Effect of Alloying Elements on Properties of Biodegradable Magnesium Composite for Implant Application
Received: 01-Oct-2017 / Accepted Date: 10-Oct-2017 / Published Date: 18-Oct-2017 DOI: 10.4172/2168-9806.1000179
Abstract
This research aims to improve the mechanical properties and corrosion resistance of magnesium based composites with addition of zinc and manganese as alloying elements. Mg-Zn-HA, Mg-Mn-HA and Mg-Zn-MnHA composites with Mg-HA composite as control have been fabricated through powder metallurgy route. 1.5wt% of alloying elements was added into magnesium matrix for each composite. The composites were prepared by mechanical alloying followed by compaction under 400 MPa and sintering at 400°C. The results showed that with additional alloying element, micro-hardness, compressive strength and corrosion resistance of composites are significantly enhanced compared with Mg-HA composite. The Mg-Zn-Mn-HA composite showed the best properties among Mg-based composite with density, micro-hardness and compressive strength of 1.77 g/cm3, 65.5 HV and 210 MPa respectively all in range of properties of human bone. Immersion test in Hank’s Balanced Salt Solution (HBSS), weight loss of Mg-Zn-Mn-HA reduces to 0.61% when immerse for 5 hours and to 6.20% for 24 hours immersion.
Keywords: Alloying elements; Magnesium-based composite; Mechanical alloying; Mechanical properties; Corrosion
Introduction
Implants are man-made devices produced to replace a missing, to support a damaged, or to enhance an existing biological structure. The use of metals in implant application is preferable as metals have high impact strength, high wear resistance, high ductility and the capacity to absorb high strain energy compared to other materials. These properties make metals suitable candidates for orthopaedic loadbearing application and fixation devices such as joint replacement, bone plates and screws, as well as dental implants, pacer and suture wires, and coronary stents [1].
Over the last decade, degradable metallic biomaterials have emerged as an alternative for medical applications. The metal proposed for biodegradable implants is magnesium (Mg) because the density of Mg metals (1.7-2.0 g/cm3) is close to the density of bone (1.7-2.1 g/ cm3) while the densities of other metals such as titanium and stainless steel are much higher and densities of polymers are much lower [2]. In addition, Mg is biocompatible which mean that it does not have toxic or inflammable effects to the tissue and its surrounding. Therefore, it is totally safe to be placed in human body. Mg also naturally occurs in humans since our body contains about 25 g of this element and 50-60% is found in bone. The normal serum Mg concentration is 1.8 to 2.3 mg/ dl and people consume about 380-850 mg of Mg daily. Excess Mg is not stored in the body and is removed readily by the kidneys.
Stainless steel has least corrosion resistant, and is usually used for temporary implants while titanium and Co-Cr alloys do not corrode in the body; however, metal ions slowly diffuse through the oxide layer and accumulate in the tissue [3]. In addition, these metallic materials as implants are not biodegradable and require a second surgery in order to remove the metals implant from the body causing more pain to patient [4]. Consequently, it not only increases the hospitalization time and cost but also elevates chances of infection and complications. The metallic materials may also cause stress shielding due to the mismatch between the mechanical properties of these material and the natural bone. The mechanical forces and loads are retained by implants and are not transferred to the healing bone [5].
The major issue with biodegradable Mg is that it may degrade too quickly in the physiological system, producing hydrogen gas in the corrosion process leading to lose mechanical integrity before the tissue has fully healed [6]. The other problems of Mg are low strength, low Young modulus and limited ductility. These could restrict the wide applicability of this material. However, the addition of alloying elements such as cadmium (Cd), manganese (Mn), tin (Sn), zinc (Zn), aluminum (Al), zirconium (Zr) and some of rare earth (RE) elements like cerium (Ce), lutetium (Lu), and praseodymium (Pr) seems to improve the mechanical strength and corrosion resistance of Mg [7]. Al is considered as most effective strengthener and also improves corrosion resistance of Mg but it has been found to cause Alzheimer’s disease and bone fragility [8]. Therefore, Al is not really suitable to be used in medical implants. Zr can act as a grain refiner when added in Mg; however it has been found to influence breast and lung cancer. Other RE elements like Ce, Lu, and Pr can improve the corrosion resistance and mechanical properties of Mg but they show toxicity in human body [9].
Zn is considered as an essential element in the human body and it also has positive strengthening effect on Mg alloys. Zn is considered next to Al in strengthening effectiveness as an alloying element in Mg. Addition of Zn to Mg is known to improve the corrosion resistance apart from contributing to strengthening [10]. Zn is one of the most abundant nutritionally essential elements in the human body, and considered to be safe element for biomedical applications while Mn is a widely used alloying element in Mg alloys to improve their corrosion resistance, tensile strength and weldability.
In producing MMC, an appropriate reinforcement particle can be added into Mg-based alloy matrix in order to produce a good combination of corrosion resistance and mechanical properties. Therefore the bioactive ceramic such as Hydroxyapatite (HA) with formula Ca10(PO4)6(OH)2 has been widely employed in medical applications. In addition, HA possesses low solubility in the body environment and has been proven to be an excellent carrier for tissue growth and it promises a great utility as a bioactive agent delivery vehicle. Hence, it is reasonable to assume that HA can be a suitable reinforcement in Mg-based MMCs [11]. HA crystallizes in the hexagonal crystal system and over 50% by volume and 70% by weight of human bone is a modified form of HA known as natural bone mineral.
Most studies on the fabrication of Mg alloys or Mg-HA composites commonly used casting method and followed by heat treatment. Casting method is simple but it produces high in porosity and defect, difficult to handle, poor final properties and high power consumption. Furthermore not all alloys can be cast. Due to a numerous limitations found in casting method, mechanical alloying (MA) technique have been used to overcome the shortcomings of casting. MA is a solid-state powder processing method in which powder particles blended in a high-energy ball mill through repeated cold welding, fracturing, and re-welding which ensures high reaction interface areas between powder particles in order to produce a homogeneous material [12]. In addition, this method are cost effective, produces near net shape components which required few or no secondary operations, can achieve unique properties and has full capacity for producing a variety of alloying systems and particulate composites.
This work was conducted to investigate properties of Mg-HA composite prepared through powder metallurgy route with addition Zn and Mn as binary alloying elements and the combinations of Zn and Mn into Mg composite as ternary alloying system.
Materials and Methods
Magnesium (Mg), zinc (Zn), manganese (Mn) and hydroxyapatite (HA) powder were used as raw materials. Mg is the base and matrix in the composite material while Zn and Mn are used as alloying elements. HA was used as reinforcement in composite material as well as for its bioactive properties. An organic material which is n-heptane solution was also added as surfactant or process control agent (PCA) in the mixture to prevent agglomeration during mechanical alloying process. The details of the raw materials are shown in Table 1.
| Materials | Purity | Supplier | Particle Size (µm) |
|---|---|---|---|
| Mg | ≥98.5% | Merck | ˂380 |
| Zn | 99.9% | Alfa Aesar | ˂85.8 |
| Mn | 99.5% | Strem Chemicals | ˂56.8 |
| HA | ≥90.0% | Sigma-Aldrich | ˂22.6 |
Table 1: Details of raw materials.
Mixing of raw materials was carried out in Fritsch Pulverisette P-5 planetary ball mill. The starting materials Mg, 1.5 wt% alloying material and 10 wt.% HA powder with 3% of n-heptane were placed together into stainless steel mill vial as shown in Table 2. Stainless steel milling balls with a fixed 5:1 ball-to-powder weight ratio (BPR) also were placed together with the powders. Argon gas was applied into the vial to prevent the powders from oxidation or any contamination. The milling rotation speed was set to 300 rpm for 2 hours milling time and interval of 30 minutes milling and 30 minutes rest was set in order to control the temperature increment during milling process. According to the previous study, high milling speed of MA can produce high properties of Mg alloy while short milling time with a low BPR were set to avoid the generation of excessive heat at high milling speed [12].
| Starting material | Amount | |||
|---|---|---|---|---|
| Mg-Zn-HA composition | Mg-Mn-HA composition | Mg-ZnMn-HA composition | Mg-HA composition | |
| Mg | 98.5 wt.% | 98.5 wt.% | 98.5 wt.% | 100.0wt.% |
| Zn | 1.5 wt.% | - | 0.75 wt.% | - |
| Mn | - | 1.5 wt.% | 0.75 wt.% | - |
| HA | 10 wt.% | 10 wt.% | 10 wt.% | 10 wt.% |
| n-heptane | 3% | 3% | 3% | 3% |
Table 2: Weight percentage of starting materials.
The milled powders were uniaxial pressed under pressure of 400 MPa for 2 min holding time at room to produced green composite. Subsequently the compacted composites were sintered in Lenton tube furnace at 400°C for an hour soaking time under continuous argon flow. To ensure the samples are homogenously heated, a heating rate of 5°C/min was used for both heating and cooling cycles.
Bruker model D8 Advance, Germany X-Ray Diffractometer (XRD) was used to identify the phases present in the milled powders and sintered composites. The data was collected at diffraction angles from 10° to 90°. The phases present were identified by matching the obtained pattern with the standard pattern using EVA software. The morphology of the milled powders and sintered composites were studied using ZEISS SUPRA 35VP Field Emission Scanning Electron Microscopy (FESEM). The FESEM was equipped with Energy Dispersive X-ray (EDX) and was used to evaluate the composition of the milled powders and sintered composites. Prior to observe under SEM, the sintered composites were ground with silicon carbide (SiC) paper up to 1200 grit and then polished with 1.0 μm diamond suspension, followed by cleaning with ethanol and dried under warm airflow.
Density of sintered composites was measured by Archimedes’ principle. Firstly, the sintered composites were weighed in dry state. Then, the sintered composites were placed on metal cone in Precisa XB 220A electronic balance and weight in air was measured. Lastly, the sintered composites were submerged in the water and the weight was measured. Average of five readings was taken for each sintered Mgbased composite. The relative density (TD%) was calculated based on the theoretical rule of mixture (ROM) as eqns. (1)-(3):
TD% = Density / DensityROM x 100% (1)
DensityROM = ρMg V + ρalloy Valloy + ρHA VHA (2)
VMg + Valloy + VHA = 1 (3)
Where ρMg,ρalloy and ρHA represent the density of Mg, alloying element and HA, respectively, whereas VMg, Valloy and VHA represent the volume fraction of Mg, alloying element and HA respectively.
The hardness of the sintered pellets was measured using LECO LM 248AT Vickers micro hardness tester. The load used was 300 gf with 10 seconds of dwell time and an average of ten points was taken for each sintered composites. The sintered composites with ratio 1:1 of diameter to height were used for compression test. The compression tests were performed on sintered composites with ratio 1:1 of diameter to height according to ASTM E9-89a standard test methods of compression testing of metallic materials at room temperature using Introns 5982 universal testing machine at displacement rate of 1.0 mm/min at room temperature.
Weight loss analysis was used to study the corrosion behavior of Mg-based composites. The immersion for weight lost test was performed in the in Hank’s Balanced Salt Solution (HBSS) for 5 hours and 24 hours at a controlled temperature of 37 ± 0.5°C maintained in water bath. After each period of immersion, the samples were removed from the solution and cleaned with chromic acid to remove the corrosion products. Then, the samples were rinsed with ethanol followed by de-ionized water and dried in oven at 100°C for an hour. The weight before and after immersion was weighed using Sartorius electronic balance and percentage of weight loss was calculated.
Results and Discussion
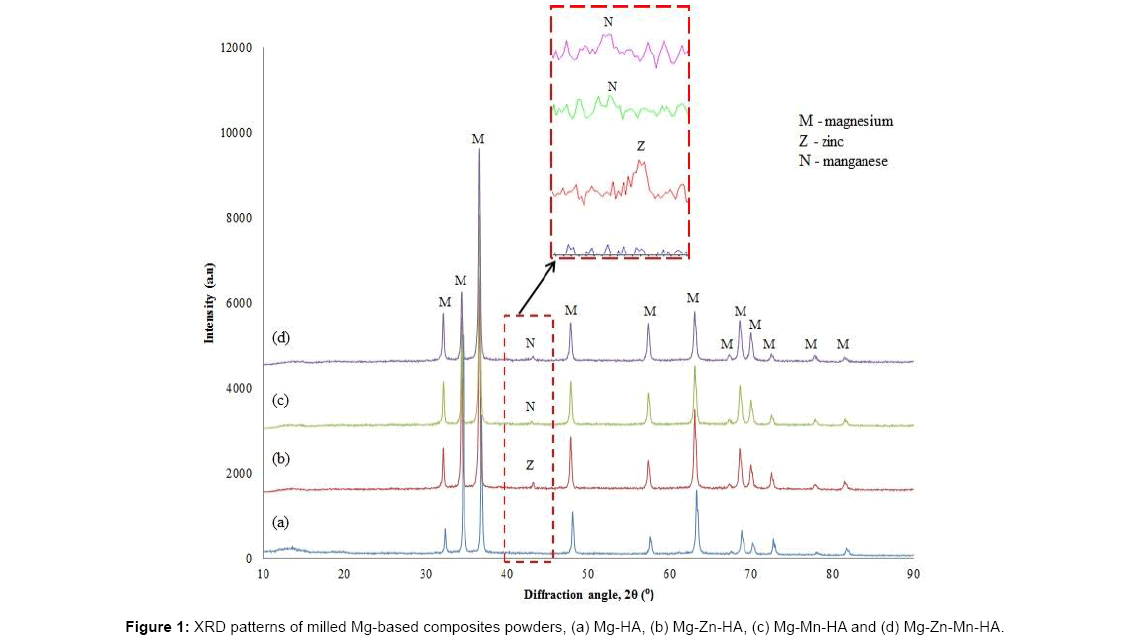
Figure 1 shows the XRD patterns of milled Mg-based composites powders. For Mg-Zn-HA composition, XRD peaks show the existence of Zn phase. The peak of Mn was seen in Mg-Mn-HA composite and Mg-Zn-Mn-HA composite. However, only a single peak of Zn or Mn existed in Mg-based composites with alloying element addition. Thus it appears that during mechanical alloying most of Zn and Mn elements are dissolved into Mg matrix forming α-Mg solid solution.
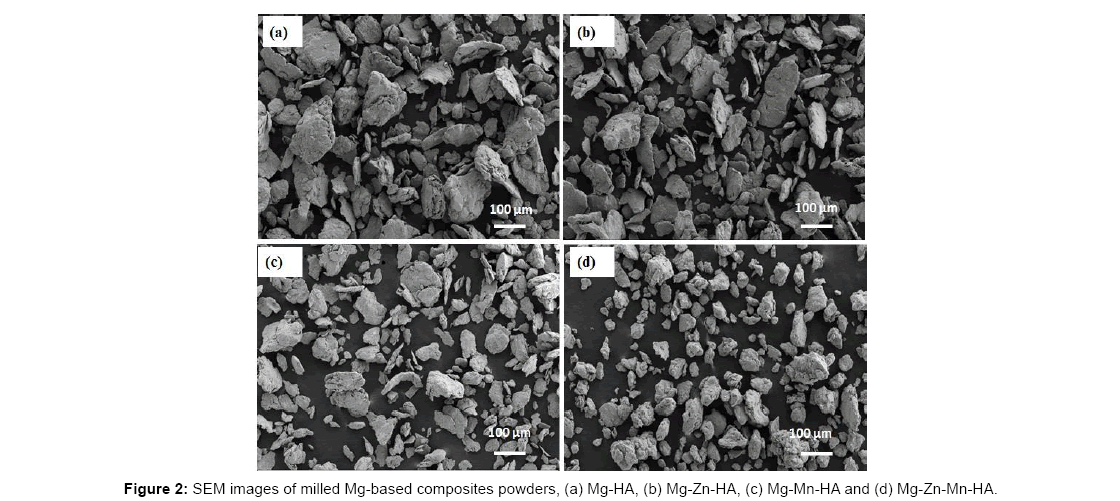
Figure 2 shows the SEM images of milled Mg-based composites powder. The Mg-based composite without alloying elements consists of agglomerates with coarse and irregular shaped particles. The less crystalline structure of HA may cause agglomeration of Mg- HA composite [13]. With the addition of alloying elements the agglomeration of the particle is significantly reduced specially with the addition of Mn element. Much finer particle size is obtained with addition of Mn than with just the addition of Zn in the Mg. Only binary addition of Mn or Zn in Mg-based composite shows the irregular and flat shaped particles. The flat shape of the powder may be due to the ball and vial collision during mechanical alloying. Better refinement of particle size occurred with the addition of Zn and Mn in combination in Mg-based composite.
The ternary alloyed Mg-based composite showed less agglomeration of the particles with uniform granular shape and no flattening of the particles was observed. The addition of crystalline material such as Zn and Mn in the Mg-based composite seems to reduce the particle size as well as increase the homogeneity in size of the powder. This may be due to the resultant harder phase formation on ternary addition. The harder particles break easily to finer sizes as compared to ductile phases.
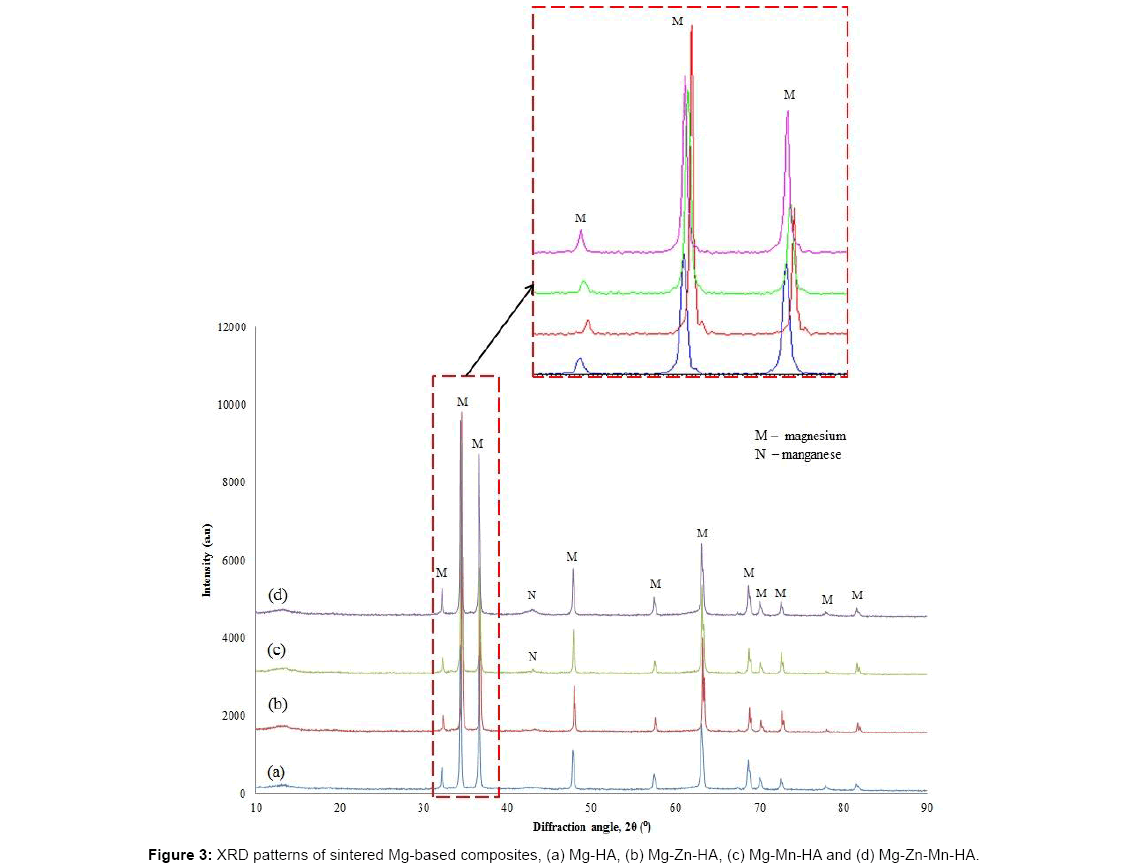
The diffractogram of Mg-Zn-HA composite powder placed in Figure 2b shows a peak of Zn observed after milling. However after sintering, no peak of Zn was detected but from SEM observation, there are some Zn particles were observed. This mean Zn not fully dissolved into Mg matrix to form solid solution of α-Mg. The α-Mg solid solution causing the Mg peaks slightly shifted to the higher diffraction angle compared to the Mg-HA composite as shown in Figure 3.
The XRD patterns of Mg-Mn-HA composite in Figures 2c and 3c show the shows the existence of Mn both before and after sintering. This shows that Mn is not fully dissolved into Mg matrix. However, the Mg peaks seem slightly shifted to the higher diffraction angle compared to the Mg-HA composite which was believed to be induced by the solid solution of Mn. When Mn dissolved in the Mg matrix, Mn atoms will be replaced the Mg atoms in the Mg lattice.
The diffractogram of Mg-Zn-Mn-HA composite before and after sintering is shows in Figures 2d and 3d respectively. The peak of Mn was detected before and after sintering while no peak of Zn was detected before and after sintering. However, the Mg peaks slightly shifted to the higher diffraction angle compared to the Mg-HA composite that might the formation of solid solution of α-Mg.
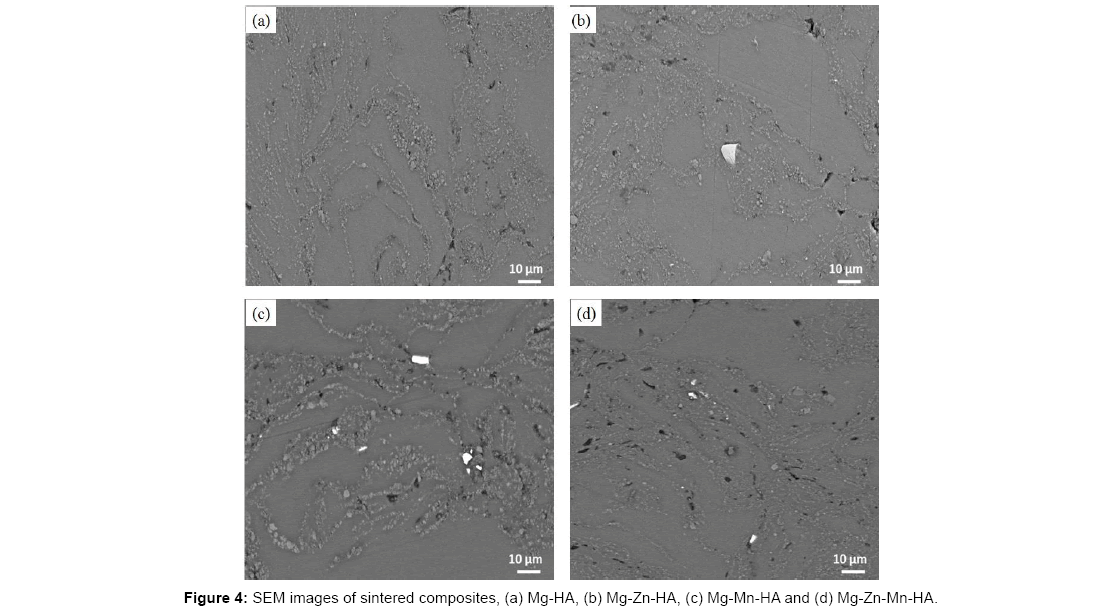
Figure 4 shows the morphology of sintered Mg-based composites. The dark phase is referred to Mg or α-Mg phase while white phase is HA and the a few bright particles are suggested to undissolve alloyed particles since they are not appear in Mg-HA composite. The Mg-Zn- HA composite shows better and more uniform distribution of HA than Mg-HA composite while Mg-Mn-HA shows large particles of HA and Mg-Zn-Mn-HA shows finer particles of HA and distribution of HA is not uniform. The different distributions obtained of HA in Mgbased composite may be due to the shape and particle size obtained after milling as was shown in Figure 2. There are some pores visible on the composites after sintering which may be due the burning out of the impurities or any organic materials decomposing at temperature of 400°C.
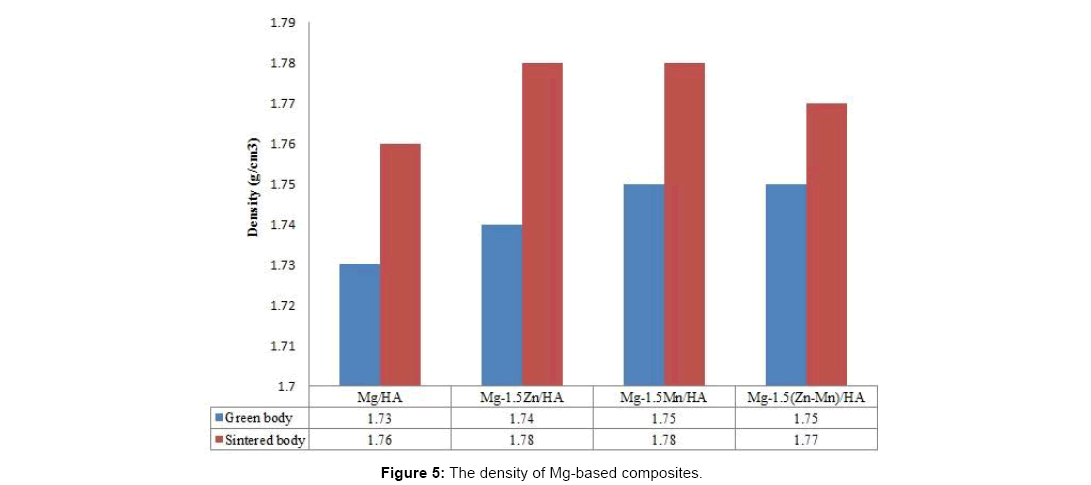
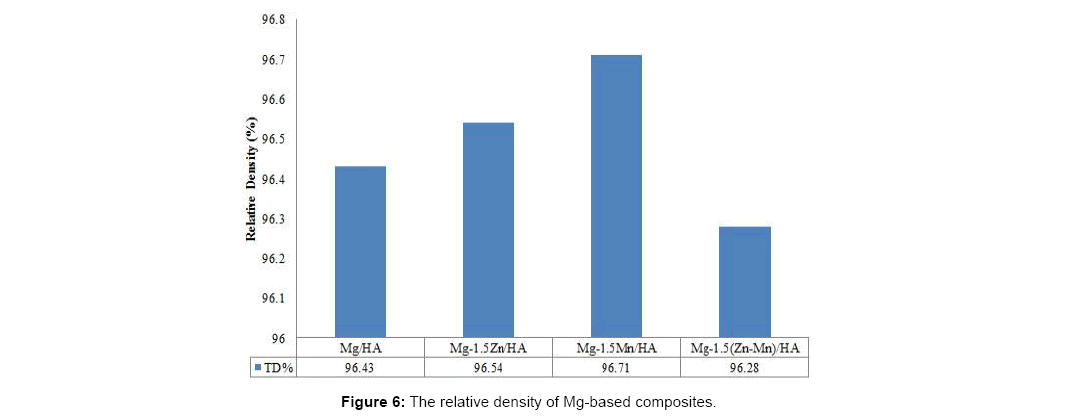
Figure 5 shows that the density of sintered Mg-based composites increases after sintering since the green bonded particles allow diffusion of species into each other, forming necking and thus reducing pores during the sintering process. In addition, the density of the composites increases with the addition of alloying elements as the alloying element diffuse into Mg matrix and reduce some pore in the matrix. The addition of 1.5% w.t. alloying elements did not significantly affect the densification of Mg-based composites which increase to 1.78 g/cm3 for binary alloyed Mg-based composites and 1.77 g/cm3 for ternary alloyed Mg-based composites which still in range of natural bone density (1.70-2.10 g/cm3). The addition of Mn element shows slightly higher relative density of 96.71% as shown in Figure 6.
Relative density is percentage ratio of the Mg-based composite density to the theoretical density calculated according to rule of mixture (ROM) formula. The theoretical density value of Mg-HA is 1.82 g/cm3 while theoretical values of Mg-Zn-HA, Mg-Mn-HA and Mg-Zn-Mn- HA composites are 1.84 g/cm3.
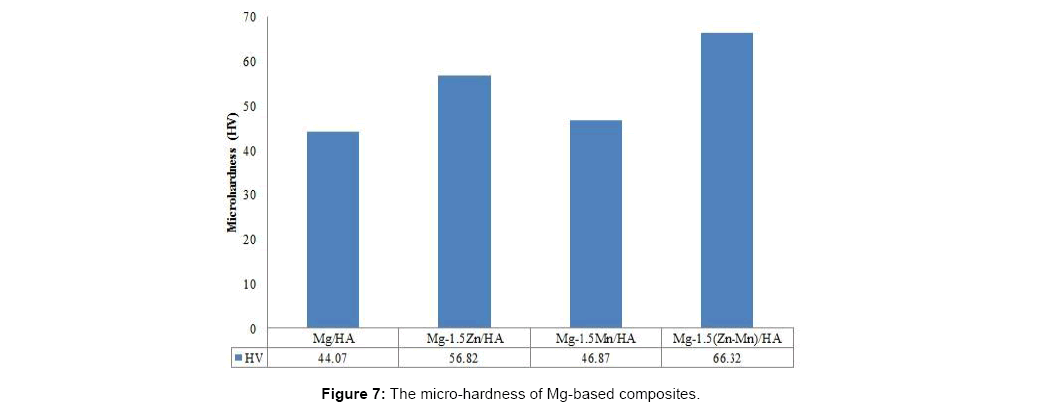
Microhardness of Mg-based composites increases as alloying elements are added into the Mg-based composite. This can be seen in Figure 7. The microhardness of Mg-Zn-Mn-HA composite shows the highest value among the alloyed Mg-based composites. This may due to lattice distortion effect where multi-element lattice is highly distorted because all the atoms are solute atoms and their atomic sizes are all different from one another [14]. For binary alloyed composite, the additional of Zn shows better effect on hardness than the additional of Mn.
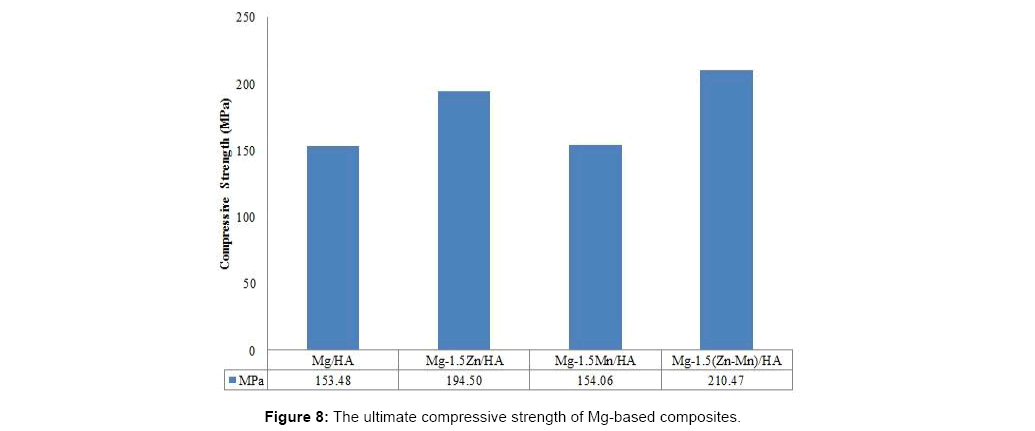
Mechanical testing is done in order to determine failure strength under uniaxial compressive loading of the sintered Mg-based composite. Figure 8 indicate the ultimate strength of the Mg-based composites and it clearly shows that by adding the alloying elements into Mg-based composite, ultimate compressive strength of the composites increased. The addition of ternary alloying elements in Mgbased composite shows the highest ultimate compressive strength that increases from approximately 150 MPa without alloying elements to 210 MPa with alloying elements. This is because the dispersion of both Zn and Mn as alloying elements in the Mg matrix give solid solution strengthening effect as on deformation dislocation movement is obstructed by the stressed matrix increasing the compressive strength of the composites [10].
It can also be inferred that the compressive strength is directly proportional to the hardness as increasing the hardness may increase the compressive strength. In binary alloying of Mg-based composite, the addition of Zn show better strengthening than the addition of Mn since Zn is considered next to Al in strengthening effectiveness as an alloying element in Mg.
Immersion test was performed in order to measure the weight loss of the Mg-based composites in HBSS solution. Mg degrades rapidly in the physiological system. Therefore, weight loss measurement in HBSS was done to study the corrosion behaviour of the Mg-based composite. The weight loss was obtained after removing the corrosion products from the corroded samples by cleaning with chromic acid to remove the corrosion products from the corroded sample without removing any amount of metallic Mg [15].
Figure 9 indicate the percentage weight loss of the Mg-based composite after 5 hours and 24 hours immersion in HBSS. The graph clearly shows that the weight loss was significantly increased as immersion time was increased. This is because chloride ion from HBSS aggressively attack the composite surface by producing Mg(OH)2 which encourage Mg matrix to dissolve in HBSS leading to weight loss of the composite [16].
Figure 9 also clearly shows that the weight loss can be reduced by adding alloying element into Mg-based composite. The Mg-based composite with addition of Zn and Mn as alloying elements showed the lowest weight loss among the Mg-based composite followed by Mn and Zn as individual alloying elements. This results show similar trends as reported by Xu et al. [17] who studied corrosion behaviour of Mg-Mn- Zn alloy. They concluded that the combination of alloying elements in Mg alloy can reduce the weight loss since more elements can react and form a protective layer on the surface of the composite.
In the present studies also it is observed that the addition of alloying elements into Mg-based composite can increase the corrosion resistance. The binary alloying elements in Mg-based composites show that element addition of Mn causes less weight loss than composite containing Zn. Therefore, it seems that Mn is more effective to enhance corrosion resistance of Mg-based composite since Mn have been improving the saltwater resistance of magnesium alloy by removing iron and other heavy-metal elements while Zn has tendency to fast corroded rather than Mn [18].
Conclusion
The binary addition of alloying element Mn in Mg-Mn-HA sintered composite leads to higher density of Mg-based composite with 96.71% relative density (1.78 g/cm3) compared to Mg-Zn- HA composite. However, higher microhardness in binary alloyed composite is obtained by adding Zn as alloying element in Mg-based composite with a value 56.82 HV as well compressive strength of 195 MPa. Mg-Mn-HA composite shows 0.9% and 9.1% of weight loss when immersed for 5 hours and 24 hours in HBSS showing increase in the corrosion resistance.
Addition of ternary alloying elements to Mg-based composite shows excellent properties of Mg-based composite with 65.49 HV microhardness and compressive strength of 210 MPa which is comparable to compressive strength of bone (170 MPa-200 MPa). The density of Mg-Zn-Mn-HA composite is 1.77 g/cm3 and the relative density is 96.28% which is slightly lower than the binary Mg-Mn-HA, however it is still in the range of natural bone density (1.70-2.10 g/cm3). In immersion test, Mg-Zn-Mn-HA shows reduced weight loss of 0.6% to 6.2% when immersed for 5 hours and 24 hours in HBSS.
Acknowledgement
The authors would like to thank Ministry of High Education, Malaysia (FRGS Grant No. 6071304) and scholarship scheme (MyBrain15) for the financial support.
References
- Moravej M, Mantovani D (2011) Biodegradable metals for cardiovascular stent application: Interests and new opportunities. International Journal of Molecular Sciences 12: 4250-4270.
- Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, et al. (2015) Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 8: 5744-5794.
- Gotman I (1997) Characteristics of metals used in implants. Journal of Endourology 11: 383-389
- Ye X, Chen M, Yang M, Wei J, Liu D (2010) In vitro corrosion resistance and cytocompatibility of nano-hydroxyapatite reinforced Mg-Zn-Zr composites. Journal of Materials Science: Materials in Medicine 21: 1321-1328.
- Yin DS, Zhang EL, Zeng SY (2008) Effect of Zn on mechanical property and corrosion property of extruded Mg-Zn-Mn alloy. Transactions of Nonferrous Metals Society of China 18: 763-768.
- Ding Y, Wen C, Hodgson P, Li Y (2014) Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: a review. Journal of Materials Chemistry B 2: 1912-1933.
- Campo R, Savoini B, Munoz A, Monge MA, Garce´s G (2014) Mechanical properties and corrosion behavior of Mg-HAP composites. Journal of the Mechanical Behavior of Biomedical Materials 39: 238-246.
- Mjöberg B, Hellquist E, Mallmin H, Lindh U (1997) Aluminum, Alzheimer’s disease and bone fragility. Acta Orthop Scand 68: 511-514.
- Brar HS, Platt MO, Sarntinoranont M, Martin PI, Manuel MV (2009) Magnesium as a biodegradable and bioabsorbable material for medical implants. Jom 61: 31-34.
- Zhang S, Zhang X, Zhao C, Li J, Song Y, et al. (2010) Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomaterialia 6: 626-640
- Gu X, Zhou W, Zheng Y, Dong L, Xi Y, et al. (2010) Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/HA composites. Materials Science and Engineering 30: 827-832.
- Salleh EM (2015) Mechanical Compatibility and Degradation of Biodegradable Mg-Zn Alloy Based Composite Fabricated Using Powder Metallurgy. PhD Thesis, Universiti Sains Malaysia, Penang.
- Karimzadeh F, Enayati MH, Tavoosi M (2008) Synthesis and characterization of Zn/Al2O3 Nano composite by mechanical alloying. Materials Science and Engineering A 486: 45-48.
- Yeh JW (2006) Recent progress in high-entropy alloys. Annales de Chimie: Science des Materiaux 31: 633-648.
- Zhao MC, Schmutz P, Brunner S, Liu M, Song GL, et al. (2009) An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing. Corrosion Science 51: 1277-1292.
- Xin Y, Liu C, Zhang X, Tang G, Tian X, et al. (2007) Corrosion behavior of biomedical AZ91 magnesium alloy in simulated body fluids. Journal of Materials Research 22: 2004-2011.
- Xu L, Zhang E, Yin D, Zeng S, Yang K (2008) In vitro corrosion behavior of Mg alloys in a phosphate buffered solution for bone implant application. Journal of Materials Science: Materials in Medicine 19: 1017-1025.
- Avedesian MM, Baker H (1999) ASM specialty handbook: magnesium and magnesium alloys, ASM international.
Citation: Hussain Z, Mohd Isa N, Dhindaw BK (2017) Effect of Alloying Elements on Properties of Biodegradable Magnesium Composite for Implant Application. J Powder Metall Min 6: 179. DOI: 10.4172/2168-9806.1000179
Copyright: © 2017 Hussain Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7020
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 5914
- PDF downloads: 1106