Review Article Open Access
EDTA: Ethylene Diamine Tetra Acetic Acid – A Review
Eleonore Kaethe Blaurock-Busch*
Micro Trace Minerals Clinical Laboratory, Germany
- *Corresponding Author:
- Blaurock-Busch EK
Research and quality control director, Micro Trace Minerals Clinical Laboratory
Röhrenstr 20, 91217, Hersbruck/Germany
Tel: +49-91-51- 816535
E-mail: ebb@microtrace.de
Received date: July 29, 2016; Accepted date: September 07, 2016; Published date: September 14, 2016
Citation: Blaurock-Busch EK (2016) EDTA: Ethylene Diamine Tetra Acetic Acid – A Review. Occup Med Health Aff 4:245. doi: 10.4172/2329-6879.1000245
Copyright: © 2016 Blaurock-Busch EK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Abstract
Chelation therapists around the world incorporate chelation therapies into their daily medical practice, frequently using EDTA (ethylene diamine tetra acetic acid) compounds, unaware of the chemical difference of the various EDTA chelating agents. With this information, we aim to clarify the different mode of action of the EDTAs, including their appropriate medical use. In the USA, medical practitioners promote EDTA chelation, often as an alternative to conventional treatments for a variety of chronic diseases, including vascular problems. German nonmedical professionals use the ‘CaEDTA push’ as promoted by US web pages, although this is against standard protocol. CaEDTA has been FDA- approved for lead intoxication only, and Na2EDTA has not been approved for the treatment of cardiovascular disease. These facts are often overlooked. Misunderstandings increase the risk of iatrogenic accidents. This information aims to prevent this.
Keywords
EDTA; CaEDTA push; Chelation; Lead; Calcium
Na2EDTA, NaMgEDTA and NaCaEDTA Chelation - Understanding the Difference
In the early 1930, the German scientist Munz first synthesized Na2EDTA (disodium ethylene diamine tetra acetate dehydrate or edetate disodium). The chemical was patented and used to soften the hard and calcium-rich waters of Germany, which helped the textile industry to develop more uniform dying processes. In the 1950s, Na2EDTA was FDA (Food and Drug Administration) approved for the treatment of hypercalcemia and digitalis intoxication. At about the same time, NaCaEDTA (disodium calcium EDTA, also called edetate calcium disodium, sold as calcium disodium versanate and herewith referred to as CaEDTA) was used to treat acute cases of lead intoxication in children and adults [1]. Since then, the FDA has issued the following warning:
“The two EDTA drugs have established names that are easily confused and both are referred to in clinical practice as "EDTA". This confusion has resulted in medication errors in which some patients have received the wrong drug, which has been fatal in some cases or caused serious adverse reactions in others. The error is especially dangerous when edetate disodium is erroneously given to a patient who is supposed to receive edetate calcium disodium.
The two EDTA drugs have different approved uses and significantly different effects. For example, edetate disodium is more likely to cause severe decreases in blood calcium levels. A severe decrease in blood calcium levels due to the erroneous administration of edetate disodium has resulted in death, predominantly among pediatric patients, who were to be treated for lead poisoning with edetate calcium disodium. As noted, FDA has special concerns regarding the use of edetate disodium and is reconsidering the overall risks and benefits of the drug” [2].
Since the 1970s, American medical doctors have added magnesium to Na2EDTA, changing it into NaMgEDTA. Members of The American College of Advancement in Medicine (ACAM) actively promoted the use of NaMgEDTA (disodium magnesium EDTA) infusions for the treatment of cardiac disease and started to develop protocols. However, the difference between the EDTA chelation agents was, and still is, not clear to many chelation therapists.
Since 2000, the chelation therapy movement has gained wide acceptance among environmental physicians and natural healthcare practitioners. In Germany, nonmedical practitioners are fond of using CaEDTA, either as a ‘push’ or a fast infusion, all of which is against protocol. Few are aware of the risks involved. The fact that NaMgEDTA should not be administered to children, and that (however few) mortalities happened is overlooked. Pharmacies provide EDTAs without Rx to nonmedical professionals and without proper drug information.
In 2003, the FDA responded to the death of a child when a physician wrongly applied Na2EDTA instead of CaEDTA, a true medical error [3].
CaEDTA has been approved for the treatment of acute lead intoxication, and while the FDA has issued a public health advisory around reported accidents following the administration of ‘EDTA’, it clearly states that “Seven of the 11 deaths resulted from confusion of edetate disodium with another drug. In five cases, edetate disodium was administered instead of edetate calcium disodium. In two cases, edetate disodium was administered instead of the drug, Etomidate. Etomidate is not a form of EDTA” [3].
With this information, we aim to clarify the different mode of action of the EDTAs and their appropriate medical use. In Germany, nonmedical practitioners widely use EDTA chelation, not being aware of the differences of the various substances. In the USA, medical practitioners promote EDTA chelation, often as an alternative to conventional treatments for a variety of chronic diseases and these easily accessed website information are used as teaching material. Some websites promote the administration of the “CaEDTA push”, which is clearly against protocol, increasing the risk of iatrogenic accidents.
Materials and Method
To analytically demonstrate the differences and similarities of the chelating agents Na2EDTA and CaEDTA we statistically evaluated our data base. Over the past 10 years, we methodically supplied physicians with detailed information about chelating agents and proper protocols, asked clinics to submit samples with treatment details, including amounts of chelating agents used, and patient history. We provided urine sample collection protocols that allows proper information regarding metal binding and urinary excretion.
For this review, the urine data utilized is based on samples received during 2014-2015 from mostly German chelation therapists. Protocol instructions, including sampling instructions were provided. The samples included in our study are from chronically exposed adult patients. Acutely intoxicated patients were not included. To avoid external contamination, samples were collected into metal-free tubes, provided by the laboratory. Samples were shipped to the laboratory via regular post or courier.
Sample Testing
For urine sample digestion the following method was used:
• 500 μL Urine were pipetted in a 15 ml tube
• +50 μL of Internal Standard Solution was added (Sc, Y, Ho) à 200 ppb
• +500 μL nitric acid (HNO3) Supra Quality, 69%
• +8.95 ml Millipore-Water was added after approximately 2 min for final dilution
Urine metal analysis was performed using the 7700 Series Inductively Coupled Mass Spectrophotometer (ICP-MS) with Agilent’s Octopole Reaction System (ORS), an improved type of mass spectrometer, which provides sensitive, robust, interference-free analysis of difficult, high-matrix samples. With five times the sensitivity of its predecessor and increased matrix tolerance, the ORS system replaces both GFAA and ICP-OES instruments in addition to older generation ICP-MS systems [4].
Certified urine standards and in-house standards were used for quality control and for the validation processes. To avoid the potentially great margin of error that can result from the patients’ fluid intake, or from incorrectly provided sample volume, results are reported in mcg/g creatinine for all elements, except calcium. For this macro-element, values are reported in mg/g creatinine. Patient age and sex was used to determine urine creatinine levels [5].
Statistics
From our data bank we randomly selected provocation test results that had been obtained after the intravenous application of CaEDTA or NaMgEDTA.
We compared the mean value of the urine metal concentration before and after provocation tests. Baseline urines represent a morning or spot urine that has not been provoked with any chelating agent. Patients were instructed not to take supplements or algae products to avoid the oral intake of metals.
Mean results of the urine provocation test results were compared to mean baseline urine values (Tables 1-3).
| # of tests | Cd | Cu | Fe | Ni | Lead | Mn | Mo | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| 2618 | Baseline urine | 0.29 | 8.95 | 23.56 | 6.04 | 1.31 | 2.94 | 2.94 | 0.44 |
| 93 | CaEDTA 1.9g | 1.03 | 24.59 | 283.92 | 9.52 | 10.25 | 40.13 | 24.26 | 14.16 |
| 131 | NaMgEDTA 3g | 1.06 | 62.74 | 282.26 | 9.81 | 17.85 | 27.93 | 23.29 | 14.04 |
Source: MTM laboratory data base 2015
Table 1: Na2EDTA and NaCaEDTA Comparison of Urine Excretion mean values after chelation.
| # of tests | As | Ba | Hg | Pd | Sb | Se | |
|---|---|---|---|---|---|---|---|
| 2618 | Baseline urine | 26.19 | 5.89 | 0.44 | 0.27 | 0.09 | 22 |
| 93 | CaEDTA 1.9g | 21.43 | 5.02 | 0.87 | 0.27 | 0.1 | 24.2 |
| 131 | NaMgEDTA 3g | 16.02 | 4.23 | 1.17 | 0.2 | 0.19 | 27.32 |
Source: MTM laboratory data base 2015
Table 2: Na2EDTA and NaCaEDTA Comparison of Urine Excretion mean values after chelation.
| # of tests | Sn | Sr | Tl | U | W | |
|---|---|---|---|---|---|---|
| 2618 | Baseline urine | 0.57 | 150 | 0.25 | 0.07 | 0.17 |
| 93 | CaEDTA 1.9g | 0.44 | 147 | 0.22 | 0.05 | 0.13 |
| 131 | NaMgEDTA 3g | 0.59 | 152 | 0.27 | 0.01 | 0.11 |
Table 3: Na2EDTA and NaCaEDTA Comparison of Urine Excretion mean values after chelation.
Similarities of CaEDTA and NaMgEDTA
Natrium Magnesium EDTA (NaMgEDTA) is Na2EDTA with added magnesium sulfate. Both compounds are added to an isotone 500 ml saline solution (0.9%) for infusion purposes. Because magnesium has the ability to reduce vascular spasms, the combination provided positive effects in patients with vascular problems. NaMgEDTA treatments have been used over 50 years in the treatment of cardiac disease. Positive reactions include its ability to bind calcium and due to this calcium binding, Na2EDTA or NaMgEDTA act as an anticoagulant, or rather a platelet anti-aggregation agent. “Platelets are key components of all blood clots propagating within the arterial circulation, hence they are an obvious therapeutic target in attempts to inhibit coronary artery thrombosis,” writes Peter van der Schaar, MD, PhD, retired chairman of the International Board of Clinical Metal Toxicology [6].
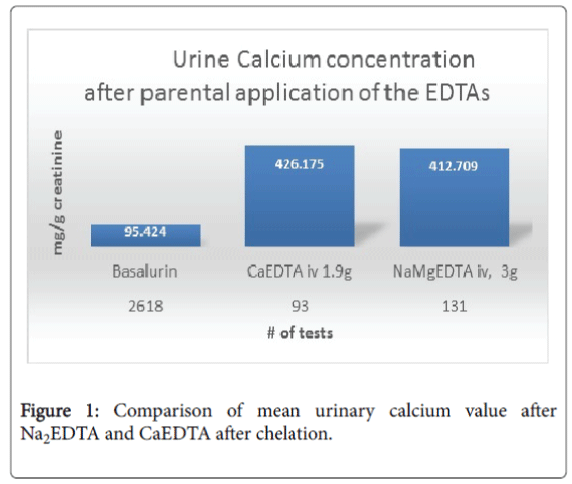
NaMgEDTA is also effective in improving vasodilation, however Ciccone MM et al. suggest that treatment with Beniparin is a safe and effective alternative treatment, especially for deep vein thrombosis [7]. CaEDTA does not noticeably improve vasodilation, and it does not bind calcium. When it comes to binding lead or other potentially toxic metals, CaEDTA, Na2EDTA and NaMgEDTA show similar metal binding abilities. Na2EDTA is rarely administered without the addition of magnesium, hence our data bank not have sufficient data to include Na2EDTA in the comparison as outlined below (Figure 1, Tables 1-3).
Table 1 indicates that CaEDTA and NaMgEDTA are useful for the chelation of cadmium (Cd). Urine cadmium concentration after provocation with NaMgEDTA and CaEDTA show equal results for cadmium.
The binding of nickel (Ni) may be considered marginal for both chelating agents. For the chelation of lead (Pb), CaEDTA is generally preferred, however our data indicates that NaMgEDTA may also be considered for the detoxification of lead-intoxicated adults. (Caution: because of the strong calcium binding, Na2EDTA and NaMgEDTA should NOT be used for the treatment of children!).
The highlighted mean values for the elements copper (Cu), Lead (Pb) and manganese (Mn) may be due to the fact that 3 g of NaMgEDTA was used compared to 1.9 g of CaEDTA. The 5 ml Na2EDTA Ampules generally supply 3 g of Na2EDTA while the 5 ml CaEDTA supplied by pharmacies contain 1.9 g CaEDTA.
It should be of interest to physician involved in environmental medicine that urine provocation with CaEDTA or NaMgEDTA did not result in an increase in metal binding for the elements As (Arsenic), Ba (Barium), Pd (Palladium), Sb (Antimon), Se (Selenium), Sn (Tin), Sr (Strontium), Tl (Thallium), U (Uranium) and W (Tungsten).
However, the mean value for mercury increased, but not to the level of a DMPS (2,3-Dimercapto-1-proponesulfonic acid) provocation test. Our statistical evaluation of 2659 intravenously administered DMPS tests (1 Ampule Dimaval = 250 mg DMPS) showed a mean mercury value of 16.7 mcg/g creatinine, compared to the EDTA mean mercury value of around 1 mcg/g creatinine (Table 2). Again, none of the data involved came from acutely intoxicated patients.
Differences of CaEDTA and Na2EDTA
As pointed out before, CaEDTA is bound to Calcium, while Na2EDTA or NaMgEDTA is not. One 5 ml ampule CaEDTA, containing 1.9 g CaEDTA releases about 203 mg Calcium into the blood stream. The Textbook of Pediatric Medicine warns that “Side effects of NaCaEDTA include local reactions at injection sites, fever, hypercalcemia” [8].
Na2EDTA or NaMgEDTA only bind calcium. Figure 1 indicates that the intravenous administration of 1.9 g CaEDTA, either as a ‘push’ or short infusion) increased the urine calcium concentration above 400 mg/g creatinine. The infusion of 3 g NaMgEDTA in a 500 ml carrier solution released no calcium, but resulted in calcium binding. The urine calcium concentration following the administration of 3 g NaMgEDTA is nearly the same as that from the administration of 1.9 g CaEDTA.
Patients suffering from hyperparathyroidism should not be treated with CaEDTA. Primary hyperparathyroidism occurs in 25 per 100,000 persons in the general population and in 75 per 100,000 hospitalized patients.
Hypercalcemia is a fairly common metabolic emergency. Between 20% and 40% of patients with cancer develop hypercalcemia at some point in their disease (this may be decreasing with the use of bisphosphates, but data are lacking). Because hypercalcemia is associated with malignancies and not uncommonly found in cancer patients, CaEDTA chelation should not be an option for the treatment of cancer [9].
Na2EDTA and Hypocalcemia
Because of its strong calcium binding ability, Na2EDTA was approved to treat digitalis intoxication. In the 1950s, the FDA also approved Na2EDTA for the emergency lowering of hypercalcinosis, a rare disease.
Na2EDTA infusions cause a temporary lowering of serum calcium levels. Patients with parathyroid disorders and particularly children are at risk of developing hypocalcemia during treatment. Consequently, Na2EDTA or NaMgEDTA infusions must be administered slowly, about 1 g/h. Most importantly, the intravenous application of Na2EDTA or NaMgEDTA is not a treatment option for children.
Case Report
Texas: In February 2005, a girl aged 2 years who was tested for blood lead during routine health surveillance had a capillary BLL (blood lead level) of 47 μg/dL. A venous BLL of 48 μg/dL obtained 12 days later confirmed the elevated BLL. A complete blood count and iron study conducted concurrently revealed low serum iron levels and borderline anemia. On February 28, 2005, the girl was admitted to a local medical center for combined oral and IV chelation therapy.
The patient's blood electrolytes at admission were within normal limits. Initial medication orders included IV Na2EDTA and oral Succimer (DMSA (Meso-2-3-Dimercaptosuccinic acid), the agent primarily used for treatment of lead poisoning in children). The medication order was corrected by the pediatric resident to IV CaEDTA.
At 4:00 p.m. on the day of admission, the patient received her first dose of IV CaEDTA (300 mg in 100 ml normal saline at 25 ml/h). At 4:35 p.m., she was administered 200 mg of oral Succimer. Her vital signs remained normal throughout the night. At 4:00 a.m. the next day, a dose of IV Na2EDTA (instead of IV CaEDTA) was administered. An hour later, the patient's serum calcium had decreased to 5.2 mg/dL (normal value for pediatric patients: 8.5-10.5 mg/dL). At 7:05 a.m., the child's mother noticed that the child was limp and not breathing. Bedside procedures did not restore a normal cardiac rhythm, and a cardiac resuscitation code was called at 7:25 a.m. The child had no palpable pulse or audible heartbeat. Repeat laboratory values for serum drawn at 7:55 a.m. indicated that the serum calcium level was <5.0 mg/dL despite repeated doses of calcium chloride. All attempts at resuscitation failed, and the girl was pronounced dead at 8:12 a.m.
The cause of death was recorded as sudden cardiac arrest resulting from hypocalcemia associated with chelation therapy. The hospital's child mortality review board findings indicated that a dose of IV Na2EDTA was unintentionally administered to the child [9].
NaMgEDTA, Diabetes and Vascular Disease
It is estimated that during the past 50 years, over one million patients have received intravenous chelation therapy with NaMgEDTA. Atherosclerotic patients improved, which surprised even those who had administered the infusions. Consequently, the American College of Advanced Medicine introduced thousands of medical doctors to NaMgEDTA chelation treatment, courses were taught, attracting physicians from around the world. NaMgEDTA infusions are now used around the globe, largely for the treatment of cardiovascular disease or vascular problems as seen in diabetics.
The role of NaMgEDTA Chelation therapy in Diabetes and Heart Disease has long been debated. A Google search leads to nearly 500,000 pages of pro and con information. To prove or disprove NaMgEDTA’s use in cardiovascular medicine, the National Institutes of Health has spent $30 million on a major clinical trial. The study was finalized in 2013. The chief investigator Prof. Gervasio A Lamas MD from Mount Sinai Medical Center found a significant 15% decrease in risk of the primary composite end point among diabetes patients. NaMgEDTA treatment caused a 40% reduction in total mortality, a 40% reduction in recurrent MI, and about a 50% reduction in mortality in patients with diabetes [10].
There are several mechanisms known that benefit cardiovascular disease patients. Research published by Yasuyuki Fujiwara of the Department of Environmental Health, Faculty of Pharmaceutical Sciences, Hokuriku University, Japan indicates that lead and cadmium inhibit repair of vascular cells. EDTA (CaEDTA, Na2EDTA, NaMgEDTA) effectively removes lead and cadmium [11].
Summary and Conclusion
Both of the chelating agents, CaEDTA and NaMgEDTA, can be used for the detoxification of Cadmium, Copper, Iron, Nickel, Lead and Zinc. Because of their chemical and pharmacological difference, the agents differently affect the calcium metabolism. Na2EDTA or NaMgEDTA infusions cause a temporary lowering of serum calcium levels, which can be life-threatening if used in children.
From our data we suggest that neither CaEDTA nor NaMgEDTA are useful for the detoxification of As (Arsenic), Ba (Barium), Pd (Palladium), Sb (Antimon), Se (Selenium), Sn (Tin), Sr (Strontium), Tl (Thallium), U (Uranium) and W (Tungsten). The EDTAs Mercury binding ability is not promising.
All the EDTAs should be used with care. One consequence of administering CaEDTA is the release of calcium into the bloodstream, which may lead to hypercalcemia, a condition associated with malignancies.
In Germany, the EDTAs are listed as prescription items (Rx) and thus are officially available to medical physicians only. However, inofficially, pharmacies provide these Rx drugs to nonmedical practitioners who generally have little or no training in parenteral applications of such chemicals and are largely unaware of the pharmacological differences of the EDTA chelating agents discussed here. We recommend that teaching institutions provide more thorough information regarding the use of these chelating agents. We also recommend that pharmacists and internet pharmacies are more closely regulated when supplying the EDTAs.
References
- Marcus AC, Spencer AG (1995) Treatment of chronic lead-poisoning with calcium disodium versenate. Br Med J 2:883-885.
- FDA (2013) Questions and Answers on Edetate Disodium (marketed as Endrate and generic products).
- CDC (2006)Morbidity and Mortality Weekly Report. MMWR Weekly55: 204-207.
- Wilbur S, Soffey E (2004) Performance characteristics of the agilent 7500ce – The ORS advantage for high matrix analysis. Agilent Tech.
- Thomas L (2005)Laboratory and diagnostic: Indication and evaluation of laboratory testing for medical diagnostics, 6thedn. TH Books. pp:533-543.
- Van der Schaar P (2003) The IBCMT protocol for the safe and effective administration of EDTA and other chelating agents. IBCMT 2003: 28
- Ciccone MM, Cortese F, Corbo F, Corrales NE, Al-Momen AK, et al. (2014)Bemiparin, an effective and safe low molecular weight heparin: A review. VasculPharmacol62:32-37.
- Fleisher GR, Ludwig S (2012) Textbook of Pediatric Emergency Medicine. 6th edn. Lippincott Williams and Wilkins, Philadelphia, USA.pp:1202.
- Gallacher SJ, Fraser WD, Farquharson MA, Logue FC, McArdle C, et al. (1993) Coincidental occurrance of primary hyperparathyroidism and cancer-associated hypercalcaemia in a middle-aged man. ClinEndocrinol (Oxf) 38:433-437.
- Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, et al. (2013) Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction; the TACT randomized trial. JAMA 309:1241-1250.
- Yasuyuki Fujiwara (2004) Cell biological study on abnormal proteoglycan synthesis in vascular cells exposed to heavy metals. Glob J Health Sci 50: 197-204.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 14884
- [From(publication date):
October-2016 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 13883
- PDF downloads : 1001