Research Article Open Access
Ecological Consequences of Oysters Culture
Ahmed OO1 and Solomon OO2*1Aquaculture and Fisheries Management, University of Ibadan, Nigeria
2International Studies in Aquatic Tropical Ecology, University of Bremen, Germany
- *Corresponding Author:
- Solomon OO
MSc International Studies in Aquatic Tropical Ecology, University of Bremen, Germany
Tel: +49 421 218-1
E-mail: solomonunilag@yahoo.com
Received date: April 12, 2016; Accepted date: August 10, 2016; Published date: August 15, 2016
Citation: Ahmed OO, Solomon OO (2016) Ecological Consequences of Oysters Culture. J Fisheries Livest Prod 4:198. doi: 10.4172/2332-2608.1000198
Copyright: © 2016 Ahmed OO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The oyster cultivation is a profitable and operationally diverse industry that has prevailed in many countries of the world. Different culture techniques have been employed from farm to farm, with growers using either, the rack and rail method, longlines, or a combination of two or more methods to grow oysters. They are grown in either intertidal or subtidal marine zones, with post-harvest activities taking place predominantly at land-based facilities. There are a number of environmental issues that are relevant to the cultivation of oysters in the coastal waters. These are typically associated with the operation of the farm or the land-based service facility. If the farm is designed or managed inappropriately, there is potential for ecological harm. Therefore, this paper reviewed some of the ecological damage associated with oyster cultivation and proffers solutions to the problems.
Keywords
Oyster; Cultivation; Techniques; Ecological damage; Solutions
Introduction
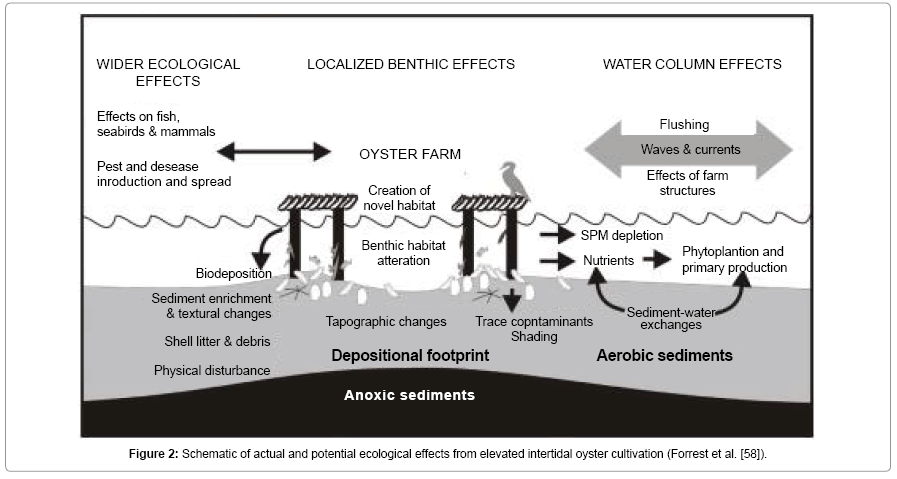
Oyster cultivation is one of the most significant bivalve aquaculture industries world-wide (FAO, 2006). While the global industry is based on a range of species, Crassostrea gigas, Ostrea edulis, Crassostrea virginica, for instance, are by far the most dominant, having been spread either deliberately or inadvertently (e.g. via shipping) to many countries [1]. Over the last 30 years, aquaculture in marine waters such as oysters farming has greatly increased partly driven by the need for greater self-sufficiency in marine food production [2]. One of the great barriers to the development of shellfish or mollusc aquaculture is the perception that industry expansion could have negative environmental effects on our coastal waters. Although, there have been very few studies on the effects of oyster cultures on intertidal habitats [3-7]. However, it is now stated that aquaculture activities cause environmental disturbances [6,8,9]. Oysters farming usually results in a net removal of nutrients from the water column and may also compete with other organisms for survival (e.g seagrass) and this is generally considered to cause environmental damage. In the Pertuis Charentais (SW France), oysters have traditionally been cultivated directly on the sediment, hereafter called on-bottom culture, but currently the most common technique is on tray culture (Figure 1), hereafter called off-bottom culture. This involves placing the oysters in plastic mesh bags tied to metal trestles. The presence of trestles arranged in parallel rows in the intertidal area [10] significantly reduces the strength of tidal currents [4]. This limits the dispersal of pseudo-faeces and faeces in the water column and thus increases the natural sedimentation process by several orders of magnitude [11]. The adverse effects of aquaculture-derived organic matter loads on subtidal benthic assemblages are known [12], so in view of the features of onbottom and off-bottom culture methods, it is plausible that off-bottom cultures cause more disturbance than on-bottom cultures to intertidal benthic environments. Other potential negative impacts associated with oyster farming include; physical impacts associated with farming structures and farm operations, reductions in native stocks caused by the collection of result wild seed and impacts associated with the introduction of exotic or invasive species, etc as seen in Figure 2. As stated earlier, there is little information on the impacts cultured oysters on the environment except for a few studies on mussels and the northern quahog [1,4,13-17].
The purpose of this article is to review some of the negative impacts of oyster farming on the coastal habitats and provide remediation strategies that could be employed to curb the problems.
Impacts of Oysters Farming
Competition for phytoplankton through filter-feeding
One of the greatest potential impacts of filter feeder cultivation such as oysters is the net loss of energy, in the form of phytoplankton, from the ecosystem. Many research conducted have shown that bivalve species such as oysters can filter, on average, 15-55 litres/day (4-14.5 gallons/day) of seawater [18]. Filtration has been shown to control phytoplankton growth by removing them from the water [19,20] this process is referred to as “top-down” population control. They also consume detritus and can thus have an impact on their abundance and composition in the water. Competition for phytoplankton and detritus can affect wild species as the cultured species, being the most predominant, manage to filter out the most of the phytoplankton and so the wild species which also depend on the same resources may suffer. Haure and Baud [21] showed that in the bay of Bourgneuf as the stock of oysters (C. gigas) increased from 37,821 to 46,343 tonnes from 1986-88. The wild population of mussels dropped from 40,068 to 6,700 tonnes in the same period. They postulated that this reduction is due to trophic competition with oysters as they feed on approximately the same phytoplankton.
Bio-deposition and changes in seabed topography and sedimentation
Bio-deposition is the term given to the accumulation of faeces and pseudo-faeces under the oysters’ beds [22]. These biodeposits are rich in nitrogen and phosphorus and may represent a significant proportion of the energy potentially available to consumer invertebrates as a food resource. Therefore, Oysters may stimulate primary productivity through bio-deposition. They do this by exerting control over the amount of available mineral nitrogen and phosphorus to phytoplankton by sequestering them as protein in their meat and shell tissues [23]. Not only that, they deposit organic nitrogen-rich bio-deposits to the bottom sediments that bacteria decompose, thus forming ammonium; ammonium is converted by nitrifying bacteria in oxygen-rich sediments to nitrate, which denitrifying bacteria in deeper sediment layers then convert to nitrogen gas [14,24]. Due to bio-deposition by oysters, organic enrichment has been recorded at some sites. According to Nugues et al. [4] noted an increase in organic and silt composition sediment beneath the oysters’ trestles. Martin et al. [25] have shown that trestle cultivation of oysters is responsible for increased sedimentation (Figure 3) of both organic matter and contaminants. According to Sornin et al. [26] went as far as to say that the accumulation of bio-deposits by oysters brings about noticeable geological modifications of the underlying sediment. He recorded an increase in the organic, silt and phaeo-pigment content beneath the trestles which was again probably related to the recorded decrease in current velocity at both sites.
Introduction of invasive species, pests and diseases
There are great concerns that the widespread movement of cultured species (broodstock, seed, or planting stock) will facilitate the movement of disease-causing organisms and exotic species [27], which may pose potential dangers for both cultured and wild stocks [28]. For instance, American whelk tingle (Urosalpinx cinera) was introduced into England along with American oyster (Crassostrea virginica). This gastropod became established in some areas of Essex and Kent and caused a great deal of damage to the juvenile stages of the European flat oyster (Ostrea edulis) [29]. Pacific oysters may be invasive primarily in rocky habitats and artificial structures, and there is also evidence that they can invade soft-sediment estuarine habitats and their distributional range [30,31]. Mytilicola orientalis was not known in Irish waters until prior to the transfer of Pacific oysters from France in 1993. Introductions of Pacific oysters with Mytilicola orientalis must have consequences for other marine populations. Mytilicola orientalis is a potentially serious pest, not least because it is capable of transferring to the native oyster (Ostrea edulis) [32]. According to Minchin et al. [33] made the point that the discovery of Ostrea edulis and Mytilus edulis in Pacific oyster consignments is horrible as they are both vectors of Martelia refringens and in the case of the protozoan Bonamia ostreae, Ostrea edulis is a vector. Invasive or transported species may breed with other distinct populations of the same species, possibly resulting in a genetic shift in the local population and a loss of genetic diversity. Likewise, hybridisation may result when the endemic species and translocated species are genetically compatible. Several diseases and parasites associated with introduced oysters have been reported, most of which are also globally ubiquitous and pose some commercial threat to oyster production. These include various species of flatworm and mud-worm [34,35] and herpes virus, which infects oyster larvae and spat.
Impact on the benthos
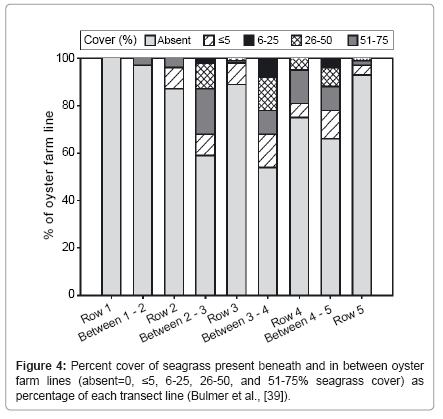
Decreases in macro-faunal abundance have been detected in areas of extensive intertidal oyster cultivation [3,36,37]. Shading by the Oysters, farm infrastructure and farm activities such as boat and vehicular traffic may have a detrimental impact on benthos such as the seagrass beds. For instance, Thorne [38] reported a reduction in seagrass cover (presumably Heterozostera tasmanica) under stocked oyster racks in Tasmania. He also showed that the seagrass appeared to recover in areas left un-stocked for a long period of time. According to Castel et al. [3] investigated the influence of oyster (Crassostrea gigas) parks on the abundance and biomass patterns of meio- and macrobenthos in tidal flats. He found out that when compared to the adjacent sandbanks, oysters clearly enhanced the meiofaunal abundance (from 1130 to 4170 individuals 10cm-2) but depressed macrofaunal densities (from 640 to 370 individuals 10cm-2). According to Moore [34] looked at the impact of an intertidal oyster farm on the benthos in Dungarvan Harbour. He reported that fauna in the lanes between trestles was more diverse than the control. According to Bulmer et al. [39] also reported a slight decline of sea grass cover (Figure 4) in their assessment of the effects of hanging basket Oysters farming on the seagrass distribution in the southern arm of the Kaipara Harbour, New Zealand.
Habitat exclusion and modification and effects on sea-birds
Areas which would normally be available for birds and other animals may be occupied by shellfish culture. Oyster farming can occupy a large amount of space on the intertidal flats and there is no reason to suppose that a similar reduction in a species dependant on the lower tidal zone could not occur. Loss of habitat can arise from the presence of structures used for growing oysters on intertidal feeding ground. These structures include frames used for holding small spat, bags held on trestles, areas under netting, tractors, trailers, sorters, trestles and boxes may be scattered over a wide area of the shoreline. These may interfere with roosting at different stages of the tide [40]. It is well recognised and documented that loss of habitat causes reduction in the species dependant on it. Some birds are more likely to be affected by habitat loss than others. The species most likely to be affected by loss of habitat are birds whose feeding and breeding habitats are suitable for oysters farming and which feed or breed on the low shore to mid shore. This may increase their energy requirements, and hence adversely affect survival [41]. Nearly all the wader species fit into this category. All species feeding on the lower shore area are likely to be affected by habitat loss to oyster farming [42]. According to Zydelis et al. [43] suggested that shellfish culture racks or stacked bags/nets could block large intertidal regions from wading shorebirds such as oystercatchers, plovers, stilts and potentially dotterels. Disturbance from intertidal shellfish culture affects few breeding birds. It principally impinges on wintering birds. This is because intertidal flats (mud and sand), although a minor summer habitat for breeding birds, are of major importance as a habitat for many winter visitors [41,44]. According to Watson-Capps & Mann [45] reported significant habitat exclusion of Indian Ocean bottlenose dolphins (Tursiops aduncus) by pearl oyster farms in Western Australia, in a bay where racks were suspended or fixed to the seabed in relatively shallow water (~2-4 m).
Accumulation of shell litter, debris and associated organisms and physical disturbance
Live oysters, shell litter, farm debris (e.g. oyster growing sticks) and fouling or epi-benthic organisms tend to accumulate beneath growing racks (sea-bed) and are best visible in oyster farms during low tide. Therefore, such accumulated materials may potentially provide novel habitats for fouling organisms and associated mobile biota and persist for many years even after the cessation of farming thereby resulting in long-term shifts in benthic community composition [46-49].
Physical disturbance, particularly from vessel movements (e.g. propeller wash) and farm personnel walking between cultivation structures, has a strong influence on benthic changes beneath oyster farm sites [5,7]. According to Forrest & Creese [7] described an association between benthic macrofaunal composition and decreased sediment shear strength (increased ability for sediments to erode or resuspend) beneath Pacific oyster cultures in Mahurangi Harbour, which they suggested could reflect physical disturbance beneath racks (Figure 5).
Suggested Solutions
Proper site selection
The choice of site is absolutely critical as this determines to a large extent the impact of the farm. The nature and magnitude of effects largely depend on site-specific conditions relating to the intensity of farming, flushing characteristics of the environment, and the proximity of the farm to valued habitats (e.g. rocky reefs) and species (e.g. nesting shorebirds) [50]. Effects to the seabed may be reduced by siting oyster farms away from the breeding and foraging areas of birds and in high current environments or open coastal situations at sufficient depths such that increased currents and wave action enhances dispersion of farm-generated wastes over a wider area.
Effective farm management
Effective environmental code of practice (ECOP) for managing inputs of debris associated with the development and maintenance of farm structures should be put in place and adopted by the oyster farmers. Once a farm is in place there will be various impacts associated with its mere presence. However, the impact that an individual farm will have on an area will depend to a large degree on the farmers’ attitude. Some farmers leave equipment and abandoned trestles scattered on the shore and drive all over the shore. Other farmers have good sensitive and sensible farm-keeping practices and respect all the other shore users. Seabed effects and water column reduction of phytoplanktons from individual farms can be managed through the development of environmental criteria and maintaining an appropriate stocking density, which can be integrated into adaptive management plans (AMPs). Education of, and interaction with, farmers should be a key management goal.
Integrated culture
Integrated culture involves the cultivation of two or more species, usually of different trophic levels, in close proximity to one another. It is a rapidly advancing area of research and has considerable scope for mitigation of environmental effects in the future. The potential combinations of species are many and varied. Combinations with particular scope for mitigation of environmental effects include: growing sea cucumbers beneath Oysters farm to process deposited organic material [51,52] and culturing oysters around finfish farms to intercept organic particulates and dissolved nutrients [53-55]. Uptake by industry is presently constrained by environmental necessity and/or proven economic incentives.
Managing pests and diseases
The adverse effects of pest and disease introduction and spread, can have profound non-local and irreversible consequences, are arguably more significant than the commonly cited seabed effects. They have the potential to impact on wild conspecifics and conceivably associated fisheries. Good biosecurity practices and surveillance are probably the best ways of managing the threat [56].
Managing genetic diversity
These risks are manageable through identifying the genetic structuring within the wild population and regulating transfer between regions accordingly, and through careful management of selectively bred stock to ensure adequate genetic diversity. For example, steps can also be taken at the grow-out stage to manage and maintain diversity of farmed stock within farms/bays/regions by setting standards for combinations of families within a bay (or other relevant area based on progeny dispersal range). In some instances, farmers may also adopt triploidy, which theoretically negates genetic contamination issues. Triploids are safe way to test e.g with Crassostrea gigas with little or no risk of reproduction and use of F1 generation or greater progeny reduces the risk of disease [50].
Conclusion and Recommendations
Some oyster farming practices, if managed inappropriately, may result in longer term aquatic ecological impacts. Over-stocking of oysters can seriously affect phytoplankton availability for other aquatic animals and plants. In a small, localised area this is considered to be exceeding ‘carrying capacity’ and can affect the economic return of a particular farm. The larger flow-on effect is the potential to exceed the carrying capacity of a whole bay, whereby total productivity of a bay is reduced to the point where its ecological balance is disrupted. Physical shading of seagrass by oyster infrastructure can lead to seagrass loss by reducing light below necessary levels. A report by Madigan et al. [57] indicates that the traditional ‘rack and rail’ system of intertidal oyster culture can adversely affect seagrass health and survival. Nonetheless, it is important for growers to understand the high sensitivity of seagrass to human interference. The impact of vehicle wheels and boat propellers on sensitive ecological areas, particularly on seagrass, is an observable environmental impact of oyster culture. The presence of inappropriately positioned sea-based oyster infrastructure can potentially disrupt local hydrodynamics such as wave and current speed. This may lead to non-removal, deposition and acquisition or piling of excessive sediment and mud and consequently has adverse impacts on the benthic community.
Clear environmental planning and enactment of code of practice act for the oyster farming industry should be put in place by the government to overcome conflict between oyster farming and other potential water users. Licensing is a key regulatory mechanism and should be given to those that will adhere strictly to the act with prosecution and penalties for the violators. Oyster farmers need to be adequately educated, informed and empowered by the government and Non-Governmental Organizations (NGOs) to cultivate in an eco-friendly, sustainable manner. It is envisaged that management agreements should be reached with these farmers as part of the design and implementation of the plans. This will enhance cooperation, compliance and ensure success of the management plan is realised [58-60].
Future research needs to be conducted in finding cost-effective, ecofriendly methods of oyster cultivation with minimum environmental impact.
Acknowledgement
A special thanks to German Academic Exchange Service, Germany for their financial support.
References
- Kaiser MJ, Laing I, Utting SD, Burnell GM (1998) Environmental impacts of bivalve mariculture. Journal of Shellfish Research 17: 59-66.
- Gallardi D (2014) Effects of Bivalve Aquaculture on the Environment and Their Possible Mitigation: A Review. Fish Aquac J 5: 105. doi: 10.4172/2150-3508.1000105.
- Castel J, Labourg PJ, Escaravage V, Aubey I, Garcia ME (1989) Influence of Seagrass Beds and Oyster Parks on the Abundance and Biomass Patterns of 138 Meio and Macrobenthos in Tidal Flats. Estuarine,Coastal and Shelf Science 28: 71-85.
- Nugues MM, Kaiser MJ, Spencer BE, Edwards DB (1996) Benthic community changes associated with intertidal oyster cultivation. Aquaculture Research 27: 913-924.
- Grave DS, Moore SJ, Burnell G (1998) Changes in benthic macrofauna associated with intertidal oyster, Crassostrea gigas(Thunberg) culture. Journal of Shellfish Research17: 1137-1142.
- Kaiser MJ (2001) Ecological effects of shellfish cultivation. In: Black KD (Ed.), Environmental impacts of aquaculture. Sheffield Academic Press, Sheffield: 51-75.
- Forrest BM, Creese RG (2006) Benthic impacts of intertidal oyster culture, with consideration of taxonomic sufficiency. Environmental Monitoring and Assessment 112: 159-176.
- Chamberlain J, Fernandes TF, Read P, Nickell TD, Davies IM (2001) Impacts of biodeposits from suspended mussel (Mytilus edulisL) culture on the surrounding surficial sediments. ICES Journal of Marine Science 58: 411-416.
- Carvalho S, Barata M, Pereira F, Gaspar MB, da Fonseca LC, et al. (2006) Distribution patterns of macrobenthic species in relation to organic enrichment within aquaculture earthern ponds. Marine Pollution Bulletin 52: 1573-1584.
- Goulletquer P, Héral M (1997) Marine molluscan production trends in France: from fisheries to aquaculture. In: MacKenzie CL, Burrell VG, Rosenfield AHW (eds.) The history, present condition, and future of the Molluscan fisheries of North America and Europe. NOAA Technical Report NMFS 129, Department of Commerce, Seattle, Washington: 137-164.
- Ottman F, Sornin JM (1985) Observations on sediment accumulation as a result of mollusk culture systems in France. Proceedings of the international symposium on utilization of coastal ecosystem: planning, pollution and productivity, Rio Grande, Brasil: 329-337.
- Crawford CM, Macleod CKA, Mitchell IM (2003) Effects of shellfish farming on the benthic environment. Aquaculture 224:117-140.
- Dahlback B, Gunnarrsson LAH (1981) Sedimentation and sulfate reduction under a mussel culture. Marine Biology 63: 269-275.
- Kaspar HF, Gillespie PA, Boyer IC (1985) Effects of mussel aquaculture on the nitrogen cycle and benthic communities in Kenepuru Sound, Marlborough Sound, New Zealand. Mar Biol 85: 127-136.
- Mojica R, Nelson WG (1993) Environmental effects of a hard clam (Mercenaria mercenaria) aquaculture site in the Indian River Lagoon, Florida. Aquaculture 113: 313-329.
- Haamer J (1996) Improving water quality in eutrophied fjord system with mussel farming. Ambio 25: 357-363.
- Grant J, Hatcher A, Scott DB, Pocklington P, Schafer CT (1995) A multidisciplinary approach to evaluating impacts of shellfish aquaculture on benthic communities. Estauries 18: 124-144.
- Powell EN, Hoffmann EE, Klinck JN, Ray SM (1992) Modeling oyster populations: A commentary on filtration rate. Is faster better? Journal of Shellfish Research 11: 387-398.
- Cloern JE (1982) Does the Benthos control phytoplankton biomass in South San Francisco Bay?. Marine Ecology Progress Series 9: 191-202.
- Officer CB, Smayda TJ, Mann R (1982) Benthic filter feeding: a natural eutrophication control. Marine Ecology Progress Series 9: 203-210.
- Haure J, Baud JP (1991) Trophic competition between natural beds of mussels(Mytilus edulis), Japanese oysters (Crassostrea gigas) in the bay of Bourgneuf (Atlantic coasts of France). Implication in its management. In: De Pauw N, Joyce J (eds) Aquaculture and the environment. Short communications and abstracts presented at the International Conference Aquaculture Europe, Dublin, Ireland. EAS special 14: 146.
- DPIF (1997) Marine Farming Development Plans for Tasmania -D’Entrecasteaux Channel. Department of Primary Industry and Fisheries, Tasmania.
- Rice MA (1999) Control of eutrophication by bivalves: Filtration of particulates and removal of nitrogen through harvest of rapidly growing stocks. Journal of Shellfish Research 18: 275.
- Newel RIE, Cornwell JC, Owens MS (2004) Influence of simulated bivalve deposition and microphytobenthos on sediment nitrogen dynamics: A laboratory study. Limnology and Oceanography 47: 1367-1379.
- Martin JLM, Sornin JM, Marchand M (1991) The significence of oyster biodeposition in concentrating oganic matter and contaminents in the sediment In: De Pauw N, Joyce J (eds) Aquaculture and the environment. Short communications and abstracts presented at the International Conference Aquaculture Europe, Dublin, Ireland: 207.
- Sornin JM, Feuillet M, Heral M, Paoli DJM (1983) Effect des biodepots de l’huitre Crassostres Gigas (Thunberg) sur l’accumulation de matieres organiques dans les parcs du bassin de Marennes-Oleron. J Moll Stud: 185-197.
- Naylor RL, Williams SL, Strong DR (2001) Aquaculture: A gateway for exotic species. Science 294: 1655-1656.
- Ford SE (1996) Range extension by the oyster parasite Perkinsus marinusinto the northeastern United States: Response to climate change? Journal of Shellfish Research 15:45-56.
- Spencer BE (1991) Predators and methods of control in shellfish cultivation. In: (Eds) DePauw N, Joyce J comps. Aquaculture Europe. Aquaculture and the Environment 14: 303.
- Jenkins RJ (1997) Studies on the dynamics of colonisation of the Pacific oyster, Crassostrea gigas (Thunberg), in the Marlborough Sounds, New Zealand. Unpublished MSc thesis, University of Otago.
- Cognie B, Haure J, Barillé L (2006) Spatial distribution in a temperate coastal ecosystem of the wild stock of the farmed oyster Crassostrea gigas (Thunberg). Aquaculture 259: 249-259.
- Holmes JMC, Minchin D (1995) Two exotic copepods imported into Ireland with the Pacific oyster Crassostrea gigas (Thunberg). Irish Naturalist Journal 25: 17-20.
- Minchin D, Duggan CB, Holmes JMC, Neiland S (1993) Introductions of exotic species associated with Pacific oyster transfers from France to Ireland. International Council for the Exploration of the Sea, Copenhagen (Denmark). Mariculture Comm Counc. Dublin, Ireland.
- Handley SJ, Bergquist PR (1997) Spionid polychaete infestations of intertidal Pacific oysters Crassostrea gigas (Thunberg), Mahurangi Harbour, northern New Zealand. Aquaculture 153: 191-205.
- Handley SJ (2002) Optimizing intertidal Pacific oyster (Thunberg) culture, Houhora Harbour, northern New Zealand. Aquaculture Research 33: 1019-1030.
- Heral M, Paoli DJ, Prou J (1986) Dynamiques des production et des biomasses des huitres creuses cultivee (Crassostrea angulata et Crassostrea gigas) dans le bassin de Marennes-Oleron depuis un siecle. International Council for the Exploration of the Sea.
- Simenstad CA, Fresh KL (1995) Influence of intertidal Aquaculture on benthic communities in pacific Northwest estuaries: Scales of disturbance. Estuaries 18: 43-70.
- Thorne AJ (1998) Alterations in the structure of macrobenthic communities related to the culture of oysters (Crassostrea gigas). B Sc. (Hons.) Thesis, University of Tasmania.
- Bulmer R, Kelly S, Jeffs AG (2012) Hanging basket oyster farming: assessing effects on seagrass using aerial photography.Aquacult Environ Interact 2: 285-292.
- O’Briain M (1993) International conservation instruments and shellfish aquaculture. In: Aquaculture in Ireland -Towards sustainability. Meldon J: 38-45.
- Davidson NC, Rothwell PI (1993) Human Disturbance to waterfowl on estuaries: the conservation and coastal management implications of current knowledge. Wader Study Group Bulletin 68: 97-105.
- Heffernan ML (1995) Shellfish farming and Special Protection Areas for birds in Ireland. MSc thesis submitted in part-fulfilment to the Environmental Science Unit, Trinity college, Dublin.
- Zydelis R, Esler D, Boyd WS, Lacroix DL, Kirk M (2006) Habitat use by wintering surf and white-winged scoters: effects of environmental attributes and shellfish aquaculture. Journal of Wildlife Management 70: 1754-1762.
- Coveney J, Merne O, Wilson J, Allen D, Thomas G (1993) Towards a conservation strategy for birds in Ireland. Irish Wildbird Conservancy, Dublin.
- Watson-Capps JJ, Mann J (2005) The effects of aquaculture on bottlenose dolphin (Tursiops sp.) ranging in Shark Bay, Western Australia. Biological conservation 124: 519-526
- Peterson CH, Grabowski IH, Powers SP (2003) Estimated enhancement of fish production resulting from restoring oyster reef habitat: Quantitative valuation. Marine Ecologt Progress Series 264: 249-264.
- Escapa M, Isacch J, Daleo P, Alberti J, Iribarne O, et al (2004) The distribution and ecological effects of the introduced Pacific oyster, Crassostrea gigas (Thunberg, 1793) in northern Patagonia. J Shellfish Res23: 765-772.
- Ruesink JL, Lenihan HS, Trimble AC, Heiman KW, Micheli F (2005) Introduction of non-native oysters: Ecosystem effects and restoration implications. Annual Review of Ecology Evolution and Systematics 36: 643-689.
- Coen LD, Brumbaugh RD, Bushek D, Grizzle R, Luckenback MW, et al (2007) As we see it: ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303-307.
- Heffernan ML (1999) A review of the ecological implications of mariculture and intertidal harvesting in Ireland. Irish Wildlife Manuals.
- Slater MJ, Carton AG (2007) Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with greenlipped mussel farms. Aquaculture 272: 389-398.
- Paltzat DL, Pearce CM, Barnes PA, McKinley RS (2008) Growth and production of California sea cucumbers (Parastichopus californicus Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas Thunberg). Aquaculture 275: 124-137.
- Lefebvre S, Barille L, Clerc M (2000) Pacific oyster (Crassostrea gigas) feeding responses to fish-farm effluent. Aquaculture 187:185-198.
- Rosa LT, Mirto S, Favaloro E, Savona B, Sara G, Danovaro R, Mazzola A (2002) Impact on the water column biogeochemistry of a Mediterranean mussel and fish farm. Water Research 36: 713-721.
- Cheshuk BW, Purser GJ, Quintana R (2003) Integrated openwater mussel (Mytilus planulatus) and Atlantic salmon (Salmo Salar) culture in Tasmania, Australia. Aquaculture 218: 357- 378.
- Taylor MD, Dodgshun TJ, de Zwart E (2005) Biosecurity management plan for a proposed south Kaipara oyster farm. Cawthron Report. Cawthron Institute, Nelson, New Zealand.
- Madigan S, Venema S, Haskard K, Clarke S (2000) Oyster Environmental Monitoring Program (OEMP): Small scale seagrass health study,SARDI, Adelaide.
- Forrest B, Keeley N, Hopkins G, Webb S, Clement D (2009) Bivalve aquaculture in estuaries: Review and synthesis of oyster cultivation effects. Aquaculture 298: 1-15.
- Kaiser MJ, Edwards DB, Spencer B (1996) Infaunal community changes as a result of commercial clam cultivation and harvesting. Living Aquatic Resources 9: 57-63.
- Moore SJ (1996) The impact of an intertidal oyster farm on the benthos. BSc Thesis presented to the Faculty of Science, University College Cork, Ireland: 34.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 25136
- [From(publication date):
December-2016 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 23778
- PDF downloads : 1358