrate. An increase, all POD activity of the leaves (Figure 3) was found with increase in metal concentrations except at 7 days As [V]. The maximum increase of 51% (7 d As [III]), 46% (14 d As [III]) and 18.6% (14 d As [V]) but only decreased of the 46.7% (7 d As [V]) at 10 ppm concentration as compared to control. On the contrary, POD activity of the leaves (Figure 3) decreased with increase in metal concentrations in 7 d As [V] as compared to control and has shown negative correlation. |
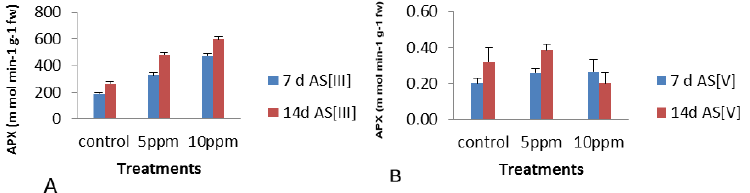
| The antioxidant enzymes are important components in preventing the oxidative stress in plants as is based on the fact that the activity of one or more of these enzymes is generally increased in plants when exposed to stressful condition and this elevated activity correlated the increased stress tolerance. APX activity was found to maximum increase of 156%, 126% and 33.6% at 10 ppm concentration of As was observed at 7 d As [III], 14 d As [III] and 7 d As [V] and the maximum decreased of 37% (p<0.05) at 14 d As [V] as per graphical analysis of Figure 4 as compared to control. Thus on the other way, APX activity increased under metal stress as compared to control with the exception at 14 d As [V]. |
| Effect on antioxidants |
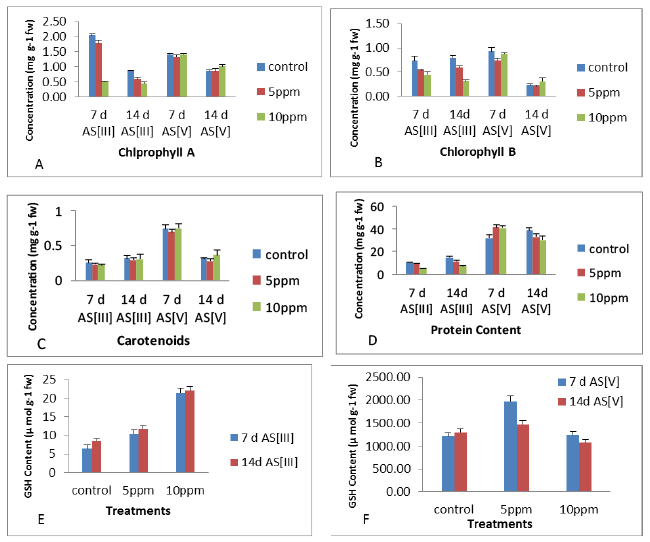
| The results showed an decrease in protein content (Figure 5) of leaves at all the exposures of As [III] and As [V] concentrations at 7 d and 14 d as compared to their respective controls with maximum significant increase of 28.8% (7 d As [V] at 5 ppm), significant (p<0.05) decrease in protein content was observed with maximum decrease of 43.3% (14 d As [III] at 10 ppm), compared to control. In leaves, the level of GSH content increased with increase in metal concentrations at 14 d, however, decrease was observed at higher metal concentrations (10 ppm after 14 d As [V]) as compared to control. The maximum increase of 232% at 10 ppm concentration of 7 days As [III] and 160 % at 10 ppm concentration of As [III] was observed at 14 days as compare to controls while the maximum increase of 232% was observed at 10 ppm concentration of As [III] after 7 days, compared to control as shown in Figure 5. In leaves, the level of chlorophyll A, B and carotenoid content (Figure 5) decreased with increase in metal concentrations at 7 days and 14 days as compared to controls. The concentration wise analysis of the result showed decrease in chlorophyll a, b and carotenoid content in the leaves with increase in concentration upto 10 ppm after 7 days as compared to controls. The maximum decrease of 74.95%, 39.23% and 12% was observed at 10 ppm after 7 days of exposure period respectively. Among antioxidant enzymes, APX was positively correlated with MDA content in the leaves at 7 days and 14 days, in contrast to POD which has shown positive correlation with MDA in leaves at 7 days and 14 days. Among non-enzymatic antioxidants, negative correlation was observed for all the parameters with MDA of the leaves at all the exposures periods with the exception of GSH at 14 days and Protein content at all the exposures. In leaves, all the antioxidants have shown positive correlation with MDA content at initial period of exposure. Thus, the correlation coefficient obtained for most of the antioxidant parameters and MDA content in leaves at initial period of exposure was found positive (Table 1). |
| Discussion |
| A marked decrease in shoot elongation and number of green leaves was observed with arsenic treatments, as compared to control, whereas accumulation of arsenic and malondialdehyde (MDA) in seedlings were increased significantly with increasing arsenic derivatives concentration, i.e., As (III) and As (V). Excess level of metal ions particularly redox metals have been reported to induce the production of ROS in plants [37]. The induction of a particular group of enzyme activities is considered to play an important role in the cellular defence strategy against oxidative stress, caused by toxic metal concentrations. The elimination of H2O2 has been shown to be an important function in the plant peroxidases that uses ascorbate as the hydrogen donor [38]. Apart from these enzymes, some non-enzymatic antioxidants like cysteine, non-protein, Proline, carotenoids and ascorbic acid may play a role in inducing resistance to metals by protecting labile macromolecules against attack by free radicals which are formed during various metabolic reactions leading to oxidative stress [39]. The induction of a particular group of enzyme activities is considered to play an important role in the cellular defence strategy against oxidative stress, caused by toxic metal concentrations. |
| Conclusion |
| The differential responses of antioxidant enzymes in the plant parts are observed on metal treatment. The increased in As [III] MDA content was found in leaves but decreased in As [V] MDA content under stress condition and consequently less induction in antioxidant levels of the leaves which is also evident from positive correlation between MDA and antioxidants levels only except of Protein. SOD and H2O2 eliminating enzymes (POD and APX) respond in a similar way at 7 days and 14 days As [III] in leaves, however, at 14 days As [V], these enzymes have shown different response. Further, the data suggest that MDA and APX play an important role in antioxidant defense in leaves of the plant which showed coordinated increase in their levels. On the basis of above data, we conclude that the protective effect of as in stressed Z. mays leaves may be mainly due to stimulation of APX and non-enzymatic antioxidants. The study provides evidence that the plants have shown high accumulation of Arsenic without any visible symptom of toxicity and grow well in metal contaminated water. In these plants, antioxidants are sufficient to prevent biological damage mediated by ROS which keeps the deleterious reaction to a minimum. |
| Acknowledgements |
| The authors are thankful to Dr. Shekhar Malick, CSIR-National Botanical Research Institute and Dr. Alok M Lal (Department of Biochemistry, SHIATSAllahabad) for providing required research facilities. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14889
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10215
- PDF downloads : 4674
