Research Article Open Access
Effect of an Integrated Nutraceutical Formula on Key Inflammatory Regulators in Subjects with Metabolic Syndrome Features: A Randomized, Double-Blind Study
Fang He1, Nikhil Kumar2, Francesco Marotta3*, Birbal Singh4, Alexander N Khokhlov5, Manoj Kumar6, Angelo Italia7, Aldo Lorenzetti3, Makoto Kantah3 and Roberto Catanzaro71Department of Nutrition and Food Hygiene, West China School of Public Health, Sichuan University, Chengdu, Sichuan, P.R. China
2Department of Life Sciences, Shri Venkateshwara University, JP Nagar, Uttar Pardesh, India
3San Babila Clinic, ReGenera Research Group for Aging-Intervention, Milano, Italy
4ICAR-Indian Veterinary, Research Institute, Regional Station, Palampur, Himachal Pradesh, India
5Evolutionary Cytogerontology Sector, School of Biology, Moscow State University, Moscow, Russia
6Department of Clinical Microbiology and Immunology, National Institute of Nutrition, ICMR Hyderabad, India
7Department of Internal Medicine, University of Catania, Catania, Italy
- *Corresponding Author:
- Francesco Marotta
San Babila Clinic, ReGenera Research Group for Aging-Intervention
Corso Matteotti, 1/A, 20121 Milano, Italy
Tel: +39-024077243
E-mail: fmarchimede@libero.it
Received date: February 01, 2017; Accepted date: February 20, 2017; Published date: February 25, 2017
Citation: He F, Kumar N, Marotta F, Singh B, Khokhlov AN, et al. (2017) Effect of an Integrated Nutraceutical Formula on Key Inflammatory Regulators in Subjects with Metabolic Syndrome Features: A Randomized, Double-Blind Study. Clin Pharmacol Biopharm 6:167. doi: 10.4172/2167-065X.1000167
Copyright: © 2017 He F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
About one fourth of all American adults and a bit less than one sixth of Europeans are reported to be affected by metabolic syndrome. Aging seem associated to a consistent increase of this phenomenon which is now clear to be associated with a low-grade pro-inflammatory cascade. Dyslipidemia is a characteristic factor involved in this multifaceted metabolic setting and this provides new avenues to non-chemical biopharmaceutical interventions. Eighty-two patients (66 males and 16 females, 38-69 as age range) with metabolic syndrome were selected randomly. Patients meeting inclusion criteria were advised to adhere to a standard balanced diet but no specific dietary calorie-restriction or life-style modifications. Two matched patients groups were assigned either to 1 tab/ dinner of drug A and another group received 1 tab/dinner of drug B, both for 3 months. Physicians and patients were blinded as for the content of the tablets except being aware that one being P3/GB-2016 while the other a looking alike placebo. A third group of 25 healthy, normal-weight subjects without any biochemical abnormalities served as control. The mean HbA1c level showed a trend decrease in group I at 3-month observation but this group showed a significant decrease of total and LDL cholesterol, non-HDL aliquot and triglycerides throughout the study period (p<0.05). Serum concentration of either MIP1α or MIF were significantly higher in dysmetabolic patients as compared to healthy control (p<0.01). Treatment with P3/GB-2016 proved to significantly decrease both parameters at 3-month observation (p<0.01). Whereas circulating levels of MCP-1 appeared showed a wide dispersion of data and appearing to be higher in those subjects with highest (n.s.). Overall, P3/GB-2016 seems to be an effective oral agent in controlling dyslipidemia and key regulatory modulator within the management of metabolic syndrome.
Keywords
Dyslipidemia; Metabolic syndrome; Cardiovascular risk; Monacolin; Caviarlieri
Introduction
Dyslipidemia is the most fundamental risk factor for atherosclerotic cardiovascular disease and its associated complications, being a major cause of premature death worldwide. Levels of lipids, particularly LDL cholesterol, increase slightly during aging process in men but also in women after menopause. On the other hand atherosclerosis is associated to high total cholesterol level increases even before it shows a clearly pathological value. This is to say that the subtle lipid abnormalities may be already affecting coronary and cerebrovascular arterial network. This is essentially due to a pattern of lipoprotein abnormalities-or atherogenic lipoprotein phenotype (high VLDL levels, high small LDL particles and low HDL-cholesterol). Although an over 30% decline in cardiovascular disease has been noted in the past 15 years [1], it still accounts for 1 out of every 3 deaths in US [2]. Along this period, dyslipidemia has stepped on the front line as risk factor for cardiovascular disease. In this regard, the Cholesterol Treatment Trialists’ Collaborators has shown that a 1.0 mmol/l reduction in low-density lipoprotein cholesterol (LDL-C) brought about a 9% reduction in all-cause mortality and a quite remarkable 25% decrease in cardiovascular events, whatever the risk profile [3]. Growing incidence of obesity [4] and diabetes [5-7] have also significantly affected the current pathophysiology of cardiovascular disease. Indeed, it is now well established that all the above conditions share a number of mechanisms within a complex metabolic picture which has come clear along fundamental studies such as Framingham Heart Study [8]. As a matter of fact, metabolic syndrome has come to be an epidemic health care of large proportion affecting industrialized [9] but also developing [10] countries. It has been calculated that, given the present trend, at least 50% of people over 60 years old may be affected. This multifaceted syndrome is an insulin resistant condition comprising a spectrum of impairments such as hyperglycemia, hypertension, dyslipidemia and accelerated cardiovascular disease. In 2013 the American College of Cardiology/ American Heart Association, not without some internal disagreements, had defined four “statins benefit groups” in whom the risk reduction benefits would significantly outweigh the potential adverse events [11]. However, in spite of a recent review, balancing pros and cons, sheds an overall reassuring opinion regarding the use of statins [12], the fact remains that other authors have pointed out some warnings about its controversial widespread use [13,14]. Indeed, besides the already well-reported and not infrequent cases of myopathy [15], some concern has been also raised about the possibility that statins may negatively affect glucose level. This had lead Food and Drug Administration (FDA) to issue a specific warning on labels of this drug (http://www.fda.gov/ForConsumers/ConsumerUpdates/ Ucm293330.htm). Furthermore, a recent work from the group of Serafini has highlighted the still underestimated issue of detrimental potential interaction between statins and some nutrients [16]. Quite recently, several national and European agencies as well as collaborative research groups have also variably stratified patients in “high risk” and “low risk group [17]. This would thus suggest that a large cohort of not “high risk” patients or those who have experienced side effects from the usage of statins may be amenable to different approaches, such as phytochemicals intervention [18-21]. While several of such studies with phytochemicals may suffer from methodological limitations, it is still worth reporting what stated by Marshal et al. [13] that “As the statin-cholesterol controversy shows, it is time to recognize the benefits of natural medicine (i.e., functional medicine) for disease prevention and the maintenance of optimal health and wellness”. This position holds particularly true when considering the expanding scenario of metabolic syndrome in younger age population [22,23] where dietary and life-style modifications are more liable to be implemented. In this regard, there have been a number of studies where nutraceuticals intervention has been understandably associate to other robust therapeutic factors such as weight loss program, gut microbiota modulators and physical exercise [24-26]. While this is appreciable and meets a rational strategy, on the other hand may blur the specific significativity of the nutraceuticals intervention per sè. After a series of positive in-house experimental studies, we planned this study aiming to use an integrated phytocompound (P3/GB-2016, batch n. 607090, containing per tablet the followings: fermented red yeast 200 mg, artichoke dry extract 500 mg, vitamin B12 0.83 mcg, vitamin B6 1.4 mg, vitamin B3 9 mg, coenzime Q10 50 mg, banaba dry extract 75 mg and folic acid 110 mcg, Cardionam®, Named SpA, Lesmo, Italy) in patients with clustered risk factors for metabolic syndrome in view of potential benefits on key inflammatory markers besides on the lipid profile as well [27,28].
Materials and Methods
Selection of patients
Information’s collected at the time of the first recruitment baseline evaluations comprised demographic data, personal and family medical history, alcohol and/or cigarette consumption and duration, oral contraceptive use and hormone replacement therapy.
Exclusion criteria: Patients with secondary hypertension, cardiomyopathy, liver or kidney function, cerebrovascular or neurodegenerative diseases. Moreover, grossly abnormal total cholesterol (>280 mg/dl or LDL >180 mg/dl) under established lipid lowering drugs, past or concurrent malignancies or ongoing insulin treatment were excluded as well. Alcohol consumption over 12 g/day represented further exclusion criteria.
Patients group: Eighty-two patients (66 males and 16 females, 38-69 as age range) with metabolic syndrome were selected randomly. Metabolic syndrome was diagnosed by NCEP ATP guidelines: Blood Pressure ≥140/≥90 mmHg Males >102 cm Waist Circumference Females >88 cm Triglycerides >150 mg/dl Males <40 mg/dl HDL Cholesterol Females <50 mg/dl Fasting Plasma Glucose >110 mg/dl (Table 1). Written informed consent was obtained from all subjects beforehand and the study met the ethical guidelines of the 1975 Declaration of Helsinki. The study was carried out in a randomised double blind placebo controlled fashion. All patients were kept on their usually prescribed oral hypoglycaemics and/or anti-hypertensive drugs) but without any anti-hyperlipidemic medications.
| Blood Pressure ≥ 140/ ≥ 90 mmHg | Blood Pressure ≥ 140/ ≥90 mmHg | Blood Pressure ≥ 140/ ≥ 90 mmHg |
| Waist Males >102 cm | Waist Males >102 cm | Waist Males >102 cm |
| Circtunference Females >88 cm | Circumference Females >88 cm | Circumference Females • 88 cm |
| Triglycerides >150 mg dl | Triglycerides >150 mg/dl | Triglycerides >150 mg/dl |
| HDL Cholesterol Males <40 mg/dl | HDL Cholesterol Males <40 mg/dl | HDL Cholesterol Males <40 mg/dl |
| Females <50 mg/dl | Females <50 mg/dl | Females <50 mg/dl |
| Fasting Plasma Glucose >110 mg/dl | Fasting Plasma Glucose >110 mg/dl | Fasting Plasma Glucose >110 mg/dl |
Table 1: NCEP ATP guidelines (Eckel RH, Cornier MA. Update on the NCEP ATP-III emerging cardiometabolic risk factors. BMC Med. 2014 Aug 26;12: 115).
Patients meeting inclusion criteria were advised to adhere to a standard balanced diet but no specific dietary calorie-restriction or life-style modifications (physical exercise) were attempted at this stage. Two patients groups were matched as for gender, duration of proven diagnosis and associated diseases (Table 2).
| Group | I | II |
|---|---|---|
| Age (years) | 49 ± 8 | 55 ± 9 |
| Gender (m/f) | 35/9 | 31/7 |
| BMI (kg/m2) | 25.4 ± 3.2 | 24.6 ± 3.8 |
| Fasting Blood Glucose (mg/dl) | 98.8 ± 7.8 | 101.6 ± 6.7 |
| Waist Circumference (mg/dl) | 87.8 ± 10.5 | 96.6 ± 8.4 |
| Triglycerides(mg/dl) | 177.6 ± 12.6 | 183.6 ± 13.3 |
| Total Cholesterol (mg/dl) | 251.7 ± 22.2 | 258.5 ± 12.6 |
| HDL- Cholesterol (mg/dl) | 41.4 ± 3.2 | 44.2 ± 2.4 |
| LDL- Cholesterol (mg/dl) | 161.4 ± 10.3 | 158.6± 11.2 |
| VLDL (mg/dl) | 33.6 ± 4.7 | 34.1 ± 2.2 |
| Oral Hypoglycaemics | 11 | 9 |
| Anti-hypertensives | 6 | 9 |
Table 2: Demographic, anthropometric, biochemical and clinical characteristics of studied population.
One group received 1 tab/dinner of drug A and another group received 1 tab/dinner of drug B. Physicians and patients were blinded as for the content of the tablets except being aware that one being P3/ GB-2016 while the other a perfectly outside looking alike placebo. Both tablets were bearing a distinctive code number. Patients’ visits were scheduled weekly and seven tablets were provided on each visit. On every office visit, patients’ compliance was checked by counting remaining tablets and adherence to diet was also discussed. At the end of the whole study period, all the data along with code numbers was submitted to the statistician and after decoding, the patients were divided into group I (P3/GB-2016) and group II (placebo). A third group of 25 healthy, normal-weight subjects without any biochemical abnormalities served as control.
Methods
All anthropometric assessments were carried out by the same dedicated nurse. Weight was calculated to the nearest 0.1 kg by using a digital scale with subjects stepping on barefoot in light clothing. Waist circumference was measured to the nearest 1 mm with a flexible, nonelastic tape midway between the rib cage and the iliac crest at the end of a mild expiration. Body Mass Index (BMI) was calculated as follows: weight (kg)/height squared (m2). Brachial artery systolic and diastolic blood pressure was measured by a mercury sphygmomanometer on the right arm with the subject in a sitting position after at least a 5-min rest. Blood samples were collected in the morning (8 AM to 9 AM) after a 12 h overnight fast, blood was drawn into into pyrogen-free blood collection tubes tubes and total cholesterol and high-density lipoprotein (HDL) cholesterol and triglyceride were measured by enzymatic assay (Denka-BioTech & Science, Lipokit-07532, Japan). Low-density Lipoprotein (LDL) cholesterol was calculated by the Friedwald formula LDL-C: ¼ TC-(HDL-C+TG/5) [29]. High-density lipoprotein-cholesterol was measured by a direct method (Wako Pure Chemical Industries, Osaka, Japan). Glucose, liver and renal function tests were performed by routine biochemistry (using the Hitachi 7800 Series autoanalyzer (Hitachi High-Technologies Corporation, Tokyo, Japan) as well.
Part of plasma obtained after centrifugation was also stored at -80°C in multiple aliquots until analysis. Baseline serum levels of macrophage chemoattractant protein 1 (MCP-1), Migratory Inhibitory Factor (MIF) and macrophage inflammatory protein-1α (CCL3/MIP-1α) were assayed by commercially available Elisa kits. Levels were measured by quantitative sandwich enzyme-linked immunosorbent assay (Quantikine, R&D Systems, and Minneapolis, USA) according to the manufacturer’s protocols. In particular, the assays were carried out with an OptEIA MIP-1α and MIF kit. The assayed, MIP-1α and MIF levels were derived from a standard curve using recombinant human MIP-1α and MIF and the SOFTmax curve-fitting program. The intraand inter-assay coefficients of variation for all chemokines were <10%. All samples were measured in duplicate and the average of the 2 values was used for final data analysis. The assay conditions were controlled, standardized and pre-optimized to ensure repeatability and reproducibility. Analyses were blinded to clinical diagnosis.
Statistical analysis
Statistical analysis was performed using the χ2 statistic or Fisher’s Exact Test for independence. Pairwise analysis was performed when appropriate. Two-tailed test of significance was used throughout. Correlations were assessed using linear regression analysis. Post hoc test with Bonferroni correction was used to compare post-treatment values against baseline ones. Data are presented as mean ± SD and the significance level for all tests was set at p<0.05.
Results
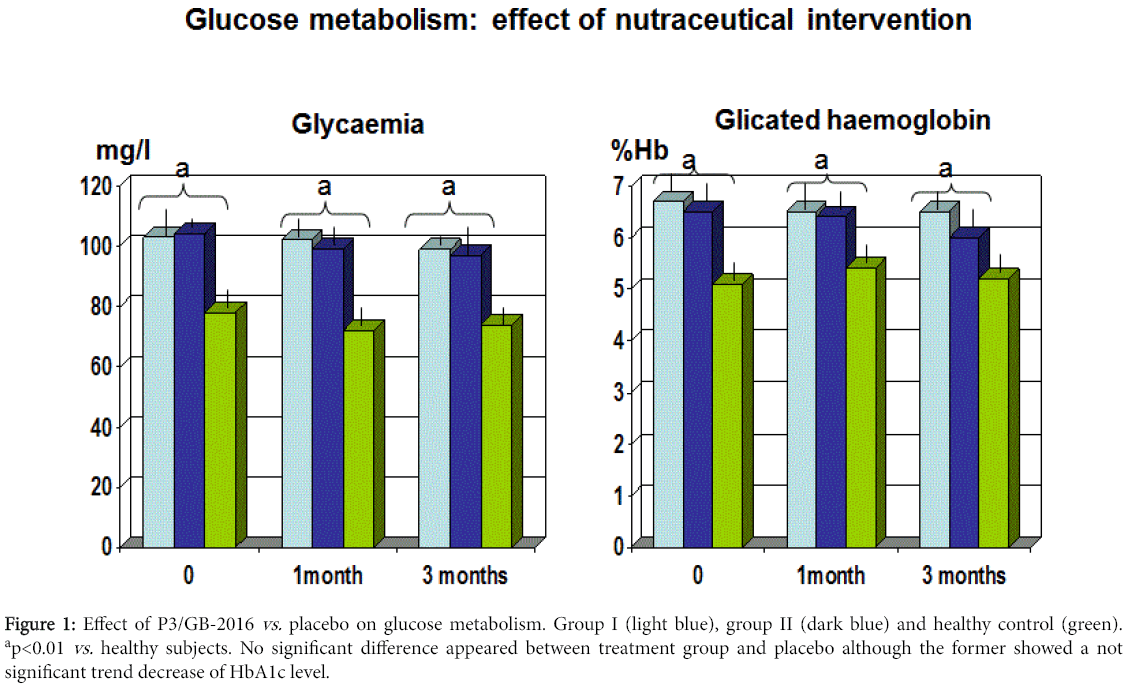
After breaking the codes kept under custody in a separate country, it appeared that group I was the compound whereas group II was the placebo. In both groups during the three months, no adverse effect was reported. The mean BMI in both the treatment groups showed a trend decreased without any significant difference between the two (data not shown). The systolic blood pressure remained stable throughout the study, irrespective of any treatment (data not shown). Both groups showed a comparable mean fasting blood sugar at 1 and 3 months control. However, the mean HbA1c level showed a trend decrease in group II at 3-month observation (p<0.061 vs . group I) (Figure 1).
Figure 1: Effect of P3/GB-2016 vs. placebo on glucose metabolism. Group I (light blue), group II (dark blue) and healthy control (green). ap<0.01 vs. healthy subjects. No significant difference appeared between treatment group and placebo although the former showed a not significant trend decrease of HbA1c level.
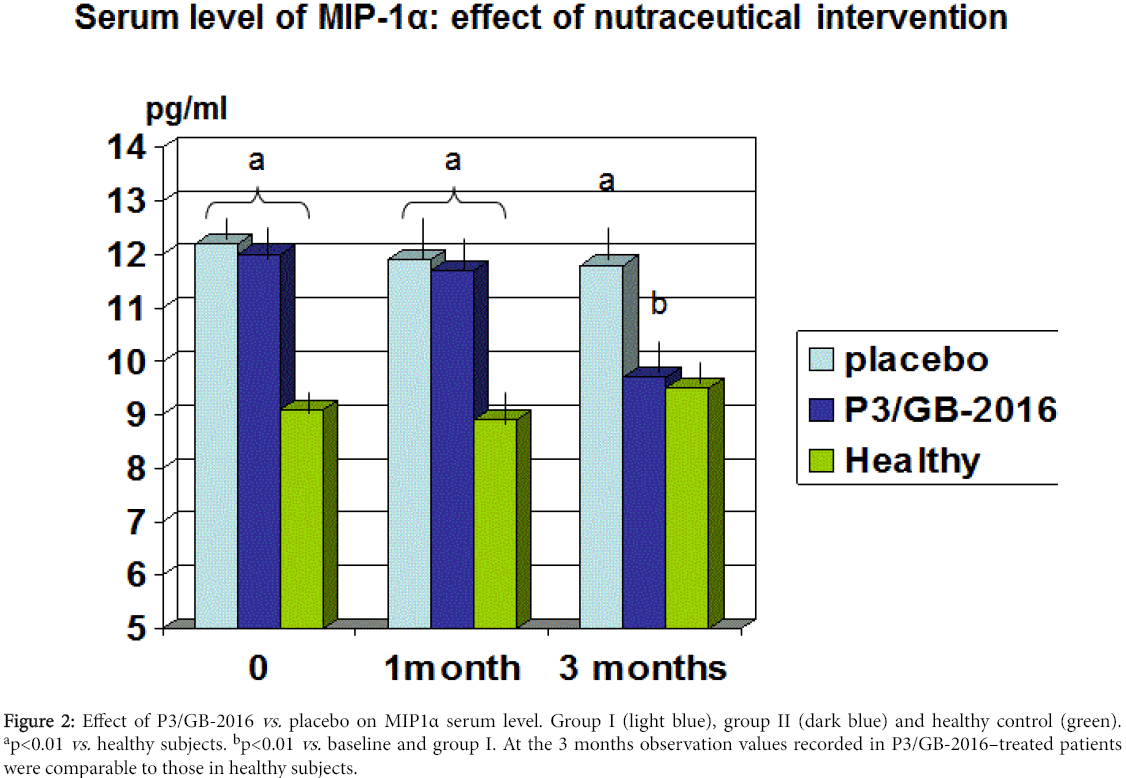
There was no statistically significant difference in the other routine biochemical parameters (data not shown). As compared to baseline and group I, group II showed a significant decrease of total and LDL cholesterol starting from the first observation visit throughout the study period (p<0.05, Table 3). At 3-month observation, group II patients showed also a not significant trend increase of HDL (p<0.067 vs. baseline and group I) and a significant decrease of VLDL aliquot and triglycerides (p<0.05 vs. baseline and group I). The circulating levels of MCP-1 were not significantly higher in our population as compared to healthy subjects (176 ± 98.5 pg/ml vs. 146.4 ±.54.4 pg/g, p<0.07). However, it appeared a wide dispersion of data and when analysing separately those subjects with BMI higher than 27 (11 patients) this group gathered the highest MCP-1 absolute values (212.2 ± 32.3 pg/ml). Statistics was not feasible to analyse this parameter in this subgroup for the limited number. Serum concentration of either MIP1α or MIF were significantly higher in dysmetabolic patients as compared to healthy control (p<0.01, Figures 2 and 3). Treatment with P3/GB-2016 proved to significantly decrease both parameters (MIP1α reaching normal values) at 3-month observation (p<0.01 vs. baseline and vs. placebo-treated group).
Figure 2: Effect of P3/GB-2016 vs. placebo on MIP1α serum level. Group I (light blue), group II (dark blue) and healthy control (green). ap<0.01 vs. healthy subjects. bp<0.01 vs. baseline and group I. At the 3 months observation values recorded in P3/GB-2016–treated patients were comparable to those in healthy subjects.
Figure 3: Effect of P3/GB-2016 vs. placebo on MIF serum level. Group I (light blue), group II (dark blue) and healthy control (green). ap<0.01 vs. healthy subjects. bp<0.01 vs. baseline and group I. At the 3 months observation values recorded in P3/GB-2016–treated patients were comparable to those in healthy subjects.
| Group | I (placebo) | II (P3/GB-2016) |
|---|---|---|
| Triglycerides(mg/dl) | 171.2 ± 11.2 | 133.2 ± 11.6* |
| Total Cholesterol (mg/dl) | 251.7 ± 22.2 | 218.7 ± 17.4* |
| HDL-Cholesterol (mg/dl) | 42.6 ± 3.6 | 47.7 ± 2.5 |
| LDL-Cholesterol (mg/dl) | 157.7 ± 8.9 | 118.8± 10.2* |
| VLDL (mg/dl) | 33.2 ± 5.2 | 28.5 ±3.6* |
Table 3: Lipid metabolism profile in studied population: effect of P3/GB-2016 supplementation for 3 months. *p<0.05 vs. baseline and group I.
Discussion
Current views indicate that about 25% of all American adults and around 15% of Europeans are estimated to suffer from metabolic syndrome [2]. The age-related increased likelihood of having metabolic syndrome brings to a picture of 40% of people in their 60s to 70s suffering from it. The metabolic syndrome is associated with a low-grade pro-inflammatory setting that may partially account for its relationship with an increased cardiovascular risk. Moreover, a growing number of data highlights the concept that obesity per sè represents a further state of ongoing subtle chronic inflammation primarily mediated by immune cells, such as macrophages and T-lymphocytes. Such situation triggers the development of insulin resistance and associated metabolic comorbidities such as non-alcoholic fatty liver disease and type 2 diabetes mellitus. To the morbidity and mortality associated to the latter, there is often a concurrent factor represented by atherosclerosis [5], and this applies either to men [6] and women [7]. Our study showed that P3/GB-2016 significantly improved the lipid profile as already shown by other authors but who also applied a more stringent dietary intervention [30], as it would understandably be advised. However, the present nutriceuticals, albeit in the absence of a synergistic dietary- and life-style-modification, proved to yield a significant improvement of lipid profile with a trend increase of HDL and a significantly lower serum level of triglycerides, LDL and of non- HDL which includes very-low-density lipoprotein (VLDL), VLDL remnants, intermediate-density lipoprotein, chylomicrons, chylomicron remnants and lipoprotein(a).
This action is likely to be due to the well-known property of red yeast [31] whose even far higher dosage has been confirmed to be safe and without muscle-toxicity when also checked at a cellular level [32]. Added to this, a concomitant lipid-lowering effect is likely to have been played by artichoke extract [33,34] whose polyphenols moieties have been recently shown to possess an efficient gastrointestinal bioavailability in vitro [35] and also an anti-hypercholesterolemiainduced oxidative stress in liver and cardiac tissue in rats [36]. Interestingly, this specific phyto-ingredient has been shown, when employed at higher dosages, to exert a cholesterol-lowering effect even in healthy athletes under high-intensity exercise [37]. This property together with its flavonoids-related endothelial function protection by iNOS upregulation [38] shared most recently by corosolic acidcontaining banaba extracts [39] may widen its potential application. Moreover, although fasting glycaemia didn’t show any significant change during treatment, group I showed a trend improvement of HbA1c. Although not reaching a statistical significance, this seemingly more favourable glucose metabolism may be advocated for by the specific contribution of corosolic acid-rich banaba ingredient which has several experimental data [40,41] although limited clinical reports so far [42,43]. On the other hand, one has to consider that about 50% of our population were already on hypoglycaemic drug treatment and this may have blunted the distinctive effect of this promising phytoingredient.
As mentioned above, during such complex metabolic derangement process, amongst other processes, leukocyte recruitment and infiltration triggered by chemokines have a key regulatory role at all stages of atherosclerosis [43] and are also expressed in atherosclerotic lesions such as shown with CCL3/MIP1a [44]. Recent investigations have unveiled a correlation among different aspects of metabolic syndrome and the most common markers of inflammation. A high correlation between CRP and Body Mass Index (BMI) has already been reported, followed by the indexes of insulin resistance-fasting insulin and insulin sensitivity [45]. Interestingly, our supplementation proved to significantly decrease the blood levels of chemokines, such as MIP1α and MIF which are key regulators of the inflammatory cascade. In particular, MIF is a multifaceted unique inflammatory cytokine controlling either intracellular as well as extracellular functions [46,47] and affecting cellular signalling in health and disease [48] where it seems related to the development and progression of metabolic syndrome-associated cardiomyopathy [49-52]. As reported by Kim et al. [53], we also found out that the serum concentration of this cytokine was significantly higher in people with metabolic syndromerisk factor than in healthy control. However, unlike his study, we couldn’t find any significant gender difference, although our female population was limited.
MCP-1 level showed only a trend decrease under trial treatment. When selecting those patients with BMI above 27, this parameter showed the highest levels, however, no statistics could be applied for the limited number of such patients (11 patients). This finding is in agreement with the data from Kim et al. [54] who had shown that the circulating levels of MCP-1 were related to obesity and its links with obesity-related metabolic complications such as atherosclerosis and diabetes. Adipose tissue is nowadays clearly regarded as an “organ” and it is liable to get inflamed this leading to increased infiltration of macrophages and enhanced release of pro-inflammatory cytokines [55] affecting all body and also a metabolically-crucial organ such as pancreas, as recently pointed out in an elegant review from Catanzaro et al. [56]. Thus, it is likely that the present nutraceutical intervention if applied to overweight-obese subjects, irrespective of any other overt associated disease, may beneficially affect a greater number of obesityrelated cytokines. We can hypothesize that these beneficial effects can be advocated for by the reported anti-inflammatory properties of either red yeast [57,58], corosolic-rich banaba extract [59,60] and artichoke [61,62] phyto-ingredients.
It can be concluded that the vitamin-polyherbal preparation P3/ GB-2016, is not only an effective and safe measure in controlling dyslipidemia but also crucial inflammatory markers of metabolic syndrome, beyond hsCRP. This result has to be taken in further consideration when bearing in mind that no other robust dietary or life style interventions were concurrently applied and that these patients were already under anti-hypertensive and hypoglycaemic drugs. This specific biochemical benefit on cyto- and chemokines has been partly reported in statin-using trials [63] and so the current study, to the best of our knowledge, is the first confirming the usefulness of well-designed nutraceuticals intervention on specific and detrimental cytokines panels.
Although statins in their pleiotropic properties occupy a definite role in the therapeutic armamentarium of dyslipidemias and associated risks, a number of controversial issues still exist [13,14,64]. Recent concern has been raised as for possible neurological adverse effects [65] while coenzyme Q10 has shown significant cytoprotective mechanisms for neuronal function and repair processes [66-68]. Given the cautions as above as well as the at times straightaway “medicalization” by physicians whatever the degree of lipid disorders, one may find in a rational nutraceutical a weapon to be deployed within a long-lasting dietary- and life-style intervention strategy also for preventative aims [69]. These data appear to be substantially more clinical impacting than what we observed in recent times with a caviar derivative (Caviarlieri) which had no effect whatsoever on lipid profile and strictly-related adipose tissue-linked inflammatory markers, besides being probably an unaffordable compound in routine clinical practice [70].
References
- Unal B, Critchley JA, Capewell S (2004) Explaining the decline in coronary heart disease mortality in england and wales between 1981 and 2000. Circulation 109:1101-1107.
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Heart disease and stroke statistics– 2015 update: a report from the American Heart Association. Circulation. 131:e29-322.
- Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, et al. (2012) The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Cholesterol Treatment Trialists' (CTT) Collaborators. Mihaylova B, Emberson J Lancet 380:581-590.
- Mandviwala T, Khalid U, Deswal A(2016) Obesity and Cardiovascular Disease: a Risk Factor or a Risk Marker? CurrAtheroscler Rep 18:21.
- Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, et al. (2008) Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 31:811-822.
- Lotufo PA, Gaziano JM, Chae CU, Ajani UA, Moreno-John G, et al. (2001) Diabetes and all-cause and coronary heart disease mortality among US male physicians. Arch Intern Med 161:242-247.
- Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, et al. (2001) The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 161:1717-1723.
- Long MT, Fox CS (2016) The Framingham Heart Study–67 years of discovery in metabolic disease. Nat Rev Endocrinol 12:177-183.
- Sellayah D, Cagampang FR, Cox RD (2014) On the evolutionary origins of obesity: a new hypothesis. Endocrinology 155:1573-1588.
- Ingaramo RA (2016) Obesity, Diabetes, and Other Cardiovascular Risk Factors in Native Populations of South America.CurrHypertens Rep 18:9.
- Stone NJ, Robinson JG, Lichtenstein AH, BaireyMerz CN, Blum CB, et al. (2014) ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S1-S45.
- ŠimiÄ? I, Reiner Ž (2015) Adverse effects of statins - myths and reality. Curr Pharm Des 21:1220-1226.
- Marshall TM (2014) New Insights into the Statin-Cholesterol Controversy. J Am PhysSurg 19:42-46.
- Sinatra ST, Teter BB, Bowden J, Houston MC, Martinez-Gonzalez MA (2014) The cholesterol and statin controversy: the new 2013 statin-cholesterol guidelines. AlternTher Health Med 20:14-17.
- Harper CR, Jacobson TA (2007) The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. CurrOpinLipidol 18:401-408.
- Peluso I, Palmery M, Serafini M (2015) Association of flavonoid-rich foods and statins in the management of hypercholesterolemia: a dangerous or helpful combination? Curr Drug Metab 16:833-846.
- Hendrani AD, Adesiyun T, Quispe R, Jones SR, Stone NJ, et al. (2016) Dyslipidemia management in primary prevention of cardiovascular disease: Current guidelines and strategies. World J Cardiol 8:201-210.
- Wang P, Xiong X, Li S (2015) Efficacy and Safety of a Traditional Chinese Herbal Formula XuefuZhuyu Decoction for Hypertension: A Systematic Review and Meta-Analysis.Medicine (Baltimore) 94:e1850.
- Moriarty PM, Roth EM, Karns A, Ye P, Zhao SP, et al. (2014) Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo-controlled study. J ClinLipidol 8:568-575.
- Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, et al. (2012) Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 19:861-867.
- Feuerstein JS, Bjerke WS (2012) Powdered red yeast rice and plant stanols and sterols to lower cholesterol. J Diet Suppl 9:110-115.
- Clemente MG, Mandato C, Poeta M, Vajro P(2016) Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol 22:8078-8093.
- Kavey RE (2016) Combined Dyslipidemia in Children and Adolescents. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, et al. (eds.)Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.
- Han TS, Lean ME (2016) A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis 5.
- Sorrentino G, Crispino P, Coppola D, De Stefano G (2015) Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R D 15:21-25.
- Lerman RH, Minich DM, Darland G, Lamb JJ, Chang JL, et al. (2010) Hsi A, Bland JS, Tripp ML. Subjects with elevated LDL cholesterol and metabolic syndrome benefit from supplementation with soy protein, phytosterols, hops rho iso-alpha acids, and Acacia niloticaproanthocyanidins. J ClinLipidol 4:59-68.
- Saha SP, Whayne TF Jr(2016) Coenzyme Q-10 in Human Health: Supporting Evidence? South Med J 109:17-21.
- Holder K (2016) Myalgias and Myopathies: Drug-Induced Myalgias and Myopathies. FP Essent 440:23-27.
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:488-502.
- Cicero AF, Colletti A, Fogacci F, Bove M, Rosticci M, et al. (2016) Effects of a Combined Nutraceutical on Lipid Pattern, Glucose Metabolism and Inflammatory Parameters in Moderately Hypercholesterolemic Subjects: A Double-blind, Cross-over, Randomized Clinical Trial. High Blood Press CardiovascPrev [Epub ahead of print].
- Mannarino MR, Ministrini S, Pirro M(2014) Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med 25:592-599.
- Barrat E, Zaïr Y, Sirvent P, Chauveau P, Maudet C, et al. (2013) Housez B, Derbord E, Lescuyer JF, Bard JM, Cazaubiel M, Peltier SL. Effect on LDL-cholesterol of a large dose of a dietary supplement with plant extracts in subjects with untreated moderate hypercholesterolaemia: a randomised, double-blind, placebo-controlled study. Eur J Nutr52:1843-1852.
- Chang WC, Jia H, Aw W, Saito K, Hasegawa S(2014) Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br J Nutr 112:709-717.
- Ben Salem M, Affes H, Ksouda K, Dhouibi R, Sahnoun Z, et al. (2015) Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum Nutr 70:441-453.
- D'Antuono I, Garbetta A, Linsalata V, Minervini F, Cardinali A (2015) Polyphenols from artichoke heads (Cynaracardunculus (L.) subsp. scolymus Hayek): in vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct 6:1268-1277.
- Küçükgergin C, Aydin AF, Ozdemirler-Erata G, Mehmetçik G, Koçak-Toker N, et al. (2010) Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol Trace Elem Res 135:264-274.
- Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielcka E, Horoszkiewicz-Hassan M (2008) The influence of supplementation with artichoke (Cynarascolymus L.) extract on selected redox parameters in rowers. Int J Sport NutrExercMetab 18:313-327.
- Li H, Xia N, Brausch I, Yao Y, Förstermann U (2004) Flavonoids from artichoke (Cynarascolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J PharmacolExpTher 310:926-932.
- Li Y, Zhou ZH, Chen MH, Yang J, Leng J, et al. (2016) Inhibition of Mitochondrial Fission and NOX2 Expression Prevent NLRP3 Inflammasome Activation in the Endothelium: The Role of Corosolic Acid Action in the Amelioration of Endothelial Dysfunction. Antioxid Redox Signal 24:893-908.
- Ríos JL, Francini F, Schinella GR (2015) Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med 81:975-994.
- Kouzi SA, Yang S, Nuzum DS, Dirks-Naylor AJ (2015) Natural supplements for improving insulin sensitivity and glucose uptake in skeletal muscle. Front Biosci (Elite Ed) 7:94-106.
- Kim HJ, Yoon KH, Kang MJ, Yim HW, Lee KS, et al. (2012) A six-month supplementation of mulberry, korean red ginseng, and banaba decreases biomarkers of systemic low-grade inflammation in subjects with impaired glucose tolerance and type 2 diabetes. Evid Based Complement Alter Med 2012:735191.
- Fukushima M, Matsuyama F, Ueda N, Egawa K, Takemoto J, et al. (2006) Effect of corosolic acid on postchallenge plasma glucose levels. Diabetes Res ClinPract 73:174-177.
- Weber C, Schober A, Zernecke A (2004) Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. ArteriosclerThrombVascBiol 24: 1997-2008.
- Hagg DA, Olson FJ, Kjelldahl J, Jernas M, Thelle DS (2009) Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis 204: e15-e20.
- Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, et al. (2000) Chronic subclinical inflammation as a part of the insulin resistance syndrome. Circulation 102:42-53.
- Bernhagen J,Krohn R,Lue H,Gregory JL,Zernecke A, et al. (2007) MIF is a non cognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine 13: 587-596.
- Rassaf T, Weber C,Bernhagen J (2014) Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc Res 102:321-328.
- SchoberA, Bernhagen J,Weber C (2008) Chemokine-like functions of MIF in atherosclerosis. J Mol Med 86:761-770.
- Matia-García I, de la Cruz-Mosso U, Muñoz-Valle JF, Parra-Rojas I (2014) Macrophage migration inhibitory factor and its relationship with obesity and diabetes. Invest Clin 55:266-277.
- Kim BS, Rongisch R, Hager S, Grieb G, Nourbakhsh M, et al. (2015) Rennekampff HO, Bucala R, Bernhagen J, Pallua N. Macrophage Migration Inhibitory Factor in Acute Adipose Tissue Inflammation. PLoS One 10:e0137366.
- Herder C, Klopp N, Baumert J, Muller M, Khuseyinova N (2008) Effect of macrophage migration inhibitory factor (MIF) gene variants and MIF serum concentrations on the risk of type 2 diabetes: results from the MONICA/KORA Augsburg Case-Cohort Study, 1984–2002. Diabetologia 51: 276-284.
- Kim H, Lee S, Kim HJ, Kong MH, Kim YR, et al. (2011) Elevated levels of macrophage migration inhibitory factor in women with metabolic syndrome. HormMetab Res 43:642-645.
- Kim CS, Park HS, Kawada T, Kim JH, Lim D, et al. (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30:1347-1355.
- Qiu Y, Shan B, Yang L, Liu Y (2016) Adipose tissue macrophage in immune regulation of metabolism. Sci China Life Sci Nov 9 [Epub ahead of print] Review.
- Catanzaro R, Cuffari B, Italia A, Marotta F(2016) Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol 22:7660-7675.
- Hsu LC, Liang YH, Hsu YW, Kuo YH, Pan TM (2013) Anti-inflammatory properties of yellow and orange pigments from Monascuspurpureus NTU 568. J Agric Food Chem 61:2796-2802.
- Xie X, Wang Y, Zhang S, Zhang G, Xu Y, et al. (2012) Chinese red yeast rice attenuates the development of angiotensin II-induced abdominal aortic aneurysm and atherosclerosis. J NutrBiochem 23:549-556.
- Yang J, Leng J, Li JJ, Tang JF, Li Y, et al. (2016) Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine 23:181-190.
- Kim SJ, Cha JY, Kang HS, Lee JH, Lee JY, et al. (2016) Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep 49:276-281.
- Tanaka YT, Tanaka K, Kojima H, Hamada T, Masutani T, et al. (2013) Cynaropicrin from Cynarascolymus L. suppresses photoaging of skin by inhibiting the transcription activity of nuclear factor-kappa B. Bioorg Med Chem Lett 23:518-523.
- Yasukawa K, Matsubara H, Sano Y (2010) Inhibitory effect of the flowers of artichoke (Cynaracardunculus) on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J Nat Med 64:388-391.
- Loughrey BV, McGinty A, Young IS, McCance DR, Powell LA (2013) Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low-dose atorvastatin treatment: evidence from a randomized controlled trial. ClinEndocrinol (Oxf) 79:800-806.
- Preiss D, Seshasai ST, Welsh P, Murphy SA, Ho JE, et al. (2011) Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 305:2556-2564.
- Lei Q, Peng WN, You H, Hu ZP, Lu W (2014) Statins in nervous system-associated diseases: angels or devils? Pharmazie 69: 448-454.
- Choi H, Park HH, Koh SH, Choi NY, Yu HJ(2012) Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology 33:85-90.
- Belousova MA, Tokareva OG, Gorodetskaya EA, Kalenikova EI, Medvedev OS (2016) Neuroprotective Effectiveness of Intravenous Ubiquinone in Rat Model of Irreversible Cerebral Ischemia. Bull ExpBiol Med 161: 245-247.
- Yang X, Zhang Y, Xu H, Luo X, Yu J, et al. (2016) Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases.Curr Top Med Chem 16:858-866.
- Herder C, Peltonen M, Koenig W, Kräft I, Müller-Scholze S, et al. (2006) Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes 55:2340-2346.
- Marotta F, Lorenzetti A, Catanzaro R, Zerbinati N, Jain S, et al. (2013) A sturgeon-derived bioactive compound beneficially modulates nuclear receptors controlling metabolic functions in patients with metabolic syndrome.Acta Biomed 84:53-60.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 4350
- [From(publication date):
February-2017 - Jan 31, 2025] - Breakdown by view type
- HTML page views : 3603
- PDF downloads : 747