Research Article Open Access
Effect of Activating Lacto Peroxidase System (LPS) On Quality and Storage Stability of Soft Cheese
Rashida Parveen1*, Rabia Kausar2, Ayesha sameen1, Muhammad Issa khan1 and Neelum Sana31National Institute of Food Science and Technology, University of Agriculture Faisalabad, Pakistan
2Dalda Foods Private Limited Lahore, Pakistan
3Nestle Milk Pack Private Limited Lahore, Pakistan
- *Corresponding Author:
- Rashida Parveen
National Institute of Food Science and Technology
University of Agriculture
Faisalabad, Pakistan
Tel: 0092-334-5120509
E-mail: rashidaparveen73@yahoo.com
Received April 08, 2016; Accepted April 26, 2016; Published May 03, 2016
Citation:Parveen R, Kausar R, sameen A, khan MI, Sana N (2016) Effect of Activating Lacto Peroxidase System (LPS) On Quality and Storage Stability of Soft Cheese. J Biotechnol Biomater 6:224. doi:10.4172/2155-952X.1000224
Copyright: © 2016 Parveen R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Utilization of intrinsic enzymatic activity to increase the quality and storage stability of food product is a novel biological and biochemical technique. The aim of present research was to minimize the microbial, physiochemical and protein degradation changes in soft cheese to enhance its quality and shelf life by activating lacto peroxidase system in raw buffalo milk and ultimately using it for cheese production and studying its quality and storage stability. For this purpose buffalo milk samples were collected from Diary Research Farm at University of Agriculture Faisalabad, Pakistan. In collected milk samples LPS was activated by equimolar concentration at 20 ppm of NaSCN and H2O2 and resulting samples were used for soft cheese production. Analysis at 0, 7, 14 and 21 days of storage period were conducted under 4ºC. Collected data were analyzed by using one way analysis of variance under completely randomized design (CRD). Means were compared by using LSD test at probability level of p<0.05. Results showed minimum contamination in microbial count, especially bacteria of salt tolerant, at the end of 21 days storage period. Significantly lowered yeasts, molds, Coliform and bacterial count (p<0.05) were observed in LPS activated cheese samples as compared to other. Moreover, proteolysis results determined by Urea-PAGE gel electrophoresis for casein fractions extracted from the three samples showed lower value for LPS treated cheese sample in contrast to others samples. Hence, the present study supports the Lacto peroxidase system (LPS) as a quality-cum-economical preservative technique as compared to other techniques in practice.
Keywords
Shelf life stability; Soft cheese; Protein degradation urea page; Microbiology; Acidity; Physicochemical analysis
Abbreviations
H2O2: Hydrogen Peroxide; NPN: Non Protein Nitrogen; NCN: Non Casein Nitrogen; LPS: Lacto Peroxidase System; LAB: Gram-Positive and Facultative Anaerobes
Introduction
In human, nutrition milk and milk products play an important role. Pakistan ranks 5th largest milk producing country in the world but milk hygienic quality is low due to highly perishable nature of milk. That’s why to handle milk in highly ambient summer temperature is a challenging issue for dairy industry. Different pre-treatments are given to milk before cheese preparation to confirm its microbial protection. These treatments include cold storage, chilling, bactofugation, thermisation & pasteurization [1]. Milk proteins and lipids are hydrolyzed through bacterial secretion by a number of proteases, lipases and heat stable extra cellular enzymes. These bacteria cause several problems in dairy products such as abnormal texture, off-flavors and reduction in cheese yields [2]. During milk storage by refrigeration, it is observed psychrotrophs can proliferate. Normally soft cheeses are preserved with chemical preservatives along with refrigeration for three weeks but preservatives cause undesirable off-flavors. Pathogenic microorganisms cause various off-flavors, abnormal texture and reduction in cheese yield during storage due to its high moisture content. Normal moisture content (i.e. 80%) in cheese not only supports the survival of a variety of decomposing and pathogenic microorganisms like Psychotropic bacteria, yeast and mold but also increases the decomposition rate of the cheese [3].
The hygienic quality of milk and milk products has been sustained by the use of LPS. LPS is a naturally occurring antibacterialsystem found in milk, which is the most effective against microorganisms [4]. Peptide present in the milk protein such as lactoferrin, lacto peroxidase, immunoglobulins have specific non-nutritional physiological functions. Peroxidase (LP) in the case of human infants is undoubtedly important, but it may have highly significant and functional role in the milk industry. LPS is also important for the native immune system in milk and mucosal secretions killing bacteria, thereby enhancing the LPS application in the treatment of shelf life stability [5]. Codex Alimentarius Commission has approved Lacto peroxidase method to save the raw milk which is based on the exogenous activation of natural systems, the molar concentration of thiocyanate and hydrogen peroxide. For the most excellent threshold of enzyme activity bacteriostatic effects on both Gram-positive and Gram-negative bacteria including psychrotrophs, decrease the shelf life of liquid milk at refrigeration temperatures [6]. Quality of milk products can be improved by using LPS. 0.5kg/100L increase in cheese yields is reported by LPS [7]. Excellent results are shown in cooled milk over long periods by applying LPS at industrial scale [8,9]. The LPS is a natural antimicrobial system in milk which results from the interaction between three components; the enzyme LP, thiocyanate ion (SCN) and hydrogen peroxides (H2O2). Thiocynate is oxidized in its intermediate antimicrobial substances (for example hypothiocyanite, OSCN-) in the presence of H2O2 during LP activation. All mammals have naturally LP enzyme in their milk. It has heat stability & antibacterial activity in the presence of LPS [10]. Biologically LP acts as natural defense system against the microbial invasion. It has bactericidal and bacteriostatic effect on variety of microorganism, which have no effect on protein and enzymes of the organism which produce it [11]. Efficiency of LPS improves in presence of milk preservatives. LP is present in milk up to required concentration while thiocyanate & H2O2 are externally added [7]. Microbial quality of raw milk increases by activation of LP because of some intrinsic oxidized thiocyanate, in presence of hydrogen peroxide which can be produced by certain bacteria. So the total microbial load decreases due to LPS activation. Due to the growing interest of producers; cheese is manufactured with the help of such milk which is activated by LPS [12]. Temperature during treatment and initial microbial of milk improves the working efficacy & activity of LPS [11].
Methods used to inhibit psychotropic bacteria include the use of activation of the lacto peroxidase system (LPS) and lactic acid bacteria (LAB) for the development of antimicrobial function in food [13]. Thus, the present research was premeditated to evaluate the impact of LPS on the quality and storage stability of soft cheese prepared from Lacto peroxidase activated milk.
Material and Methods
Raw materials procurement
Raw buffalo milk was procured from Dairy Research Farm, University of Agriculture, Faisalabad, for the preparation of cottage cheese. Composition of raw buffalo milk used for cheese preparation is given in Table 1. Soft cottage cheese samples were prepared from buffalo milk with different conditions and preserved by activation of Lacto peroxidase system (on equimolar doses of 20 ppm ((SCN-& H2O2).PS) as mentioned in Table 2.
| Composition | Percentage % |
|---|---|
| Fat% | 6.6 |
| Protein | 4.23 |
| pH | 6.7 |
| Moisture | 82.50 |
| Acidity% | 0.14 |
| (Non protein nitrogen)NPN | 0.1 |
| (Non casein nitrogen) NCN | 0.84 |
Table 1: Physicochemical composition of raw buffalo milk used for cheese preparation.
| Treatments | Conditions |
|---|---|
| T0 | Controlled without activation of LPS & Refrigeration |
| T1 | LPS activated raw milk Cheese |
| T2 | LPS activated, refrigerated (4°C) & Pasteurized (63-65°C)for 30 min. raw milk cheese |
| T3 | LPS activated & refrigerated (4°C) raw milk cheese |
| T4 | Refrigerated raw milk cheese without LPS activation |
Table 2: Treatment plan used for sampling of cheese during storage study.
Activation of lacto peroxidase system (LPS) and cheese preparation
At the start of each treatment before cheese transformation, buffalo raw milk samples were stabilized through addition of equal molar concentration of sodium thiocyanate and H2O2 for the activation of Lactoperoxidase System (LPS) by the procedure described by Mouna et al. [11]. Then the milk samples were kept for 2 hours at room temperature (25°C) for free activation of the LPS and to promote interaction among its functional units, like Lacto peroxidase enzyme (LP), NaSCN and H2O2. LP treated milk was refrigerated at 4°C for 48 hours before cheese making in some treatments. Preparation of cheese was done following the protocol of Aneja [14]. Milk was pasteurized at 72°C for 15 seconds followed by cooling at 21-23°C for the mesophilic culture addition. Ripening of the mixture was done at 31-32°C in water bath for 4-5 hrs. Then the rennet was added and mixture was mixed for several minutes. Time was given to mixture for acidification till its pH 4.7 attained. Curd was cut & healing time of 10-15 minutes was provided. Heating of curd was done at 55°C with gently stirring. Whey was drained by hopping of coagulum in muslin cloth then salt added and pH noted. For analysis of all cheese treatments at 0, 7, 14 and 21 days of storage at 4°C were selected randomly. Analyses were carried out in triplicate in all treatments.
Physicochemical analysis of cheese
For analysis during storage at 4°C the soft cheese samples of different treatments were drawn at regular interval. The pH of cheese samples were measured with a penetration electrode according to method of AOAC [15]. The acidity and the moisture content were determined by the method of AOAC [15]. The fat content of cheese samples of the different treatments were measured by Gerber method following the procedure of Marshall [16]. Total nitrogen, Non-protein nitrogen (NPN) and Non-casein nitrogen (NCN) were determined by Kjeldhal method of AOAC [15] at 0, 7, 14, 21 days of storage at 4°C. The protein content was determined by multiplying the total nitrogen of cheese samples of all treatments with factor of 6.38 in all cheese sample of all treatments whereas NPN and NCN content was calculated by multiplying the factor of 3.60 and 6.25 respectively. The % values of nitrogen over the total nitrogen were used as indicators of proteolysis during storage period.
Microbiological analysis
By following the methods described by Mouna et al. [11], various selective media were used for the microbiological analysis of soft cheese during storage. One gram of cheese samples was grated and dispersed in 9 mL of sodium citrate 2% (w⁄v). Then appropriate dilutions (102– 107) were carried out in nutrient agar for total plate count in cheese samples. By using De Man Rogosa and Sharp Agar medium (MRSA) after incubation of plates at 37°C for 48 hrs, lactic acid bacteria (LAB) were counted. By using Mackonkey agar, coliformswere estimated.
Electrophoresis analysis
In the samples of different chees treatments protein degradation was evaluated by Urea page gel electrophoresis (UREA-PAGE) [17,18]. 15% polyacrylamide gel slabs were prepared and run with urea. For electrophoresis analysis of protein samples were prepared by the method of Manka et al. [19]. Standards used in SDS-PAGE proteins were from Biorad (France).
Statistical analysis
Statistical analysis was carried out using analysis of variance by applying complete randomized factorial design. The mean of all treatments were also compared by using LSD test as described by Tarakci [20].
Results and Discussion
Effects of treatments on pH of soft cheese during storage
Table 3 indicates the mean value of pH of different treatments of soft cheese. Mean value of pH demonstrate that the maximum pH (5.97) was found in T4 and minimum value of pH (4.64) in T0. Similarly, the maximum pH value (5.64) was observed at 0 day and minimum value (5.41) was observed at 14 day. The decrease in pH after 21 days in T0 was 0.8, in T1 was 0.2, whereas increase in T2 was 0.4, in T3 was 0.4 and in T4 was 0.6. These differences demonstrate maximum decrease in T1 and minimum increase in T4. These findings are in accordance with the results of Mouna et al. [11] reporting minimum decrease in pH of soft cheese with storage interval. pH value is also dependent on acidity of cheese. If acidity of cheese is higher, its pH value will be lower and if less acidity then pH will be higher. Higher the lactic acid bacteria more lactose change into lactic acid and acidity decrease while pH also decrease. The activity of LPS suppress the growth of culture and lactic acid bacteria, which in turn affects the rate of acid production. Results are also according to AOAC [15] reporting LPS delayed the coagulation time and reduced the activity of starter cultures.
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 5.13 ± 0.005 | 5.17 ± 0.01 | 5.86 ± 0.005 | 6.03 ± 0.005 | 6.00 ± 0.005 | 5.64a |
| 7 | 4.58 ± 0.005 | 5.18 ± 0.005 | 5.85 ± 0.005 | 5.90 ± 0.005 | 5.99 ± 0.005 | 5.51b |
| 14 | 4.43 ± 0.005 | 5.05 ± 0.005 | 5.82 ± 0.005 | 5.81 ± 0.005 | 5.96 ± 0.005 | 5.41c |
| 21 | 4.41 ± 0.005 | 5.30 ± 0.5 | 5.79 ± 0.005 | 5.73 ± 0.005 | 5.92 ± 0.005 | 5.43bc |
| Mean | 4.64d | 5.17c | 5.83b | 5.87b | 5.97a | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05).TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 3: Effect of treatments on pH of soft cheese during storage.
Effect of treatments on acidity of soft cheese during storage
Table 4 designates the mean values of acidity of different treatments of soft cheese. Mean value of acidity show that the maximum acidity (0.29%) was observed in T0 and minimum (0.21%) in T2. During storage the acidity value varies from 0.20% to 0.29% at 0 and 21 day respectively. Milk itself has also effect on the acidity. Buffalo milk showed more resistance in pH because of its buffering capacity. Treatments T0, T1 and T2 have 0.04 difference in acidity while T3 and T4 have minimum difference in acidity value. These results are in-line with the findings of Tarakci [21] who found a significant increase in titratable acidity during the storage. The higher acidity observed in the cheese prepared from indigenous cultures showed that the activity of these starters was relatively faster because the primary function of starters is the conversion of lactose and other sugars in milk to lactic and other acids [22,23].
| Treatments Mean | ||||||
|---|---|---|---|---|---|---|
| Days | T0 | T1 | T2 | T3 | T4 | Mean |
| 0 | 0.23 ± 0.005 | 0.19 ± 0.01 | 0.19 ± 0.005 | 0.18 ± 0.005 | 0.19 ± 0.005 | 0.20d |
| 7 | 0.26 ± 0.005 | 0.24 ± 0.005 | 0.22 ± 0.005 | 0.26 ± 0.005 | 0.27 ± 0.005 | 0.25c |
| 14 | 0.31 ± 0.005 | 0.26 ± 0.005 | 0.25 ± 0.005 | 0.26 ± 0.005 | 0.26 ± 0.005 | 0.27b |
| 21 | 0.36 ± 0.005 | 0.30 ± 0.005 | 0.19 ± 0.005 | 0.30 ± 0.005 | 0.30 ± 0.005 | 0.29a |
| Mean | 0.29a | 0.25c | 0.22d | 0.25bc | 0.26b | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05).TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 4: Effect of treatments on acidity of soft cheese during storage.
Effect of treatments on moisture of soft cheese during storage
Table 5 represents the mean value of moisture percentage of different treatments of soft cheese. Mean value of moisture demonstrate that the maximum moisture (61.72%) was found in T4 and minimum (55.49%) in T3. The moisture mean value varied from 65.61 at 0 day to 55.37 at day 21 through 61.27 at day 7 and 56.67 at day 21. The variation in the moisture contents agree with the result of Licitra et al., Schor and Bosset and Montensions-Herrero [24-26] reporting that there is a drop in moisture content with the increasing storage days which may be owing to the loss of moisture contents from the surface.
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 62.36 ± | 60.01 ± | 57.68 ± | 56.95 ± | 65.05 ± | 65.61b |
| 7 | 61.71 ± | 61.77 ± | 61.76 ± | 58.79 ± | 62.32 ± | 61.27a |
| 14 | 51.76 ± | 57.27 ± | 60.37 ± | 55.16 ± | 60.71 ± | 56.67c |
| 21 | 50.38 ± | 54.77 ± | 59.98 ± | 51.05 ± | 58.77 ± | 55.37d |
| Mean | 56.55d | 58.45c | 59.95b | 55.49e | 61.72a | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05). TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 5: Effect of treatments on moisture of soft cheese during storage.
Effect of treatments on fat of soft cheese during storage
Table 6 represents the mean value of fat percentage in different treatments of soft cheese. Mean values of fat content represent that the maximum fat (25.16%) was found in T3 and minimum fat percentage was found (21.83%) in T2. During storage maximum fat percentage value (24.13%) was observed at 14 day and minimum value (23.47%) at 7 day. In treatment, fat varied 24.92%, 23.33%, 21.83%, 25.16% and 23.83 for treatments T0, T1, T2, T3 and T4 respectively. This variation in fat may be due to the acidity of the milk utilized for the production of soft cheese [27]. These results are similar with findings of Mouna et al. [11] reporting that the Lactoperoxidase system (LPS) does not affect the composition of product during storage.
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 25.33 ± 1.15 | 23.00 ± 1 | 22.67 ± 0.5 | 24.33 ± 0.5 | 23.67 ± 0.5 | 23.80ab |
| 7 | 23.66 ± 0.5 | 23.33 ± 0.5 | 21.33 ± 0.5 | 25.33 ± 0.5 | 23.67 ± 0.5 | 23.47b |
| 14 | 25.33 ± 0.5 | 23.33 ± 0.5 | 21.67 ± 0.5 | 25.67 ± 0.5 | 24.67 ± 0.5 | 24.13a |
| 21 | 25.33 ± 0.5 | 23.67 ± 0.5 | 21.67 ± 0.5 | 25.33 ± 0.5 | 23.33 ± 0.5 | 23.86ab |
| Mean | 24.92a | 23.33b | 21.83c | 25.16a | 23.83b | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05).TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 6: Effect of treatments on fat of soft cheese during storage.
Effect of treatments on protein of soft cheese during storage
Table 7 denotes the mean value of protein content of different treatments of soft cheese. Mean value of protein contents demonstrate that the maximum protein contents (14.99%) were found in T2 and minimum value of protein content (10.14%) in T3. The highest mean value (13.82%) was observed at 0 day and minimum value (11.59%) was reported at 21 days. These results indicated although that protein value remains constant during the storage intervals but there was a slight difference from 1st day to 21th day. These results correlate with the findings of Fandos et al. [28]. The decrease in the protein might be due to the enhanced growth of enzymes especially proteolytic enzyme. Comparatively, the protein in buffalo milk (4.52) is higher than in cow milk (3.30 %). It is also evident from the findings of Marit et al. [29] that the proteolytic enzyme could be more resistant towards cow milk than buffalo milk and these may have the ability to break down the peptide bond (building blocks of the protein) and convert into small peptones that produce smell observed during sensory evaluation [30].
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 11.55 ± 0.06 | 15.15 ± 0.03 | 16.37 ± 0.01 | 13.75 ± 0.01 | 12.34 ± 0.01 | 13.82a |
| 7 | 11.00 ± 0.02 | 14.57 ± 0.01 | 15.03 ± 0.1 | 12.89 ± 0.02 | 11.93 ± 0.02 | 13.08b |
| 14 | 10.73 ± 0.01 | 13.27 ± 0.01 | 14.40 ± 0.005 | 12.06 ± 0.02 | 10.81 ± 0.03 | 12.25c |
| 21 | 9.91 ± 0.02 | 11.64 ± 0.03 | 14.21 ± 0.01 | 11.87 ± 0.01 | 10.32 ± 0.01 | 11.59d |
| Mean | 10.79b | 13.66c | 14.99a | 10.14b | 11.35d | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05).TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 7: Effect of treatments on protein of soft cheese during storage.
Effect of treatments on NPN of soft cheese during storage
Table 8 represents the mean value of Non-protein nitrogen (NPN) contents of different treatments of soft cheese. Mean value of NPN demonstrate that the maximum NPN content (0.14%) was found in T4 and minimum (0.08%) in T3.These results indicated that NPN contents decrease from 0.13% to 0.07% at 0 day to 21 day. The results of NPN regarding soft cheese mismatched to the findings of Durmos et al. [31] who indicated that during storage TCA soluble nitrogen (NPN) increases in different types of cheese. This difference in findings might be due to LPS activation and concentration of hydrogen cyanide.
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 0.07 ± 0.0005 | 0.10 ± 0.0005 | 0.10 ± 0.0005 | 0.13 ± 0.0005 | 0.29 ± 0.38 | 0.14a |
| 7 | 0.10 ± 0.0005 | 0.12 ± 0.0005 | 0.07 ± 0.0005 | 0.07 ± 0.0005 | 0.10 ± 0.0005 | 0.09a |
| 14 | 0.10 ± 0.0005 | 0.10 ± 0.0005 | 0.10 ± 0.0005 | 0.07 ± 0.0005 | 0.10 ± 0.0005 | 0.09a |
| 21 | 0.07 ± 0.0004 | 0.10 ± 0.0005 | 0.07 ± 0.001 | 0.07 ± 0.0005 | 0.07 ± 0.0005 | 0.08a |
| Mean | 0.08a | 0.11a | 0.08a | 0.08a | 0.14a | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05). TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 8: Effect of treatments on NPN of soft cheese during storage.
Effect of treatments on NCN of soft cheese during storage
Table 9 represents the mean value of Non-casein nitrogen (NCN) contents of different treatments of soft cheese. Mean value of NCN demonstrate that the maximum NCN contents (0.38%) was found in T0 and minimum value of NCN content (0.30%) in T3. NCN content (0.38%) present at 0 day decreased to (0.28%) at 7 day. At 14 and 21 day the NCN content (0.34% & 0.33% respectively) almost remained constant and no significant change occurred from day 14 to day 21.
| Days | Treatments | Mean | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 0.52 ± 0.0005 | 0.35 ± 0.005 | 0.30 ± 0.0005 | 0.35 ± 0.005 | 0.39 ± 0.0005 | 0.38a |
| 7 | 0.26 ± 0.0005 | 0.34 ± 0.005 | 0.30 ± 0.0005 | 0.31 ± 0.005 | 0.22 ± 0.0005 | 0.28d |
| 14 | 0.39 ± 0.0005 | 0.35 ± 0.005 | 0.30 ± 0.0005 | 0.31 ± 0.005 | 0.35 ± 0.0005 | 0.34b |
| 21 | 0.34 ± 0.0004 | 0.34 ± 0.005 | 0.39 ± 0.0005 | 0.26 ± 0.005 | 0.34 ± 0.0005 | 0.34c |
| Mean | 0.38a | 0.34b | 0.33c | 0.31d | 0.33c | |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05). TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 9: Effect of treatments on NCN of soft cheese during storage.
Microflora of soft cheese treatments during storage period
Table 10 results indicate that bacterio statistic activity of Lacto peroxidase system and good quality of raw material results in no coliform up to 21 days of storage. Findings of Mouna et al. [11] are in accordance with our results explaining that raw milk quality, proper storage and packaging conditions can prevent the occurrence of coliform in final product.
| Viable Count | ||||||
|---|---|---|---|---|---|---|
| Days | Treatments | Mean | ||||
| T0 | T1 | T2 | T3 | T4 | ||
| 0 | 2.3333 | 1.7333 | 1.2567 | 1.1333 | 1.0333 | 1.4980a |
| 7 | 1.4333 | 1.4333 | 1.4333 | 1.7333 | 1.0667 | 1.4200b |
| 14 | 1.0667 | 1.6333 | 1.6633 | 1.0333 | 1.0333 | 1.2860c |
| 21 | 1.4333 | 1.3433 | 1.2467 | 1.0667 | 1.0333 | 1.2247d |
| Mean | 1.5667a | 1.5358a | 1.4000b | 1.2417c | 1.0417d | |
| LAB Count | ||||||
| 0 | 1.4333 | 1.2433 | 2.1133 | 1.3133 | 1.2333 | 1.4673c |
| 7 | 1.3333 | 1.3467 | 2.1833 | 1.9133 | 1.4667 | 1.6487a |
| 14 | 1.4333 | 1.4433 | 1.9033 | 1.3133 | 1.2533 | 1.4693bc |
| 21 | 1.5333 | 1.9433 | 1.2067 | 1.4367 | 1.3367 | 1.4913b |
| Mean | 1.4333c | 1.4942b | 1.8517a | 1.4942b | 1.3225d | |
| Coliform count | ||||||
| 0 | -VE | -VE | -VE | -VE | -VE | -VE |
| 7 | -VE | -VE | -VE | -VE | -VE | -VE |
| 14 | -VE | -VE | -VE | -VE | -VE | -VE |
| 21 | -VE | -VE | -VE | -VE | -VE | -VE |
| Mean | -VE | -VE | -VE | -VE | -VE | -VE |
Data represent the mean ± SD of 3 observations in each group. Means sharing similar letters in a column or in a row differ significantly from one another at (p<0.05).TO: Controlled without activation of LPS & Refrigeration; T1: LPS activated raw milk Cheese; T2: LPS activated, refrigerated (4°C) & pasteurized (63-65°C) for 30 min. raw milk cheese; T3: LPS activated & refrigerated (4°C) raw milk cheese; T4: Refrigerated raw milk cheese without LPS activation
Table 10: Microflora of Soft cheese treatments during storage period.
In cheese samples throughout ripening the average counts of different microflora found are reported in Table 10. Total viable count of all cheese samples decrease during storage interval from 1.567 log cfu/ ml to 1.041 log cfu/ml. Large numbers of TBC were observed (1.567 log cfu/ml) in T0 and less number of TBC (1.041 log cfu ⁄mL) in T4 leading to many defects observed in the body structure of the cheese. Total number of bacterial load decreased from 1.498 log cfu /ml to 1.224 log cfu/ml at 0 day to 21 day. In buffalo’s milk cheese the activation of the LPS resulted as a significant influence (p<0.05) on the growth of TBC. These findings are according to the results of Mouna et al. [11] who demonstrated that the number of total bacterial count decreased by the activation of LPS. LPS showed the bacteriostatic action against bacteria.
Table 10 represents the mean value of LAB of different treatments of soft cheese. Maximum number of lactic bacterial count (1.851 log cfu/ml) was observed in T2 and minimum number was observed (1.322 log cfu ⁄mL) in T4 resulting in many defects throughout the body of the cheese. Minimum decrease occurred in LAB count during storage. During storage number of LAB count varies as; (1.467 log cfu/ml) at 0 day, (1.648 log cfu/ml) at day 7, and minimum variation 1.469 log cfu/ml at 14 day & 1.491 log cfu/ml at 21 day. These results are similar with the results of AOAC [15] and Mouna et al. [11]. They reported that LAB was quantitatively the dominant groups during the ripening period. No significant difference (p>0.05) was found in LAB count. The heat treatment applied to the milk used for producing cheeses induced a decrease in the number of all bacterial count [15].
Protein degradation in soft cheese samples during storage
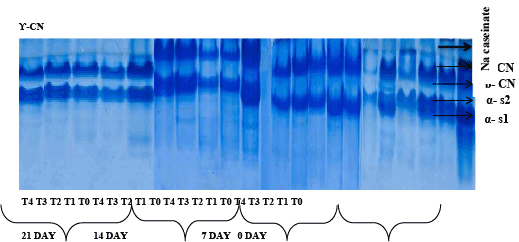
Although proteolysis in cheese during ripening is catalyzed by proteinases and peptides, however from other six sources viz., the milk, coagulant, non-starter lactic acid bacteria (NSLAB), starter lactic acid bacteria (LAB), secondary starter and exogenous proteinases and peptidases, too. It also contributes to off-flavor and flavor of cheese, directly by the production of short peptides and indirectly by the discharge of amino acids [32]. Proteolysis evaluation of soft cheese during 21 days of storage is illustrated in Figure 1. By the refrigeration of milk and the activation of the LP system; the level of protein nitrogen in cheeses was significantly affected. Thus, profiles of caseins obtained by electrophoresis showed considerable enzymatic hydrolysis of different caseins fraction (αs1, β and ?), complemented with lower molecular weights deterioration in products apparition.
The electrophoretograms showed that all the samples were very similar at the beginning of ripening where the intact bands of αs1-casein and β-casein were visible. In the present work different soft cheese samples were subjected for proteolysis analysis using Urea-PAGE after 0, 7, 14, 21 Days. During 21days of ripening the hydrolysis of αs1-casein was evidenced by the appearance of the band of the peptide αs1 and of β-casein by γ-casein [4]. Electrophoretic pattern of cheese sample during 21 days storage showed distinct difference among protein. Nacaseinate which run as standard; has four major bands named αs1, αs2, β and ? casein. After examining the concentration of Na- caseinate, it has been concluded that concentration of αs2 and ? casein was less as compare to αs1 and β casein which are almost similar in concentration.
There is also slight difference in electrophoretic mobility in cheese sample at different storage days. αs1 casein has more mobility than other protein fraction. The αs1-CN band was thicker than other. The concentration of β and αs2 casein was almost similar with slight difference. Among these β casein is more concentrated followed by αs1 and ? casein.
Conclusion
The results of current study revealed that preservation of buffalo’s milk by the LPS can be used to improve the microbiological quality of soft cheese. Prior to application of approved thermal processes, the preservation of dairy products may be extended significantly by immediately application of LP system. Combined use of refrigeration and reactivation of the LP system in buffalo milk for manufacturing of soft cheese was observed more effective for enhancing the safety and the storage stability of cheese, by controlling the excessive growth of microbial flora and preventing proteolysis of cheese produced by proteinases of Gram negative psychrotrophs. This study shows that the working activity of lactoperoxidase system becomes double with combination of other treatment like pasteurization and refrigeration. The combination of refrigeration and activation of the Lactoperoxidase system in the preparation of cottage cheese is more effective to reduce the microbial deterioration to improve safety and the storage period of cheese without any physical and biochemical changes in cheese. Lactoperoxidase system prevents the cheese from proteases action that is responsible for proteolysis of protein.
Acknowledgments
Authors are thankful to National Institute of Food Science and Technology, University of Agriculture Faisalabad (UAF), Pakistan for the financial support for this study.
References
- Seifu E, Buys EM, Donkin EF, Petzer LM (2004) Anti- bacterial activity of the lactoperoxydase system against food borne pathogens in Saanen and South African indigenous goat milk. Food Control 15:447-452.
- Mankai M, Hassouna M, Boudabous A (2003) Influence de la dure´e de re´frige´rationsur la microflorepsychrotrophe, la prote´olyse et la composition chimique et mine´rale du lait cru de collectetunisien. Industries AlimentairesetAgricoles 120:12-17.
- Ray B (2004) Fundamental food microbiology. Florida CRC press.
- Upadhyay KG(1992) Pretreatment of milk for cheese manufacture and their significance. Indian Dairyman 44: 26-40.
- Hussein J, Ali A, Behzad N (2011) Application of Lactoperoxidase system in fish and food products: A review. Amercian-Eurasian J Agric Environ Sci 10:89-96.
- Codex Alimentarius Commission (CAC) (1991) Guidelines for the preservation of raw milk by use of the lactoperoxidase system (CAC GL 13/91).
- Ndambi OA, Kamga PB, Imele H, Mendi SD, Fonteh FA (2008) Effects of milk preservation using the lactoperoxidase system on processed yogurt and cheese quality. African Journal of Food Agriculture Nutrition and Development 8:358-374.
- Food and Agriculture Organization (FAO) (1999) Manual on the use of the lactoperoxidase system in milk handling and preservation.
- International Dairy Federation (IDF)(1999) Code of Practices for the Preservation of Raw Milk by the Lactoperoxidase System. Bulletin 234 of the International Dairy Federation 1-15. Brussels: International Dairy Federation.
- Marks NE, Grandison AS, Lewis MJ (2001) Challenge testing of the lactoperoxidase system in pasteurized milk.J ApplMicrobiol 91: 735-741.
- Mouna B, Mankai M, Hassouna M (2011) Effect of thiocyanate and hydrogen peroxide on the keeping quality of ovine, bovine and caprine raw milk. International Journal of Dairy Technology 64: 52-56.
- Fonteh FA, Grandison AS, Lewis MJ (2005) Factors affecting lactoperoxidase activity. International Journal of Dairy Technology 58:233-236.
- SeifuE, Buys EM, Donkin EF (2005) Significance of the lactoperoxidase system in the dairy industry and its potential applications. Food Science and Technology 16:137-154.
- Aneja KR (2002) Experiments in microbiology, plant pathology, tissue culture and mushroom production technology. New Age Publishers, New Delhi.
- AOAC (2016) Official methods of analysis. Association of Analytical Chemist.(20thEdition), Virginia, USA.
- Marshall RT (1992) Standard methods for determination of dairy products. (16thEdition), American public health association. Washington, DC.
- Veloso ACA, Teixeira N, Ferreira IMPVO (2002) Separation and quantification of the major casein fractions by reverse-phase high-performance liquid chromatography and urea–polyacrylamide gel electrophoresis. Journal of Chromatography 967:209-218.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227: 680-685.
- Manka M, Mouna B, Olfa BM, Romdhane K, Mnasser H(2012) The effect of refrigerated storage of raw milk on the physicochemical and microbiological quality of Tunisian semihard Gouda-type cheese during ripening. International Journal of Dairy Technology 65: 25-259.
- Steel RGD, Torrie JH, Dicky DA (1997)Principles and Procedures of Statistics: A Biometrical Apporach. (3rdEdition), Mcgraw Hill Book Co. Inc., New York.
- Tarakci Z (2010) Influence of Kiwi marmalade on the rheology characteristics, color values and sensorial acceptability of fruit yoghurt. KafkasUniv Vet FakDerg 16(2): 173-178.
- Hill C, Ross P (1998). Genetic modification in food industry. Blackie Academic and Professional on imprint of Thomson Science. New York.
- Amarita F, de la Plaza M, de Palencia PF, Requena T, Pelaez C (2006) Cooperation between wild lactococcal strains for cheese aroma formation. Food Chemistry 94: 240-246.
- Licitra G, Campo P, Manenti M, Portelli G, Scuderi S, et al. (2000) Composition of Ragusano cheese during aging.J Dairy Sci 83: 404-411.
- Schor W, Bosset JO (2001) Chemical and physio-chemical changes in processed cheese and ready-made fondue during storage. A review.LWT - Food Science and Technology 35: 15-20.
- Montensions-Herrero C (2003) Properties of imitation cheese containing resistant starch. M. Agr. Sci. thesis.University College, Dublin.
- Farkye N Y (2004) Acid and Acid/Rennet–curd cheeses. Part B: Cottage cheese. In: Cheese Chemistry, Physics and Microbiology. (3rd Edition), Fox, P.F., P.L.H.
- Fandos EG, Sanz S, Olarte C (1999) Microbiological, physiochemical and sensory characteristics of cameros cheese packed under modified atmosphere. Food Microbiology 17:407-414.
- Marit SI, Waret TNPL, Garovet, Singeq AP (2005) Production of probiotic cheese. Int J Dairy 13: 148-156.
- Kebary KMK, Salem OH, Hamed AL, El-Sisa AS (1997) Flavour enhancement of direct acidified kareish cheese using attenuated lactic acid bacteria. Food Residue International30: 256-272.
- Durmos S, Ahmet A Nihat K (2007) The effect of starter culture on chemical composition, microbiological and sensory characteristics of Turkish Kasar cheese during ripening. International Journal of Dairy Technology 60:245-252.
- Fox PF, McSweeney PLH (1997) Rennets: their role in milk coagulation and cheese ripening. In: Law BA (Eds.), Microbiology and Biochemistry of Cheese and Fermented Milk. (2nd edition), Chapman & Hall, London.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 11879
- [From(publication date):
June-2016 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 10881
- PDF downloads : 998