Ecology of Rice Viruses in the South of the Russian Far East
Received: 23-Apr-2024 / Manuscript No. rroa-24-133291 / Editor assigned: 25-Apr-2024 / PreQC No. rroa-24-133291(PQ) / Reviewed: 09-May-2024 / QC No. rroa-24-133291 / Revised: 14-May-2024 / Manuscript No. rroa-24-133291(R) / Published Date: 21-May-2024
Abstract
This review summarizes the key findings from a long-term study of rice virus ecology in the southern Russian Far East, carried out at the Far Eastern Branch of the Russian Academy of Sciences’ Laboratory of Virology of the Federal Scientific Centre of East Asia Terrestrial Biodiversity (formerly the Biology and Soil Science Institute until 2018). The isolated viral strains and pertinent data related to the study were deposited in the Russian Collection of Viruses of East Asia repository. From our investigations, we discovered that four phytoviruses in the area have the ability to infect rice plantings, namely: the Russian oat mosaic virus (Bunyavirales: Phenuiviridae, Tenuivirus), the rice stripe virus (Bunyavirales: Phenuiviridae, Tenuivirus), the rice spotted mosaic virus (the taxonomic status of this virus is still unknown), and probably the northern cereal mosaic virus (Mononegavirales: Rhabdoviridae, Cytorhabdovirus). The study also examined the signs of infection, the seasonality of the disease, native wild plants that serve as natural reservoirs, and the insect fauna that inhabits the rice field, which includes vectors of varying degrees of significance. Though absent from the Far East of Russia, as indicated in scientific literature on the outcomes of phytovirological monitoring in neighbouring territories, an additional 10 viruses (from the families Caulimoviridae, Phenuiviridae, Rhabdoviridae, Secoviridae, and Sedoreoviridae) pose a potential threat to the rice planting system in the region.
Keywords
Phytovirus; Rice; Taxonomy; Ecology; Vector; Planthoppers; Aphids
Introduction
Rice cultivation in the South of the Russian Far East
The Southern part of the Russian Far East is classified as an area of moderate extratropical monsoon climate with flat-topped terrain and accumulative depressions. Very typical here are soils of heavy granulometric composition with a glued layer of low water permeability, which causes regular water-logging; this layer usually contains small amounts of organic matter and phosphorus [1]. These above-enumerated qualities make these acidic clay and loam soils suitable for rice growing due to their lower water penetration. Climatic conditions and the availability of water resources enable rice growing in Primorsky Krai on the southern tip of the Russian Far East, where the best lands for rice cultivation are mainly located in the Khanka plain and the floodplains of the adjacent Ussuri, Ilistaya, Melgunovka, and Spasovka rivers. The warm period with a minimum daily temperature of 14–22°C in this region lasts 120–130days. Significant water reserves and levelled terrain make it possible to organize effective, low-cost irrigation systems of double regulation suitable for both drainage and irrigation. This makes it possible to organize crop rotation in such systems by growing rice with other valuable farming crops such as soybeans, corn, vegetables, and potatoes, which helps to get rid of crop-specific diseases, pests, and weeds. The cultivation of grain crops has a long history in the region, as grain production became one of the key foundations for the formation of the ancient Kingdom of Bohai in the VII century on the territory of Manchuria, contemporary Primorsky Krai, and the northern part of the Korean Peninsula. Since the X century, the Jin dynasty of Jurchen State has been formed here and a land-use system similar to the one used in China has begun to form [2]. Large-scale expansion of the area of grain cultivation has taken place during the Russian government agricultural reform of Peter Stolypin (1906-1913), which was encouraged by a large-scale migration of peasants to the Russian Far East [3]. In Primorsky Krai winter crops were cultivated to a lesser extent due to an unstable snow cover and a pronounced monsoon climate. Therefore, rice cultivation was at the core of local farming. According to statistics, in 1925, Primorsky Krai had 8196ha of planted area of rice while in 1926 it had 10235ha, in 1927 it had 13895ha, in 1928 it had 17640ha, and in 1929 it had 17855ha. The gross rice harvest in 1925–1926 was 23114tonnes while in 1926–1927 it was 30998tonnes, in 1927–1928 it was 38462tonnes, and in 1928–1929 it was 45765tonnes. Rice was also planted on smaller scales in Khabarovsk Krai (Bikin and Vyazemsky districts), in the Jewish autonomous region and in the Amurskaya oblast (Tambovsky, Mikhailovsky, and Arkharinsky districts) [4]. During these years, all rice production was done by privately owned companies [5]. Changes in land-use patterns were inevitably accompanied by an intensification of the interaction of the populations of species, by the introduction of new pathogens into the local flora, and by the adaptation of natural focal viruses to the new crops. During the Soviet era, rice farming in the Russian Far East was put on a scientific basis, which allowed not only to meet the needs of the region but also to get integrated into the grain markets of the countries of the Asia Pacific. The rice planting areas had been continuously growing until the collapse of the USSR in 1991. Arbitrary decision-making in addressing agricultural problems, price dumping by the main rice producers, and the lack of funds to invest in the economy of rice-growing farms led to their bankruptcies and closures in the 1990s. And only in recent years, has there being growing evidence of rice production revival in Primorsky Krai. More advanced development of the Russian Far East implies an increase in the local yield of grain crops, especially when it comes to rice as the most suitable crop from climatic and agricultural engineering perspectives. One of the ways to achieve high yields in rice cultivation is to prevent and fight the emergence of the viral infections [6]. Suitable climatic conditions and the availability of soils led to the fact that in the recent past (in 1981–1985) the total area of rice fields was about 330thousand hectares, while the gross rice harvest exceeded 1million tonnes [4]. The modern cultivation area under rice reached 7706hectares in Primorsky Krai in 2022 and exceeded 8000hectares in 2024; the total yield amounted to 23,1thousand tonnes with an average yield of 3,0t/ha. Primorsky Krai is the northernmost region of rice cultivation in Russia (45°28'N, 130°40'E) with an effective accumulated temperature of 2300–2500°С (>10°С) and favorable conditions for the development of plant diseases and viruses [7]. An important direction of rice breeding is the creation of cold-resistant, early maturing, and high-yielding (5–6t/ha) varieties. Such varieties should be characterized by high grain quality, resistance to fungal diseases, viruses, and lodging, and high adaptability to the unfavorable conditions of the monsoon climate in Primorsky Krai. Breeders and genetic scientists pay considerable attention to these aspects [8]. Among the fungal diseases affecting rice and masking the development of viral infections, pyriculariasis provoked by Pyriculariaoryzae is the most harmful. Crop losses range from 5% to 25% in normal years and up to 60% and even up to 100% in years of epiphytotic development of the disease. The most effective type of control is the selection of rice varieties that are resistant to both fungi and viruses. Molecular and genetic methods have been used in rice breeding, particularly double haploids with several genes of resistance to the parasitic fungus P.oryzae (Pi-1, Pi-2, Pi-ta, Pi-ta2, Pi-z, and Pi-b) [9]. In recent years, the Federal Scientific Center of Agricultural Biotechnology of the Far East named after A.K.Chaika has created highly productive rice varieties that have been included in the state register of breeding achievements admitted to use in the Far Eastern region of the Russian Federation: Khankaisky52, Lugovoy, Dolinnyi, Kaskad, Almaz, and Man’chzhur (Table1). The characteristics of morphological and economically valuable characteristics of rice varieties are given according to the data of the state Register of breeding achievements of the Russian Federation approved for use in the Far Eastern region. The ultra-early maturing rice variety Kaskad was created in 2014 and has a growing period of 86–91days, short stems (68–72cm), and resistance to lodging, grain shedding, and rice blast [10]. New cold-resistant rice varieties, Almaz and Man’chzhur, generate significant interest among agricultural producers. They are characterized by a yield of 5–6t/ha, high cereal quality, a grain hardness of 95–99%, a cereal yield of 72–76%, and a high protein content in the grain (8,3%). Currently, research is being conducted towards the creation of new-generation varieties combining high yields with resistance to fungal and viral diseases using biotechnology methods.
| Characteristic | Rice variety | |||||
|---|---|---|---|---|---|---|
| Khankaisky 52 | Lugovoy | Kaskad | Dolinnyi | Almaz | Man’chzhur | |
| Subspecies. Var. | indica kato. mutica | japonica. italica Alef | japonica. italica Alef | japonica. italica Alef | japonica. italica Alef | japonica. nigro-apiculata |
| Growing period. days | 108-112 | 98-102 | 86-91 | 95-100 | 120-125 | 120-130 |
| Plant height. cm | 60-80 | 60-77 | 68-72 | 73-75 | 69-75 | 72-76 |
| Leaf color | green | green | light green | green | green | dark green |
| Leaf indumentum | sparse | sparse | sparse | sparse | sparse | sparse |
| TKW. g | 28-30 | 32-36 | 30-31 | 28-30 | 25-27 | 28-29 |
| Grain hardness. % | 95-97 | 95-96 | 89-91 | 95-97 | 95-98 | 97-99 |
| Cereal yield. % | 70-74 | 70-72 | 72-74 | 72-74 | 72-76 | 72-76 |
| Hull content. % | 17-18 | 16-17 | 16-18 | 16-17 | 16-17 | 16-17 |
| Protein content in grain. % | 10.0 | 8.2 | 8.6 | 8.2 | 8.3 | 8.3 |
| Average yield. t/ha | 4.5-5.0 | 5.5-6.0 | 5.0-6.0 | 4.5-5.0 | 5.0-6.0 | 5.5-6.0 |
| Year of inclusion in the State Register for the Far Eastern region of the Russian Federation | 2000 | 2010 | 2014 | 2014 | 2020 | 2022 |
Table 1: Morphological and biological characteristics of the modern (in XXI century) main rice varieties released in the South of the Russian Far East
Rice viruses revealed and potentially dangerous in the south of the Russian FarEast
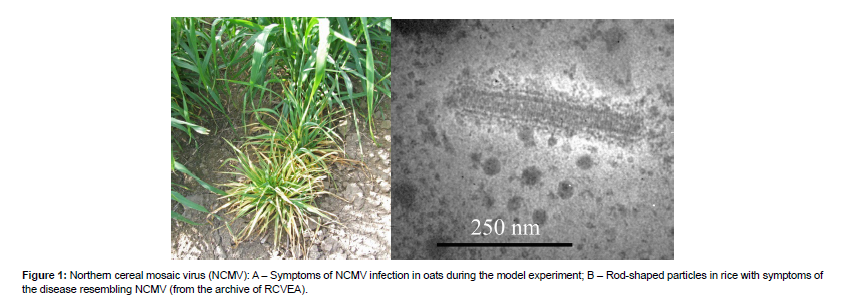
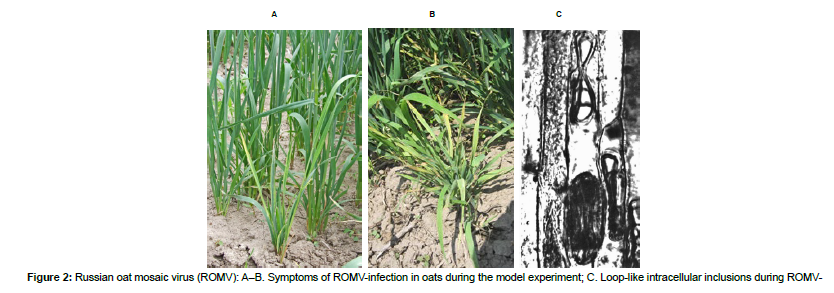
The data presented in this review on the revealed rice viruses in the South of the Russian Far East (Table 2) uses the results of a longitudinal monitoring study of cereal crop viruses conducted since 1962 by the Laboratory of Virology of the Federal Scientific Center of East Asia Terrestrial Biodiversity (known as the Biology and Soil Science Institute before 2018) of the Far Eastern Branch of the Russian Academy of Sciences. Isolated strains are deposited in the Russian Collection of Viruses of East Asia(RCVEA) [11]. Longitudinal studies have shown that rice planting offers favorable conditions for the spreading of infections due to the sparseness of the landscape, the high occupation density of the insects on the plants, and the presence of weeds, which act as the sources of the diseases. Northern Cereal Mosaic Virus (NCMV) (Mononegavirales: Rhabdoviridae, Cytorhabdovirus), which leads to yellowing of the leaves of cereals and dwarfism of plants possibly including rice (Figure1), reproduces directly inside the vector’s body, turning an infected individual into a permanent source of infection, that remains as such until its death and which, given its high mobility, can infect large numbers of plants. This pathogen spends the winter inside the infected larvae [12]. The epicenter of NCMV strain diversity is located in Amurskaya oblast. This virus is much less common in Primorsky Krai and Khabarovsk Krai, where spreading of NCMV is limited due to the presence of a number of natural barriers: the farm fields are surrounded by forests, the total area of the fields is much smaller as compared to the fields in Amurskaya oblast, and planthoppers who act as vectors reproduce much less intensively. While in Amurskaya oblast fields in August an average catch on annual cereals and oat crops exceeds 1000–1500planthoppers per 100strokes of the net, in Primorsky Krai and Khabarovsk Krai territories this catch remains at the level of only 30–40planthoppers [13]. The weak distribution of NCMV in Primorsky Krai seems to be due to the less favorable conditions of the pronounced monsoon climate. In addition, in most of the parts of Primorsky Krai and Khabarovsk Krai winter forms of grain are not planted. This undermines the feeding base of aphids in Primorsky Krai in the early spring and late autumn periods [14] (Table 2). Rice viruses revealed and potentially dangerous in the South of Russian FarEast. When it comes to the Russian Oat Mosaic Virus (ROMV) (Bunyavirales: Phenuiviridae, Tenuivirus), one should understand the history of its study and its common synonyms. This virus was described by L.Pronicheva under the name "dwarfism of oats" in 1929 in the Amur region [16] tuned to be the very first viral disease of plants discovered in the Russian Far East (Figures2.A–B) [16]. In 1940, this virus was redescribed as "cereal pupation virus" [17]. Later, Dr.V.Fedotina discovered bacillus-like virus particles and phytoplasms (Acholeplasmatales: Acholeplasmataceae) in affected plants, which allowed her to hypothesize a mixed etiology of the disease [18]. Subsequent studies aside from not adding any more clarity about the taxonomic attribution of the pathogen (some authors attributed it to tenuiviruses while others attributed it to cytorhabdoviruses), have created even more confusion due to the emergence of synonymous names such as "oat pupation virus," "barley pupation virus," "wheat pupation virus," etc. Currently, this virus, which affects a wide range of cereals and causes their dwarf bushiness, is proposed to be called ROMV [19]. However, a mixed infection of ROMV with phytoplasms whose infections are characterized by bushiness is also possible. In Primorsky Krai ROMV is most often accompanied by NCMV and is also transmitted by small brown planthoppers (Laodelphaxstriatellus) [14].
| Taxonomy | Status in Primorsky Krai | |||
|---|---|---|---|---|
| Order | family | genus | Virus | |
| Bunyavirales | Phenuiviridae | Tenuivirus | Rice Grassy Stunt Virus (RGSV) | Potentially dangerous |
| Russian Oat Mosaic Virus (ROMV) | Revealed | |||
| Rice Stripe Virus (RSV) | Revealed | |||
| Mononegavirales | Rhabdoviridae | Cytorhabdovirus | Northern Cereal Mosaic Virus (NCMV) (highlylikely) | Cytorhabdovirus particles revealed |
| Rice Yellow Stunt Virus (RYSV) | Potentially dangerous | |||
| Ortervirales | Caulimoviridae | Tungrovirus | Rice Tungro Bacilliform Virus (RTBV) | Potentially dangerous |
| Picornavirales | Secoviridae | Waikavirus | Rice Tungro Spherical Virus (RTSV) | Potentially dangerous |
| Reovirales | Sedoreoviridae | Fijivirus | Rice Black Streaked Dwarf Virus (RBSDV) | Potentially dangerous |
| Southern rice black streaked dwarf virus (SRBSDV) | Potentially dangerous | |||
| Oryzavirus | Rice Ragged Stunt Virus (RRSV) | Potentially dangerous | ||
| Phytoreovirus | Rice Dwarf Virus (RDV) | Potentially dangerous | ||
| Rice Gall Dwarf Virus (RGDV) | Potentially dangerous | |||
| Rice Bunchy Stunt Virus (RBSV) | Potentially dangerous | |||
| Unclassified | Unclassified | Unclassified | Rice Spotted Mosaic Virus (RSMV) | Revealed |
Table 2: Rice viruses revealed and potentially dangerous in the South of Russian Far East
A distinctive feature of tenuiviruses is the synthesis of a large amount of the so-called "soluble antigen," which is a low-molecular protein that forms loop-like intracellular inclusions (Figure2.C). Therefore, Sukhov’s isolation of the "soluble antigen" from the plants "sick with pupation" indicates that the pathogen belongs to tenuiviruses, while the detection of particles of a different morphology indicates a coincident infection. Since a set of symptoms of cereal diseases does not allow for unambiguous identification of the pathogen (especially in the case of mixed infections, which are often found in cereals), the isolates may differ significantly [19].
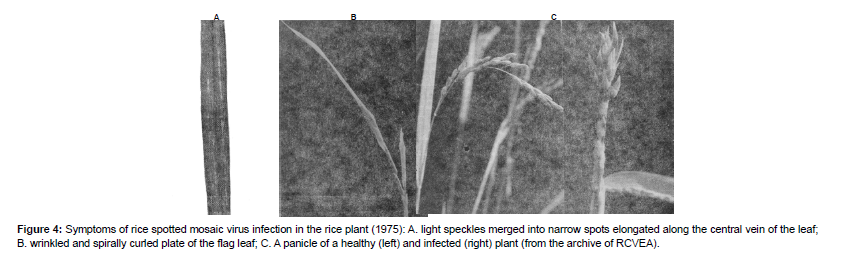
In late June or early July, rice plants show the first noticeable symptoms of the infection in the form of light specks that lengthen and turn into strokes located along the middle vein of the leaves; individual strokes stick together into solid grayish-white stripes of various widths along the entire length; the stripes are often moved to the edges of the leaf blades (Figure3). Research into the characteristics of the pathogen that causes these symptoms, led us to identify it as a tenuivirus, more specifically as a Rice Stripe Virus (RSV) (Bunyavirales: Phenuiviridae, Tenuivirus) [20,21] Raga etal., 1988).
Plants with external symptoms of RSV infection in 100% of cases gave a positive reaction with a specific antiserum to the RSV Japan strain, courtesy of Dr.R.Kishimoto. Later, Dr.R.Gnutova and Dr.N.Kakareka developed a wide antiserum panel towards RSV with a high affinity level for all variants of this virus described in Primorsky Krai (Gnutova, Pinsker, 1976). Sera had a titer up to 1:4096 being highly specific and sensitive. The nature of reactions and results of serological analyses that were attained by the Japanese counterparts and by us were similar. Using this antiserum panel, RCVEA identified the RSV on the rice varieties cultivated in PrimorskyKrai and revealed the spread of this disease in rice-growing farms here [22] [12] [16].
Inclusions in the form of loops, rings, and infinity signs were found in the epidermis cells of leaf sheaths of the RSV-affected rice plants, as well as needle-shaped crystals in the aging tissues of the affected plants and the breakdown of loop-like inclusions into needle-shaped crystals in the preparation first described by [23]. At the same time, in the preparations of the juice of affected plants with the application of a method of electron microscopy we were able to detect filamentary particles while the ultrathin sections of the tissues of the leaves revealed spherical particles with a size of 24–29nm, which indicates a co-infection. When comparing the degree of damage to test samples of rice plants on soils with different nitrogen content, it was noted that increased doses of nitrogen fertilizers introduced into the soil contribute to an increase in the manifestation of viral infection [22].
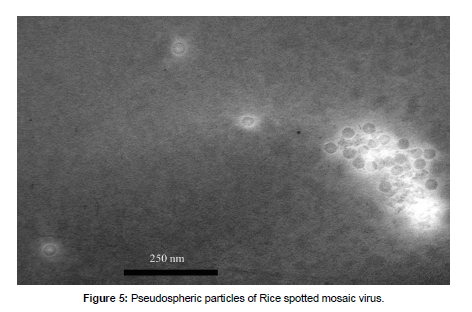
A new rice virus named Rice Spotted Mosaic Virus (RSMV), whose taxonomic affiliation has not been established, was discovered in rice plantings of the Primorsky Krai. The infectious disease of rice associated with this virus was first described in 1974 in the fields of the agricultural enterprise "50 years of the Komsomol of Primorye" (Primorsky Krai, Khorolsky district). In 1975, RSMV infection was detected in other rice farms in Khorolsky and Khankaisky districts of Primorye. Symptoms appear in individual plants or their small groups: light speckles appear on juvenal leaves (from light green to almost white), which, as the disease develops, merge into narrow spots elongated along the central vein of the leaf (Figure4.A); the spots increase in size, turn yellow, and their edges lose their sharp outline and become diffuse (as if "hiding in a yellowish haze"); gradually, the yellowness spreads to the leaf tissue around the spots, and the leaf blade is deformed; the leaf folds and droops; the plate of the flag leaf becomes wrinkled and spirally curls at the base (Figure4.B); the panicles remain underdeveloped and have an irregular, ugly shape (Figure4.C); and grains do not form in the caryopses. Electron microscopy reveals shell-free pseudospheric particles 30–50nm in diameter in the tissues of diseased plants (Figure5) similar to virions of representatives of the genus Sobemovirus [24] [12]. The data from the scientific literature allow us to identify 10more viruses (Table2) that pose a potential danger to rice planting in the south of the Russian Far East. The vectors of these pathogens are also Hemiptera insects, many of which either live in this territory or can be transmitted by the prevailing winds [25-26] [6]. Due to the intensification of rice cultivation and even more ambitious plans for the development of the farming industry in the region, special attention should be paid to the monitoring of the state of not only agrocenoses, but also natural phytocenoses in order to prevent the spread of viral diseases. The most important venue of the previously held research, which requires continuation, is the work on the selection of resistant varieties of rice with the application of state-of-the-art molecular and genetic methods.
Figure 4: Symptoms of rice spotted mosaic virus infection in the rice plant (1975): A. light speckles merged into narrow spots elongated along the central vein of the leaf; B. wrinkled and spirally curled plate of the flag leaf; C. A panicle of a healthy (left) and infected (right) plant (from the archive of RCVEA).
Reservoirs and vectors of rice viruses among wild species populations in the south of the Russian Far East
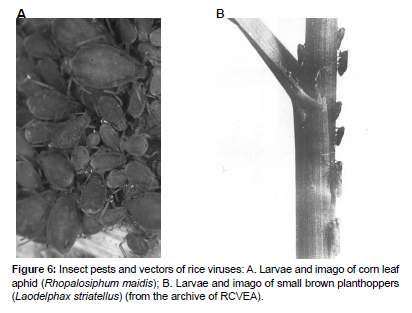
The formation of the entomofauna of rice fields happens with the participation of the insects of the meadow and marsh habitats. Many of them can be vectors of viral infections. Aphids (Hemiptera: Aphidoidea) have always been pests of cultivated cereals and effective vectors of phytoviruses that have had an impact on productivity since the very beginning of grain cultivation. The rich species diversity of cereals and various environmental conditions affecting their growth have created prerequisites for the formation of an equally diverse aphidofauna of cereal plants. These insects have switched to cultivated cereals from their wild kindred species, which act as natural reservoirs of many phytoviruses while preserving trophic connections with them [28,29]. However, there are few "cereal" viruses transmitted by aphids [12]. RCVEA has conducted investigations of the rice field aphidofauna and identification of rice virus vectors in Primorsky Krai since 1969. Key points were situated in the Far Eastern Experimental Rice Station (Spassky district), Sivakovka Rice Farms (Khorolsky district), and on the wild grasses near the rice fields, where 44 species of aphids were identified. Of these, 35species (80%) are random visitors to both the rice and the weeds growing in the rice fields. The direct inhabitants and pests of crops in the conditions of Primorsky Krai are 10 species of aphids: dogwood aphid (Anoeciacorni), rose grain aphid (Metopolophiumdirhodum), apple grain aphid (Rhopalosiphuminsertum), corn leaf aphid (Rh.maidis) (Figure6.A), water lily aphid (Rh.nymphaeae), bird cherry-oat aphid (Rh.padi), wheat aphid (Schizaphisgraminum), grain aphid (Sitobionavenae), elm leaf aphid (Tetraneuraulmi), and mealy plum aphid (Hyalopteruspruni). The following species of aphids dominate rice: Rh.maidis, Rh.padi, S.avenae, and Sch.graminum. When it comes to H.pruni, this species is not the most widespread one on rice, but, apparently, it can colonize it. Polyphage aphids appear on the rice plants incidentally; most of them develop on other feed plants, such as peach aphid (Myzuspersicae), cabbage aphid (Brevicorynebrassicae), melon aphid (Aphisgossypii), cowpea, or groundnut aphid (A.craccivora) [28]. Ecological and virological studies show that plants near forest protection strips of the rice field are most affected by the RSV virus. This indicates that forest protection strips act not only as windproof plantings and regulators of the hydrological regime but also as reservoirs and x amplifiers of rice fields. In the experiment, RSV was successfully transmitted by small brown planthoppers, and transovarial transmission of the virus was shown [21]. Wild plants that are also food for the small brown planthoppers growing in the wintering areas of the vector, along with the weeds of the rice fields (Figure6.B), were noted to be reservoirs of the RSV in the conditions of Primorsky Krai. A small brown planthopper has five nymphal stages. L.striatellus can leave for the winter in all nymphal stages; however, in the conditions of the Primorsky Krai, only nymphs of the 4th and 5th ages successfully overwinter, which are found on rice seedlings already at the end of April and have been found massively since the beginning of May. In frosty winters with weak snow cover, nymphs of the 4th age die almost in full. In July, females lay eggs in rice plants: into the inner tissues of the stems at the very edge of the water, less often into the thick central veins of large leaves (3–4clutches per plant, 2–10eggs in one clutch); clutch sites can be detected by characteristic necrotic strokes. After the eggs mature for 10–15days, around the beginning of August, the larvae hatch, and by mid-August, their abundance in the rice field can reach 500–800individuals per 100strokes of the net. In late August and early September, imago of a new generation (imago of the current year) appears which lays eggs not only on rice, but also on the overgrown surrounding vegetation. Larvae of this generation of various ages (the development period of which is significantly extended compared to the summer generation due to a decrease in temperature) can be found on wild vegetation and a warmed kidney until the end of November [28]. Planthoppers Unkanodessapporonus and brown rice planthoppers (Nilaparvatalugens), which are not widely distributed in the South of the Russian Far East, are also regularly established as RSV vectors. The rice leaf beetle (Oulemaoryzae), which has long been mistakenly associated with the cereal leaf beetle (Oulemamelanopus), is also among the RSV vectors in the southern part of the Russian Far East. In this case, the virus is transmitted in a non-persistent manner [21] [13] 1998). The research demonstrates that dominating aphids on the rice surface – Rh.maidis, Rh.padi, S.avenae, and Sch.graminum – have viroforic properties in relation to RSMV, which aggravates the harm they cause. The rice leaf beetle was also described as an effective vector for RSMV [24]. NCMV is effectively spread by the small brown planthopper, not by contact, but by transmission: the virus is capable of reproduction in the body of the vector, and the infected insect becomes a constant (up to death) source of infection. Each infected planthopper can infect a large number of plants. In winter, NCMV persists in infected larvae [30]. Moreover, it was established that a significant part of wild perennial grasses is resistant to NCMV and able to act as a natural reservoir of this virus: black bent (Agrostisgigantea), creeping bent (A.stolonifera), meadow foxtail (Alopecuruspratensis), American sloughgrass (Beckmaniasyzigachne), bushgrass (Calamagrostisepigeios), and Langsdorf’s smallweed (C.langsdorfii) [31] [12]. According to the results of serodiagnostics, the following wild plant species are effective reservoirs of ROMV: foxtail grasses (Alopecurussp.); cyperuses (Cyperussp.); American sloughgrass (Beckmanniasyzigachne); chicken millet (Echinochloacaudata); barnyard grass (Echinochloacrus-galli); Asian foxglove (Digitariaasiatica); tricolor glyceria (Glyceriatriflora); timothy grass (Phleumpratense); water grass (Phragmitescommunis); Manchurian wild rice (Zizanialatifolia). Most of the tested wild plants were symptomless during infection. External symptoms of the lesion on wild cereals have weak manifestations, mostly in the form of barely noticeable chlorotic stripes and mosaics. However, Barnyard grass has demonstrated the folding of the upper leaf into a tube along with the delay in the sweeping and bending of the panicle [31,32]. ROMV, like other tenuiviruses, is transmitted by Laodelfaxstriatellus planthoppers, but for this virus, it is a key vector. ROMV, unlike NCMV, can overwinter in the eggs of planthoppers (i.e., it can be transmitted by the transovarial pathway). In addition, among the hosts of ROMV, as noted above, there are perennial cereals on which cicadas lay eggs. In this way, persistent natural foci of ROMV infection are formed [28].
References
- Kostenkov NM, Oznobikhin VI (2006) Soils and soil resources in the Southern Far East and their assessment. Eurasian Soil Science 39: 461-469.
- Dillon M (2016) Encyclopedia of Chinese History. London-NY: Taylor & Francis 334.
- Shelokhaev VV (2016) The Stolypin variant of Russian Modernization. Russian Social Science Review 57: 350-377.
- Chaika A K (2014) Achievements of agricultural science in the Far East – strategy for development of agro-industrial complex in the region. Far Eastern Agrarian Herald 5-15.
- Sukhomirov G I (2018) Rice growing in the Russian Far East: development, problems, prospects. Regionalistika 5: 45-57.
- Shchelkanov M Yu, Kakareka N N, Volkov Yu G (2022) The formation of phytovirology in the Far East in the context of the development of the virology in Russia. FEFU 142.
- Pestereva NM (2014) Whether anomalies and the formation of rice yield in the South of the Russian Far East. AEB 8: 88-93.
- Chaika A K, Klykov A G (2016) Priority directions in the development of agro-industrial complex in the Far East of Russia. Vestnik of Far Eastern Branch of Russian Academy of Sciences 24-30.
- Ilyushko MV, Guchenko SS, Lelyavskaya VN (2022) Resistance of Far Eastern rice Oryza sativa L. varieties and competitive testing samples to Pyricularia oryzae Cav. Russian Agricultural Sciences 48: 8-12.
- Sunitskaya TV, Guchenko SS, Burundukova OL (2019) Production and morphofunctional characteristics of Primorsky intensive rice varieties of new generation. Bulletin of KrasGAU 10: 3-9.
- Shchelkanov M, Volkov G, Kakareka NN (2017) Organization of Russian State Collection of Viruses from Eastern Asia on the base of Far Eastern Branch of Russian Academy of Sciences. FEFU 466-470.
- Kakareka N N, Volkov Yu G, Sapotsky M V (2020) Viruses of cereal crops and their vectors in the South of the Russian Far East. Agricultural Biology 55: 439-450.
- Mamaev PY (1988) On the biology of the little brown leafhopper Laodelfax striatellus Fall. The role of insects in biocenoses of the Far East. USSR 63-67.
- Dyakonov K P, Sapotsky M V (2004) About phytovirological state of cereal crops at Primorsky province. Russian Agricultural Sciences 30: 92-98.
- Pronicheva L L (1929) Dwarfism of oats in the Amur district (preliminary message). Protection of plants from pests 6: 141-148.
- Shchelkanov M Yu, Kakareka N N, Volkov Yu G (2022) The formation of phytovirology in the Far East in the context of the development of the virology in Russia. FEFU 142.
- Sukhov KS, Sukhova MN (1940) The relationship between the pupation virus and the vector – Delphax striatella Fallen. USSR Acad Sci 26: 485-488.
- Fedotina V L (1974) Pupation of cereals is a mixed infection. USSR Aca of Sci218: 1211-1213.
- Sapotsky M V, Kakareka N N (2004) Oat Russian mosaic. In: Viruses and virus diseases of Poaceae. Paris. 498-500.
- Kuribayashi K (1931) Studies on the stripe disease. Bulletin of the Nagano Agricultural Experiment Station 2: 45-46.
- Pinsker NI, Reifman VG (1975) Rice streak virus and its vector cicada Laodelphax striatellus Fallen. Virological studies in the Far East. Vladivostok 161-164.
- Pleshakova T I, Kakareka N N, Sapotsky M V (2018) Variety of viruses affecting cereals in the Far East. In: Abstract Book of the 1-st International Conference “North-East Asia Biodiversity”. Vladivostok. 57-58.
- Hirai T, Suzuki N, Kimura I (1964) Large inclusion bodies associated with virus diseases of rice. Phytopathology 54: 367-368.
- Nemilostiva N I, Reifman VG (1977) New virus-like disease of rice. Proc Biol Sic 48: 108-110.
- Otuka A (2013) Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Frontiers in Microbiology 4: 309.
- Wei J, Jia D, Mao Q (2018) Complex interactions between insect-borne rice viruses and their vectors. CURR OPIN VIROL 33:18-23.
- Wang P, Liu J, Lyu Y (2022) A Review of vector-borne rice viruses. Viruses 14: 2258
- Lebedeva EG, Dyakonov KP, Nemilostiva NI (1982) Insects are vectors of plant viruses in the Far East. Vladivostok. 195.
- Dyakonov KP, Volkov YG, Kakareka NN (2005) Romanova SA. Relationships in the "Virus - vector - agrobiocenosis" system. Proceedings of Timiryazev Agricultural Academy 107-115.
- Volkov Y G, Kakareka N N, Kozlovskaya Z N (2011) Evaluation of plant virus infection of cereal crops and prediction of spread of disease in Primorsky Krai. Russ Agric Sci 37:392-394.
- Kostin VD (2005) Viroses of wild plants of the Russian Far East. Vladivostok: Dalnauka 121.
- Kostin VD, Volkov Yu G (1989) Etiology of some mosaic diseases of wild plants of the Far East. Plant protection in the Far East. Vladivostok 3-6.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Shchelkanov MY (2024) Ecology of Rice Viruses in the South of the Russian Far East. J Rice Res 12: 409.
Copyright: © 2024 Shchelkanov MY. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1701
- [From(publication date): 0-2024 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 1380
- PDF downloads: 321