Mini Review Open Access
Ecological, Biological, Behavioral and Genetic Adaptation to Xeric Habitats of Triturus Vittatus Vittatus (Urodela) on the Southern Border of its Distribution
1MIGAL - Galilee Research Institute, Kiryat Shmona, Israel
2Faculty of Science and Technology, Tel-Hai Academic College, Kiryat Shmona, Israel
- *Corresponding Author:
- Gad Degani
MIGAL-Galilee Research Institute, Faculty of Science and Technology
Tel-Hai Academic College, Kiryat Shmona, Israel
Tel: 97246953544
E-mail: gad@migal.org.il
Received date: March 24, 2017; Accepted date: April 19, 2017; Published date: April 27, 2017
Citation: Degani G (2017) Ecological, Biological, Behavioral and Genetic Adaptation to Xeric Habitats of Triturus Vittatus Vittatus (Urodela) on the Southern Border of its Distribution. J Marine Sci Res Dev 7:226. doi: 10.4172/2155-9910.1000226
Copyright: © 2017 Degani G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
In the present mini-review, the results and unpublished data that were collected on Triturus vittatus vittatus in Israel on the southern border of its distribution, and on its adaptation to the Mediterranean semi-arid climate and to arid climate environments on the southern border of its distribution were presented. The contribution of the present paper is in building a model based on the results collected on the distribution, life cycle, behaviour and genetic variations among different populations in northern Israel down to the central coastal plains and near the desert of this species. More specifically, this model is based on the morphology, biology, behaviour and life cycle of T. v. vittatus adaptation. By considering these many parameters, one hypothesis was raised and is supported. The adaptation to and selection of habitats depends mainly on the terrestrial phase and less on the aquatic phases. There are various breeding places in all of the habitats, however, the newts are mainly used to winter ponds, many of which dry up in summer where the larvae can grow and complete metamorphosis. The adaptation of the breeding ponds is not under ecological conditions during larvae growth and complete metamorphosis, but the time of adult breeding and larvae growth occur year-round. The molecular genetic variation in the different areas support our hypothesis that climate is affected by altitude and proximity to the desert. During the life cycle the newts have two habitats aquatic and terrestrial and the adaption to terrestrial habitats is more affected on the newts distribution than the aquatic habitat. The quality model of fitness T. v. vittatus to extreme conditions was suggested.
Keywords
Genetic variation; Urodela; Triturus vittatus vittatus
Introduction
Ecology defines the relationships and interactions between organisms and their environment. In order to explain the adaptation of Triturus vittatus vittatus to a habitat, as many biological parameters as possible, e.g., physiology, behaviour and life cycle, growth and reproduction parameters that are important to these characteristics, must be studied in understanding adaptation to the natural habitat. The banded newt Triturus vittatus [1] is distributed throughout Western Caucasus, Turkey, Lebanon, Syria, Israel and Iraq. The banded newt consists of two species, T. ophryticus and T. vittatus, based on trunk vertebrae count, genome size and allozyme data. The northern taxon, T. ophryticus, is subdivided into two geographic fragments: “western group” populations from western Anatolian Turkey; and “eastern group” populations distributed in the remaining area of Pontic Turkey and Western Caucasus. According to the above criteria, the T. vittatus species is found in Israel. The biology of T. vittatus in the Mediterranean area has been described by Olgun [2]. As indicated by their data, there are two known subspecies in the genus Triturus: T. v. vittatus along the eastern edge of the Mediterranean Sea from Turkey to Israel, where it reaches its southern limit; and T. v. ophryticus in the Caucasus, east and south of the Black Sea. The banded newt, T. v. vittatus, is an endangered species in Israel [3] at the southern limit of its distribution. The adaptation of T. v. vittatus on the southern border of newt populations in Israel has been scarcely described. The purpose of the present paper is to review the data collected and add some unpublished data in order to enhance our knowledge about the adaptation of this species to extreme conditions.
Distribution in Israel
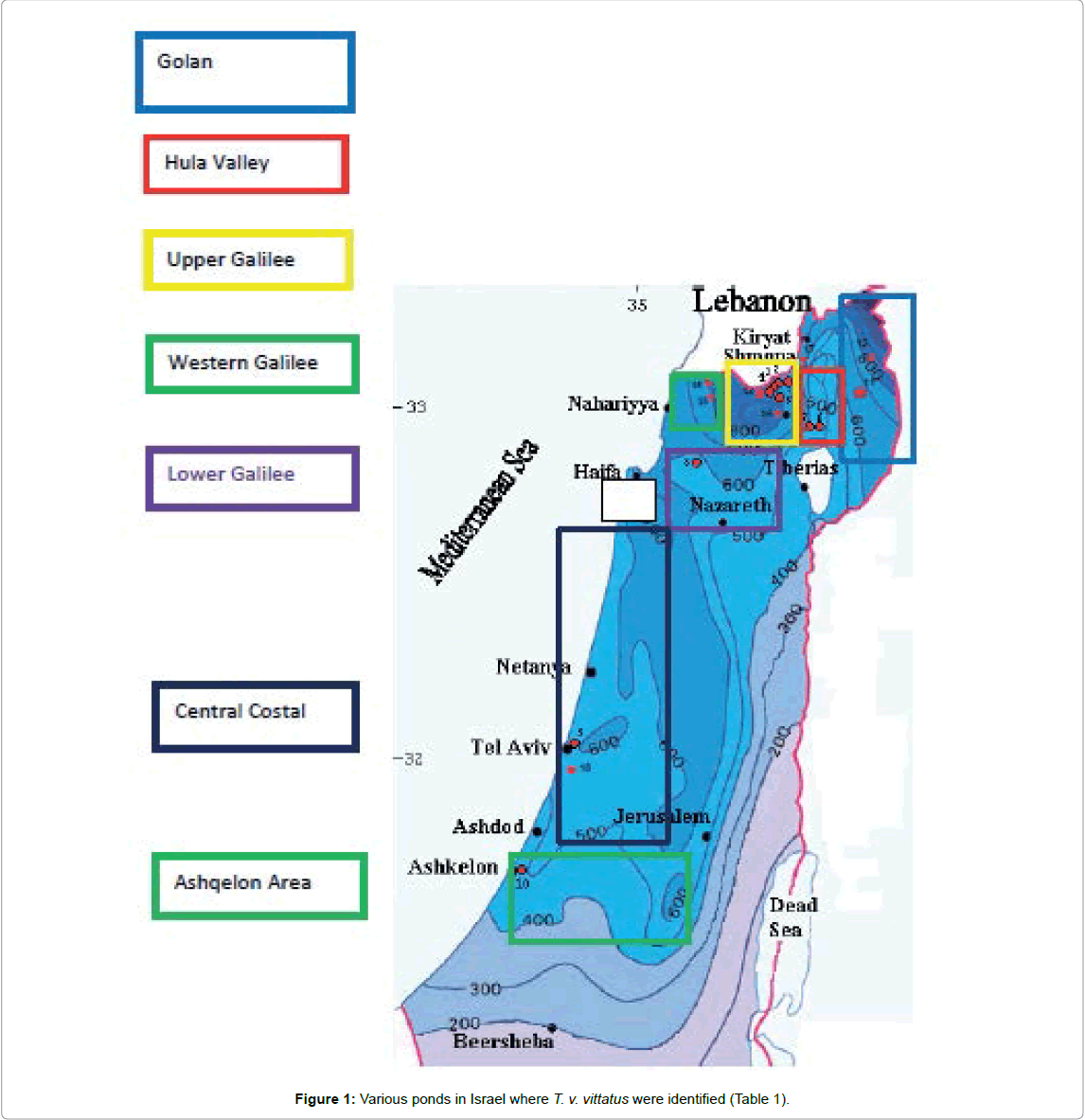
In Israel, newt populations are found under more extreme conditions than in other geographic areas of the distribution of populations belonging to T. vittatus species. The population in the center near southern Israel adapted to more extreme conditions than in northern populations in Israel. The Mediterranean climate has about 1,000 mm of rain annually in northern Israel down to the central coastal plains, to about 400 mm of rain annually in close proximity to semidesert conditions [4-10], where conditions are most extreme (Figure 1 and Table 1). Populations of newts in Israel are found around the breeding places, however, not much information has been published on the adaptation to terrestrial life, which includes physiology and environmental behavior [11-15].
| Breeding Site | Area | Reference | Latitude | Longitude | Altitude |
| Nahalit Pond 1 | Upper | 776401 | 243657 | 665 | |
| Galilee | [3] | ||||
| Matityahu | Upper | 774855 | 242783 | 670 | |
| Pond 2 | Galilee | [3] | |||
| Dovev Pond 4 | Upper | 772801 | 239158 | 740 | |
| Galilee | [3] | ||||
| Pharaa Pond 3 | Upper | [3] | 774580 | 242784 | 682 |
| Galilee | |||||
| Amiad Water | Hula Valley | 757994 | 251721 | 212 | |
| Holes 7 | [3] | ||||
| Jaudha Pond | Hula Valley | [3] | 761398 | 257589 | 110 |
| Kash Pond 5 | Upper | [3] | 770659 | 246258 | 815 |
| Galilee | |||||
| Leshem Pond 8 | Lower | [3] | 750976 | 225612 | 300 |
| Galilee | |||||
| Afeka Pond 9 | Central | 670453 | 182364 | 20 | |
| Costal | [3] | ||||
| Berekhya Pond | Ashkelon | [3] | 618578 | 166738 | 15 |
| 10 | Area | ||||
| Katzarin Pond | Central | [5] | |||
| 264566 | 765449 | 320 | |||
| 11 | Golan | ||||
| Central | |||||
| Paras Pond 12 | |||||
| Golan | |||||
| [5] | |||||
| Sasa Pond 13 | Upper | 236948 | 770859 | 800 | |
| Galilee | [3] | ||||
| Fasuta Pond 14 | Western | [3] | 229597 | 773378 | 500 |
| Galilee | |||||
| Sumara Pond | Western | 227494 | 776115 | 540 | |
| 15 | Galilee | [3] | |||
| Beitzan Pond | Upper | ||||
| [5] | |||||
| 16 | Galilee | ||||
| Raihaniya Pond | Upper | [19-22] | 195791 | 272861 | 665 |
| 17 | Galilee | ||||
| NirGalim | Central | 169748 | 536396 | 20 | |
| Pond 18 | Coast | ||||
Table 1: The breeding sites of T. v. vittatus on the southern border of its distribution.
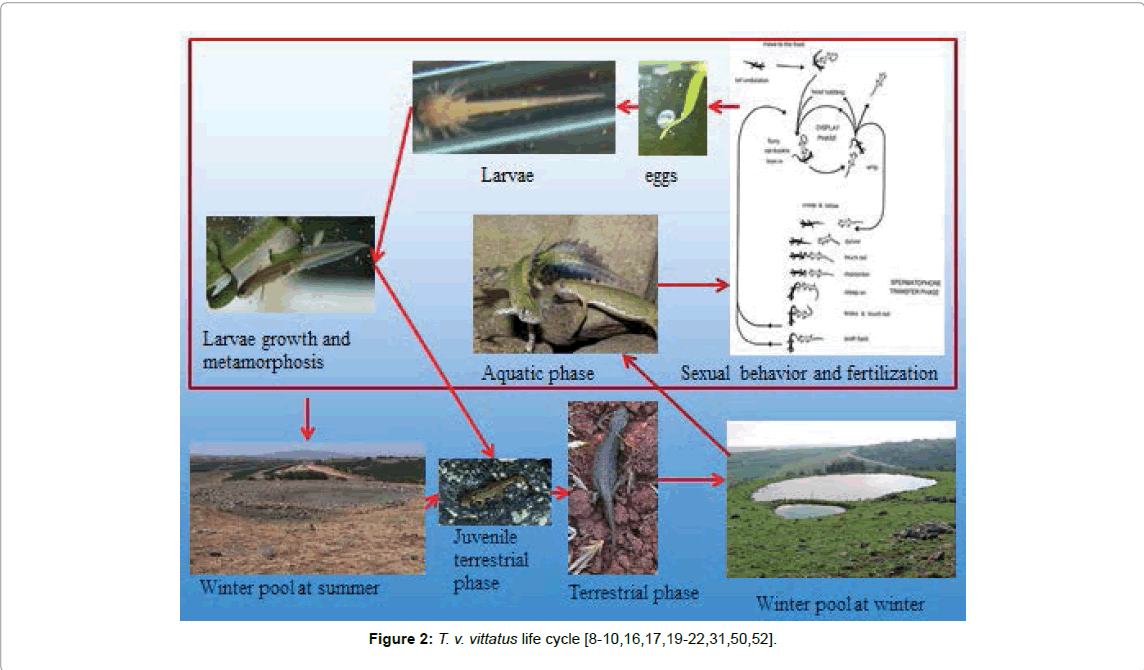
Life Cycle
The life cycle of T. v. vittatus in Israel has been described in several studies (Figure 2). In northern Israel and the Upper Galilee, the life cycle has been described by Degani and Mendelssohn [4-17]. In central in Israel, the life cycle has been described by Geffen [18]. T. v. vittatus in the aquatic phase is reproduced in mainly unpredictable habitats such as winter pools that contain water only until the beginning of the summer, although occasionally these pools contain water throughout the year [5,8-10,16,19-23]. Like other newts, T. v. vittatus require water bodies surrounded by an adequate terrestrial habitat to support both terrestrial and aquatic life phases. If either habitat is damaged, a population may be unable to survive.
Terrestrial adult newts reach the pond area at the beginning of the rainy season before the ponds fill up with water and subsequently enter the ponds in the aquatic phase after they are full. In the Upper Galilee, males inhabit the ponds from January to March, leaving them after mating. Females may remain in the water until May, when they deposit between 18-68 eggs on plant or rock surfaces until they move on to the terrestrial stage [4,8]. Afterwards, the larvae hatch 19-29 days later, depending on water temperature [16].
The period of activity, population parameters and food habits of mature T. v. vittatus in central Israel have been studied by Geffen et al. [18], who discovered that during November to December, adult newts appear on land and enter the pond after it has filled up, and remain in the water until late February in the Upper Galilee (Tables 2 and 3, Figure 2) [4].
| Breeding | 2001-2 | 2002-3 | 2003-4 | 2004-5 | ||||
| Site | ||||||||
| Months | Days | Months | Days | Months | Days | Months | Days | |
| Matitiyahu | May- | 65 | April- | 60 | April- | 40 | ||
| Pond | July | June | June | |||||
| Dovev Pond | May- | 60 | May- | 65 | April- | 75 | April- | 75 |
| July | July | July | July | |||||
| Pharaa Pond | May- | 60 | May- | 50 | April- | 50 | ||
| July | July | May | ||||||
| Amiad Water | June | 35 | ||||||
| Hole | ||||||||
| Nahalit Pond | April- | 60 | ||||||
| June | ||||||||
Table 2: The duration of larvae found in various breeding places[4,24,46].
| Breeding Site | Sample Size | Male | Female | Period in the ponds | ||||
| Male | Female | Weight | Length | Weight | Length | |||
| Matityahu Pond | 3 | 7 | 4.27 ± 1.75 | 9.90 ±1.49 | 4.07 ± 1.02 | 9.64 ± 1.14 | Jan-March | |
| Dovev Pond | 6 | 11 | 5.33 ± 0.96 | 10.87 ± 0.64 | 3.15 ± 0.4 | 9.09 ± 0.6 | Jan-March | |
| Pharaa Pond | 1 | 4 | 6.4 | 10 | 3.93 ± 0.4 | 8.67 ± 0.58 | Jan-March | |
| Amiad Water Holes | 3 | 2 | 4.27 ± 1.85 | 10.17 ± 0.29 | 3.35 ± 1.06 | 8.50 ± 0.71 | Dec-April | |
| Nahalit Pond | 2 | 2 | 5.35 ± 0.21 | 10 | 3.20 ± 1.41 | 8.25 ± 1.06 | Jan-March | |
Table 3: Size of adult newts at the breeding sites studied[4].
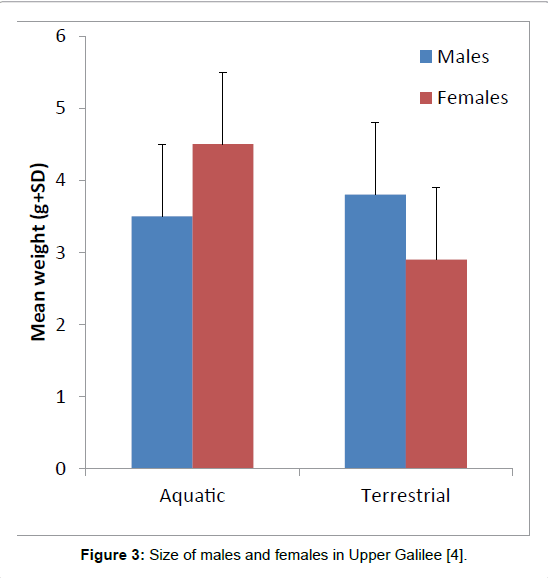
Based on the skeletochronology in two breeding populations of Triturus vittatus ophryticus inhabiting altitudes ranging from 300 m (in Gurbulak) to 1,300 m (in Hidirnebi) in northeastern Turkey, the maturation age was between 4 to 8 years but differed between the two populations at the different altitudes [23]. Differences between snoutvent length and sex dimorphismdey were found in Turkey between the populations at different altitudes. In conclusion, from the many papers, it seems that the age and size of newts are affected by ecological and climate conditions, which are affected by geographical distribution. In the Upper Galilee in Israel, the males are larger than the females, and differences in body size were significant (Figure 3) [4]. In the aquatic phase, when the females were full of eggs, they were heavier than the males. In the Upper Galilee, spawning occurred from February to May, and different amounts of eggs were deposited, up to 68 eggs per female. The larvae in the pond were also affected by altitude; they were found at the lowest place at the beginning of winter and later at a high altitude at the end of winter (Figure 3) [4,8-10].
Gonadal Cycle of T. v. vittatus
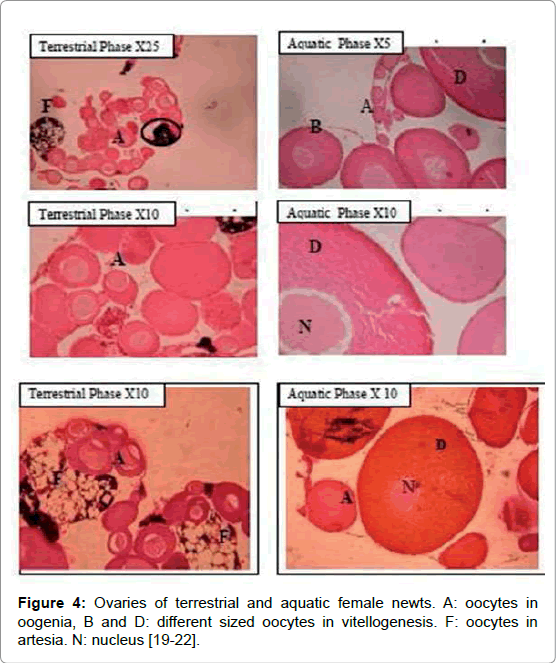
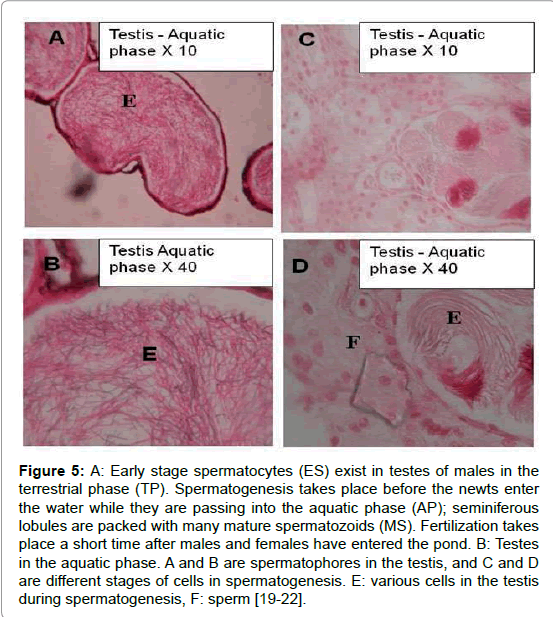
The gonadal cycle of the terrestrial and aquatic phases of T. v. vittatus has been described in various habitats in the Upper Galilee (Figure 1) for both males and females [6,7,19-23]. In female gonads, different stages were recognized: oogonia, chromatin nucleolus, perinuclear and vitellogenic oocyte maturation (Figure 4). However, there are different oogenesis states of oocytes development among individual females. Moreover, there are differences in oocytes development between aquatic and terrestrial females. In aquatic females, most of the females contained only oocytes at different stages of vitellogenesis. In contrast, ovaries of terrestrial forms had only pre-vitellogenic and atretic oocytes (Figure 4) [19-21]. Male gonads consisted of various cells at different stages of development during spermatogenesis: spermatogonia, spermacytes, spermatides and spermatozoa were observed in the gonads of aquatic forms (Figure 4A and 4B), and more developed specimens had lobuli packed with mature spermatozoa. On the other hand, lobuli of testes from terrestrial forms included mainly cells in the early stages: spermatogonia, spermacytes and early spermatids (Figure 5).
Figure 5: A: Early stage spermatocytes (ES) exist in testes of males in the terrestrial phase (TP). Spermatogenesis takes place before the newts enter the water while they are passing into the aquatic phase (AP); seminiferous lobules are packed with many mature spermatozoids (MS). Fertilization takes place a short time after males and females have entered the pond. B: Testes in the aquatic phase. A and B are spermatophores in the testis, and C and D are different stages of cells in spermatogenesis. E: various cells in the testis during spermatogenesis, F: sperm [19-22].
T. v. vittatus adults were found in ponds at low temperatures during the winter, but the eggs and larvae developed only when the temperature was above 20ºC [19-22]. The larvae hatched later and remained in the rain pool for 30-75 days. Hatching time and duration in the pool depended on water temperature, with development being slower at higher altitudes.
Larvae Growth and Complete Metamorphosis
The larvae of T. v. vittatus growth and compete metamorphosis have been studied intensively in Israel [4-16]. In Israel, larvae growth at various breeding sites is described in detail on the southern border of its distribution (Figure 6).
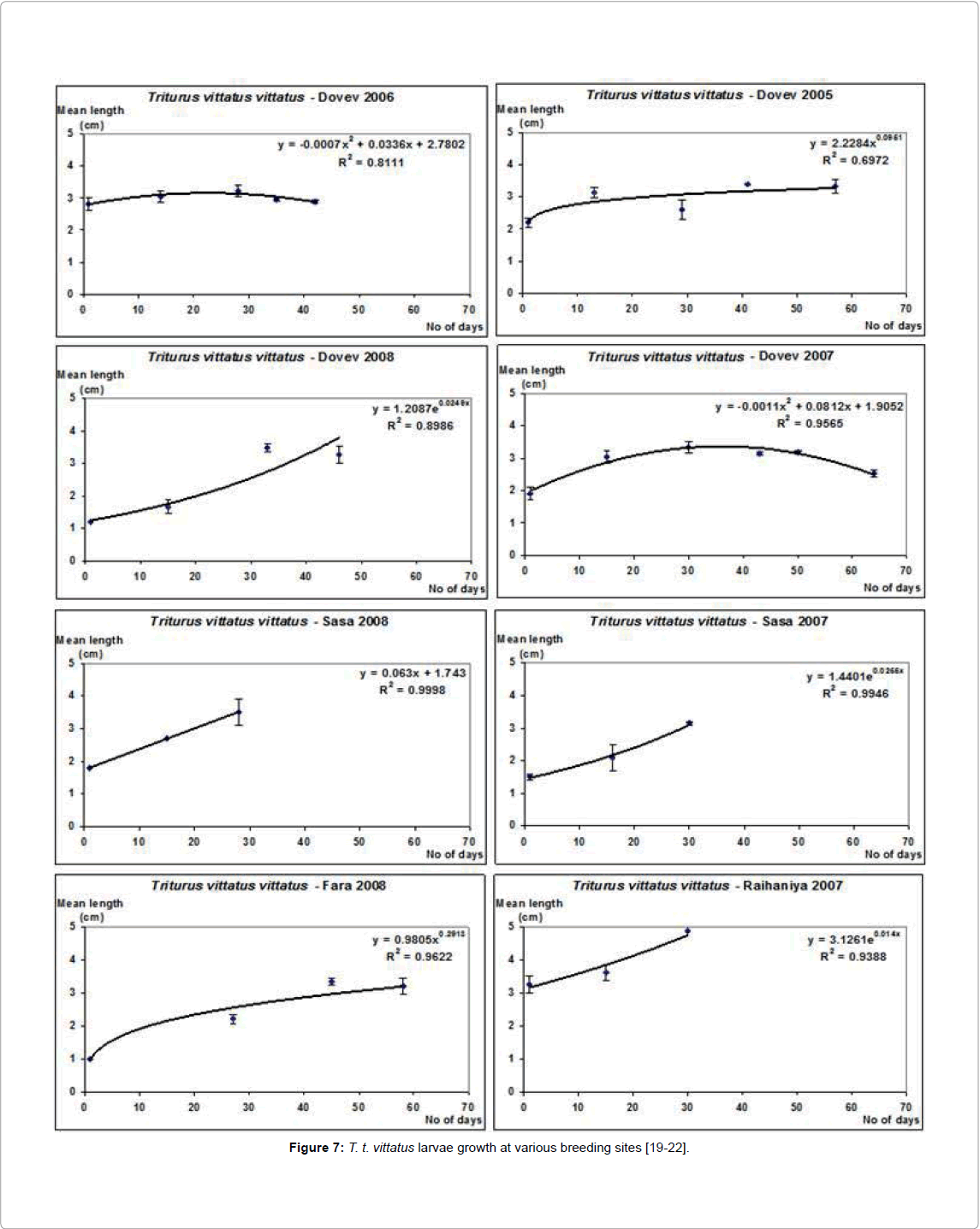
Many different water body sites in Israel were examined, and the larvae of newts were found mainly in unpredictable habitats, most of them winter pools where water is available only during part of the year, and the available water varies from one year to the other [9,19-23]. The larval growth period of T. v. vittatus differs between the various breeding sites according to the ecological conditions in the ponds [24]. These conditions at the breeding sites are affected by growth and metamorphosis (Figure 7).
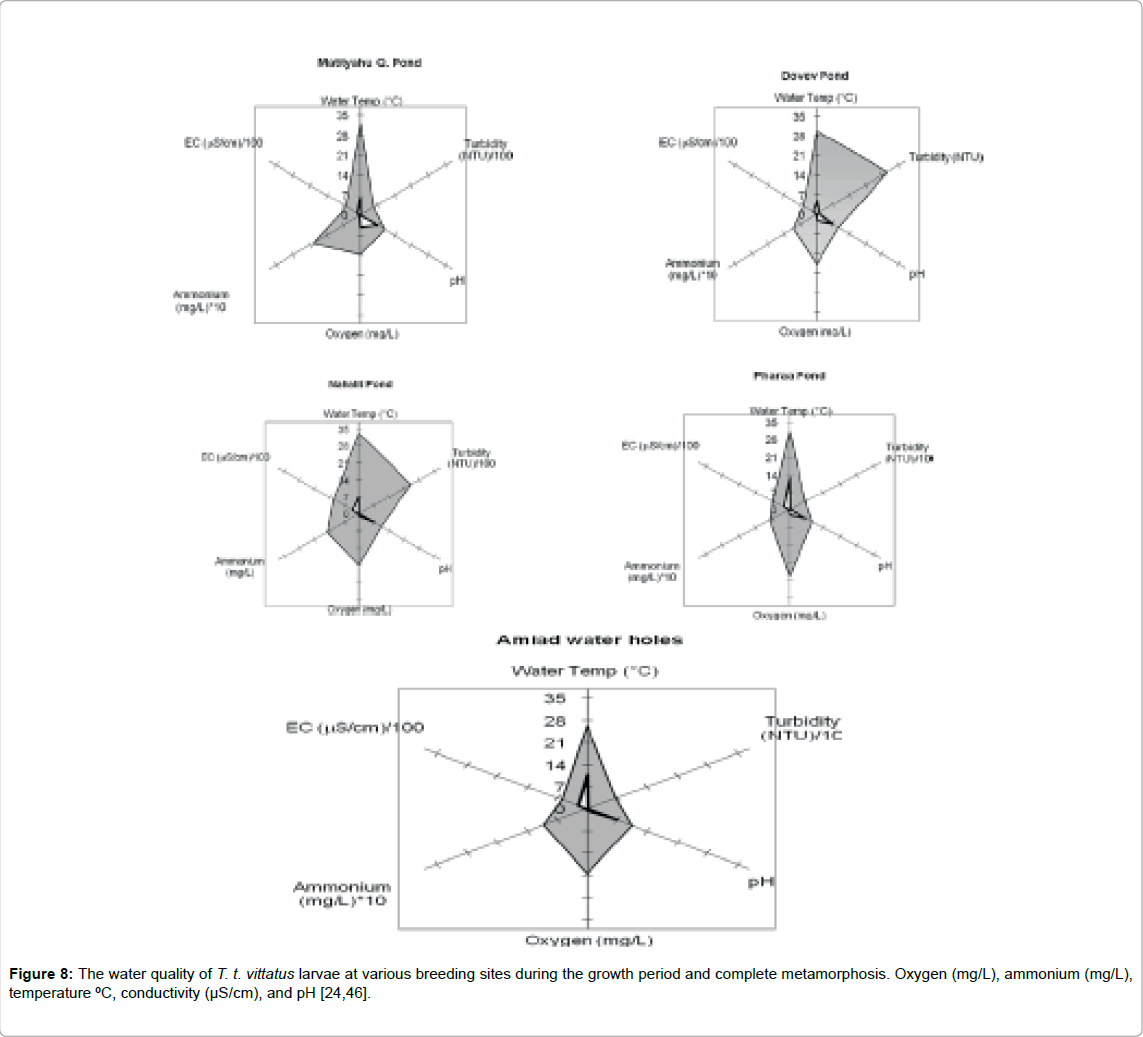
The larvae growth period and complete metamorphosis changed according to ecological conditions of the water in the water body; most changed between 1.5 to 3 months (Figure 7). The size of the breeding places, most of them winter ponds where larvae growth varied, was between 78 and 1,017 m2 and the depth reached 2.5 m. The water quality at the various breeding places in Israel has been described by Pearlson and Degani (Figure 8) [24].
Temperatures vary between 5 and 34ºC, dissolved oxygen varies between 0.6 and 27 mg/L, pH varies between 6.5 and 10.4, conductivity varies between 130 and 1,210 and ammonium is less than 1 mg/L (Figure 8) [24].
Environmental Behaviour of T. t. vittatus
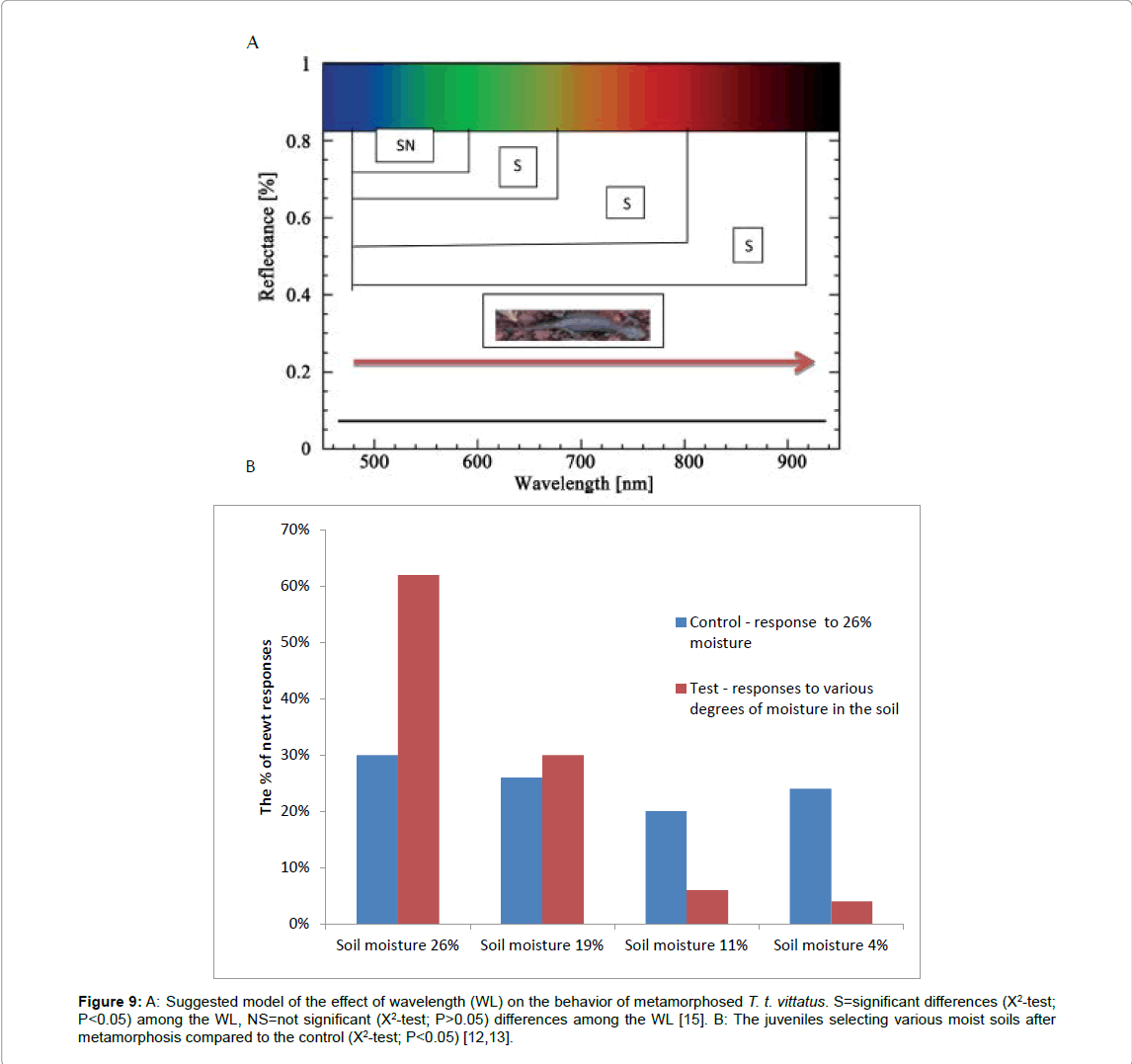
At the end of metamorphosis, the newts become terrestrial animals and are in danger of dehydration, especially at the southern border of their distribution in Israel [11]. The adaptation to terrestrial metamorphosed newts has been studied less compared to the aquatic phase and larvae growth [15]. Environmental behaviour and the ability to find hiding places to prevent dehydration are crucial for newts to survive in semi-arid habitats on the southern border of their distribution [12,13].
Metamorphosed T. t. vittatus are active at night and during the day in selected hiding places in holes and under shelters such as stones to prevent dehydration. The parameters affecting the selection of hiding places, which are very important for adaptation to terrestrial life, particularly at the southern border of its distribution where the newts are found in relatively extreme conditions, are very important. The hypothesis examined by Degani [12,13] showed that the selection of hiding places by newts is affected significantly by soil moisture and negative light (negative photo taxis) (Figure 9) [15]. Post -metamorphosis newts selected only moist soil in the hiding places and under stones and holes in the ground [4,12,13], and the effect of light in hiding places is negative photo taxis (Figure 9). This behavior might help the newts in preventing dehydration and survive the long, dry summer in natural terrestrial habitats.
Figure 9: A: Suggested model of the effect of wavelength (WL) on the behavior of metamorphosed T. t. vittatus. S=significant differences (X2-test; P< 0.05) among the WL, NS=not significant (X2-test; P>0.05) differences among the WL [15]. B: The juveniles selecting various moist soils after metamorphosis compared to the control (X2-test; P< 0.05) [12,13].
Effect of Habitat on Genetic Variation of T. t. vittatus
A relatively large number of interesting studies have been carried out on the genetic variation of urodelan taxa including the genus Triturus regarding various aspects, e.g., systematic, phylogenetic and geographic divergence [25-29]. In order to examine genetic variation among populations, various biochemical and molecular biology methods were used such as serum albumin [17], enzyme isozymes [30], randomly amplified polymorphic DNA chain reaction (RAPD PCR) [31,32], mitochondrial DNA [26,33-35], Amplified Fragment Length Polymorphism (AFLP) [19-22] and sequencing of transcriptome-based genetic markers [36].
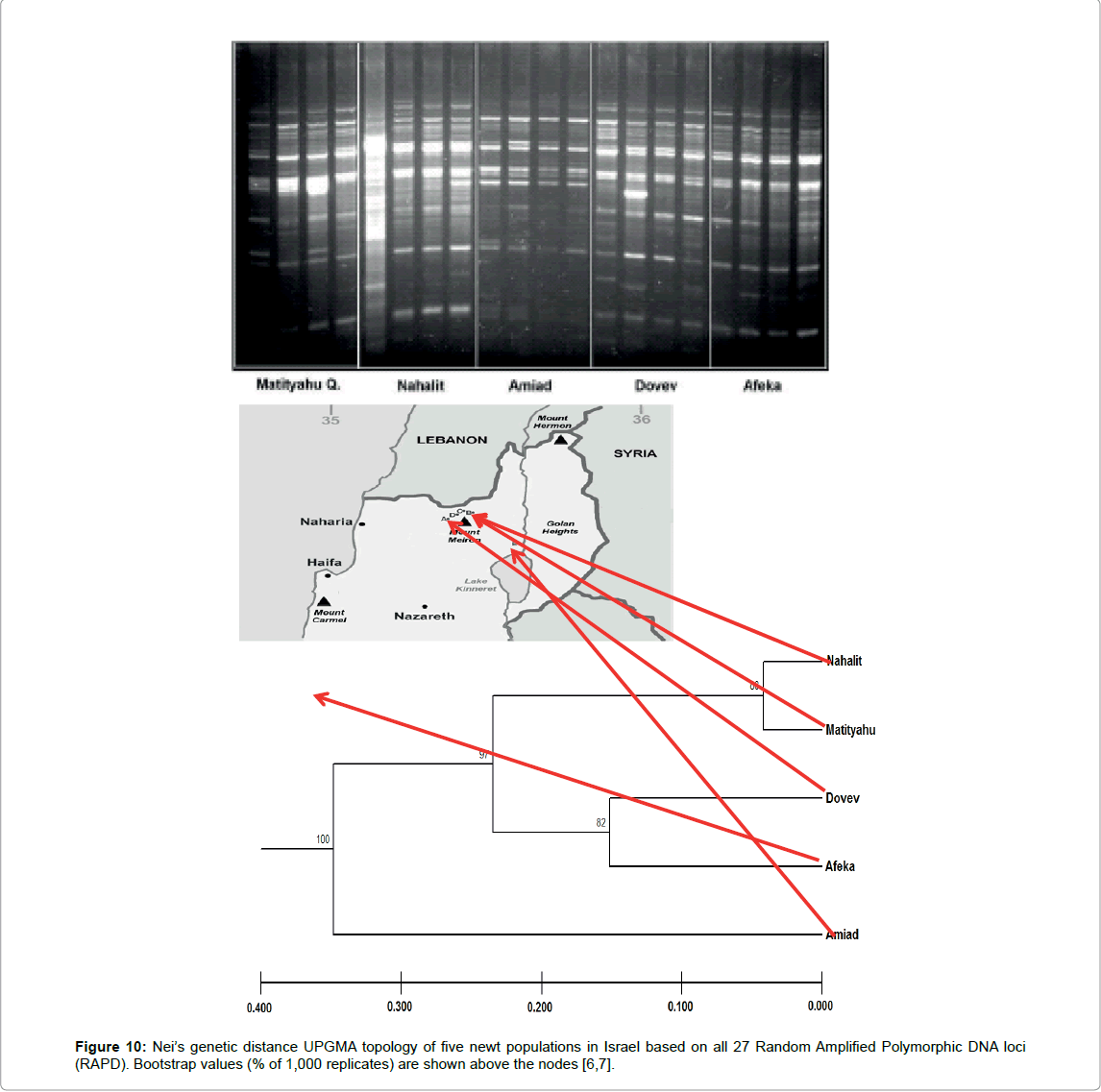
The molecular DNA variation of T. t. vittatus in different populations in relatively small areas on the southern border of its distribution where the environmental conditions are most extreme were described in several studies and various methods. Using the random RAPD PCR method, variations were found in the larvae population at different breeding sites located at altitudes ranging between 15 and 740 m above sea level (ASL) [6,7], and of the 20 primers employed by this method [37], only one was found suitable (OPA-16) to separate among the various populations.
Various bands were found among the different habitats (Figure 10), but high genetic similarity was calculated by band sharing (BS=2x (Nab)/(Na + Nb), where BS=level of band sharing between individuals a and b, Nab=number of bands shard by individuals, a and b, Na=total number of bands of individual a, and Nb=total number of bands of individual b [6,7,38]. High genetic similarity in populations were found between the larvae of newts in the breeding places in ponds at the high altitude (Table 4). A lesser similarity was found between newt larvae from the high altitude to the low altitude (Figure 10).
| Nahalit | Amiad Water | Dovev | Afeka | |
| Pond | Hole | Pond | Pond | |
| Matityahu Pond | 90% | 96% | 92% | 88% |
| Nahalit Pond | 80% | 83% | 80% | |
| Amiad Water Hole | 88% | 92% | ||
| Dovev Pond | 88% |
Table 4: Band sharing (%BS) of newts in the various ponds (Figure 6)[6,7].
The Afeka and Amiad Ponds, which are located at relatively low altitudes and at a greater distance from the other breeding sites, differed compared to other high breeding places (Figure 10).
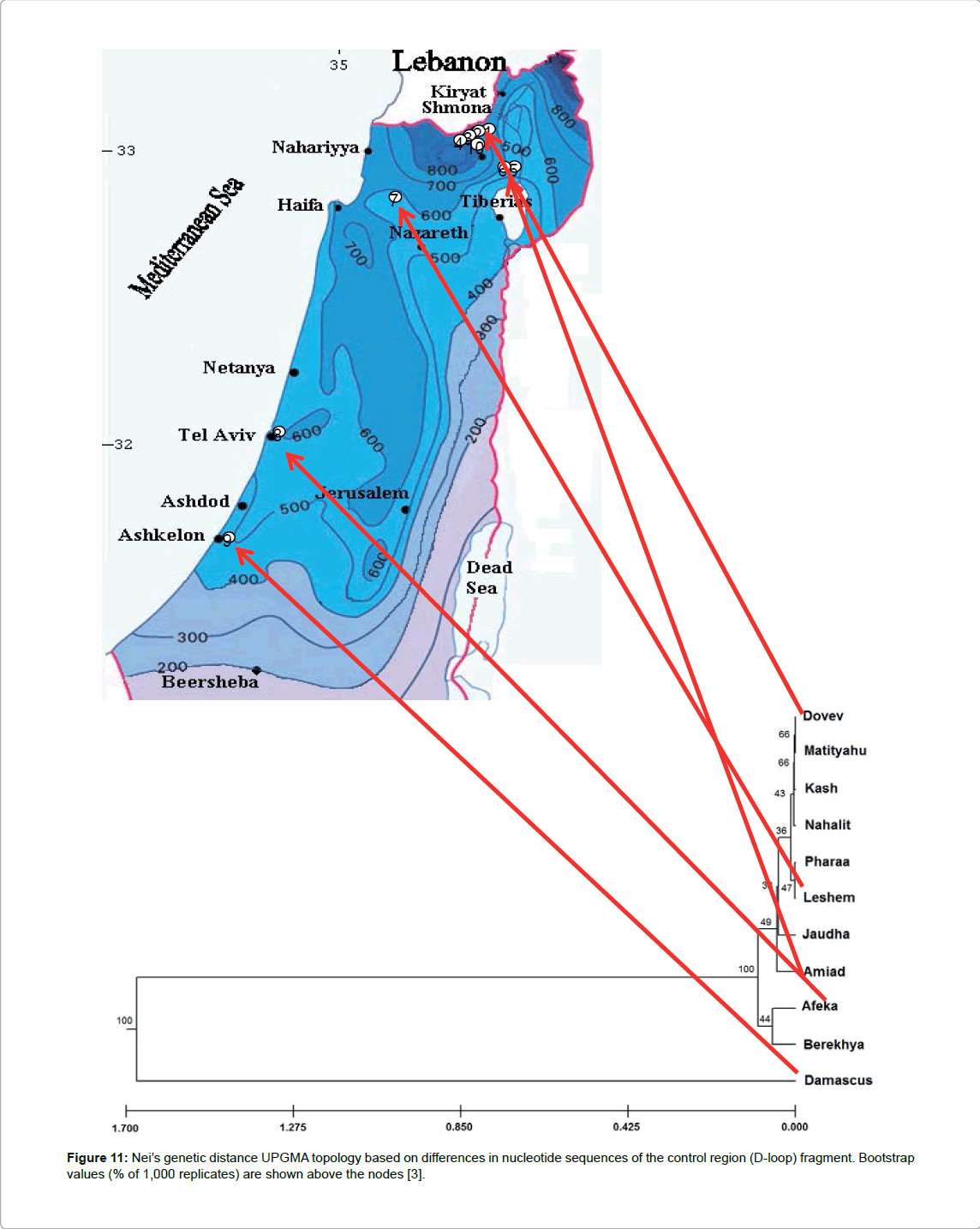
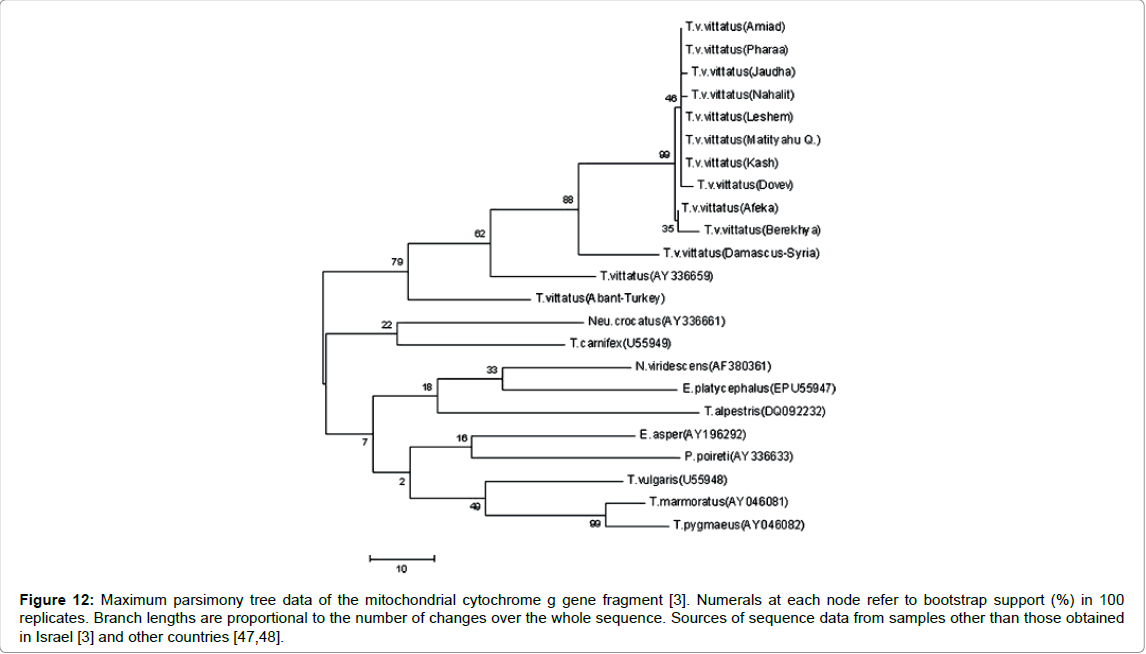
The variations in nucleotide sequences of the mitochondrial cytochrome gene and control region (D-loop) of T. v. vittatus larvae from different habitats along the area of its distribution from northern through central Israel have been described by Pearlson [3]. The results of this study support the analysis of results from RAPD [38]. The nucleotides of the D-loop and cytochrome b gene at the lowest altitude and greatest distance from other habitats are more divergent from the other newt populations (Figure 11). Moreover, the sequence analysis of mitochondrial DNA newts from the same species, T. vittatus from Syria (Damascus) and from Turkey (European part,) where the conditions seem to be less extreme or different than in Israel, is very different from Israel’s populations (Figure 12).
Figure 12: Maximum parsimony tree data of the mitochondrial cytochrome g gene fragment [3]. Numerals at each node refer to bootstrap support (%) in 100 replicates. Branch lengths are proportional to the number of changes over the whole sequence. Sources of sequence data from samples other than those obtained in Israel [3] and other countries [47,48].
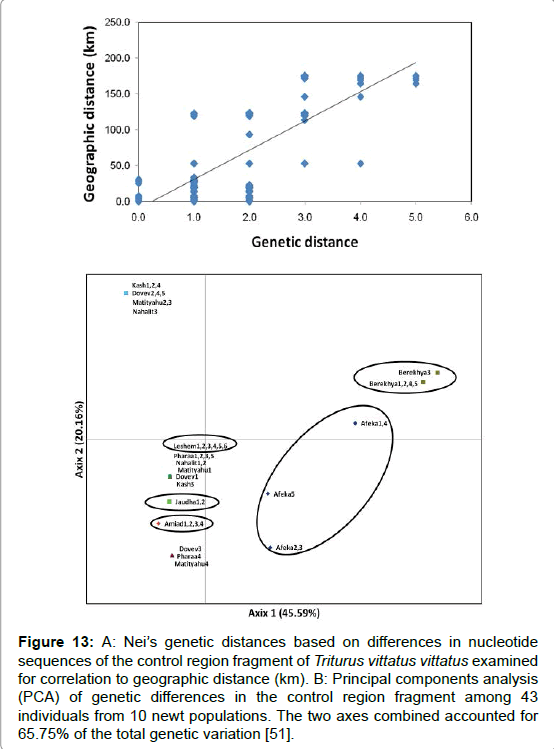
It is difficult to separate between geographical distribution and variation phenomena to various habitats based on variation in nucleotide sequences collection of T. v. vittatus in Israel. The analysis of these data by correlation between genetic distances to the geographical distribution (Figure 13) might show if there are genetic differences among those habitats. However, in some cases, habitats that were found very close to one another had different molecular markers as was found between habitats located far away from each other. The great distance might show that there is no, or very low, genetic flow among habitats, therefore the newts selected different ecological conditions.
Figure 13: A: Nei’s genetic distances based on differences in nucleotide sequences of the control region fragment of Triturus vittatus vittatus examined for correlation to geographic distance (km). B: Principal components analysis (PCA) of genetic differences in the control region fragment among 43 individuals from 10 newt populations. The two axes combined accounted for 65.75% of the total genetic variation [51].
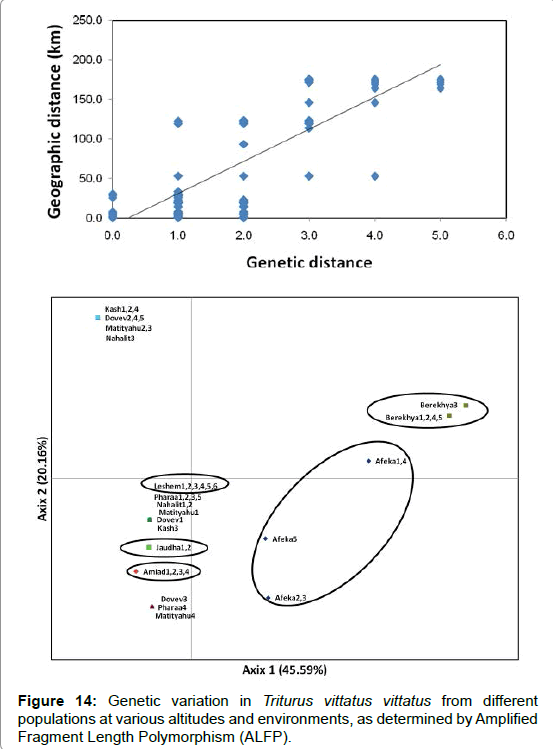
Amplified Fragment Length Polymorphism (ALFP) methods (unpublished data) support the hypothesis that the southern populations found under more extreme conditions are less variable, however, additional studies are required in order to support this hypothesis (Figure 14).
Discussion
The ecology of the adaptation of Triturus vittatus vittatus in natural habitats refers to its study in natural systems, emphasizing the interdependence on the ecological system, other elements and characteristics of the animal. In the present paper, we will consider the relatively many different characteristics from different fields of biology of T. v. vittatus to adaptation to various environments under relatively extreme conditions by comparing these characteristics to the various altitudes, habitats and climates in Israel.
Fewer studies on the adaptation of newts T. v. vittatus at the southern border of its distribution have been carried out compared to Salamandra infraimmaculata of other Urodela in Israel [39]. The distribution of this species in Israel is much less than newts and does not reach the very extreme conditions like T. v. vittatus. T. v. vittatus exist in all S. infraimmaculata habitats [5,40-42].
The T. v. vittatus population in this area is concentrated mainly around rain pools that are unstable breeding places. T. v. vittatus is a relatively small Urodela (Table 2) compared to S. infraimmaculata, for example [40,43]. No studies have been carried out on morphological variation among the various populations as was done for S. infraimmaculata [30]. The genetic consequences of habitat fragmentation are an important component of population extinction risk assessment for threatened and endangered T. v. vittatus, similar to what was found in other amphibians in the same area [5,9,10,16,17,39,40,44]. The genetic variation is also described in some Anurans, e.g., tree frog (Hyla savignyi) [45], Pseudepidalea Viridis (Syn. Bufo Viridis), Spadefoot Toads (Pelobates syriacus, Boettger, 1869) [19] and Rana bedreagae [19-22].
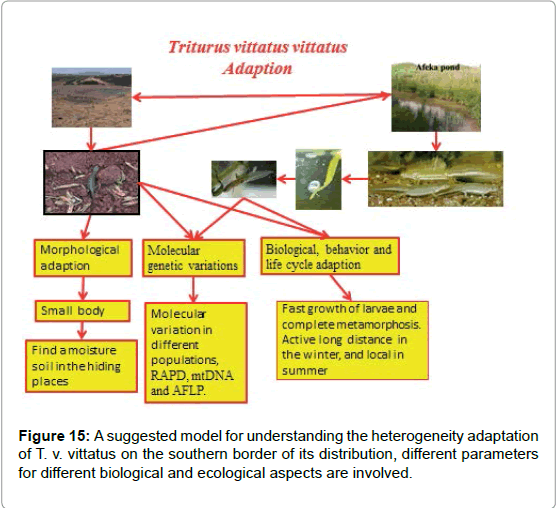
In summary, the adaptation of newts (T. v. vittatus) to xeric habitats in the proposed model (Figure 15) is based on more than 30 papers (cited here) and unpublished data showing the involvement of ecological ability adaptation of this species under extreme conditions. There is not only a connection between the ecological and biological adaptation of the different parameters, but also between the various biological parameters interacting with one another. However, no significant differences were found in the ecological conditions in the aquatic phase and larvae growth in various habitats [6,7,46] compared to the terrestrial phase of the newts. There are variations in the time of the breeding and spawning periods and larvae growth but not in ecological conditions [8]. Therefore, my hypothesis is that the adaptation and selection among the populations are stronger in the terrestrial phase compared to the aquatic phase in S. infraimmaculata [39]. Most of the breeding places of newts were winter ponds, and the larvae growth periods were relatively short [6-10,16,17,31,46-52]. A summary of this paper proposed the suggested model (Figure 15) for understanding the heterogeneity adaptation among T. v. vittatus populations on the southern border of its distribution in Israel, and different parameters for different biological and ecological aspects are involved. However, greater information on the terrestrial phase will provide us with a better understanding of the adapted of the xeric habitats.
Conclusion
The conclusion to this manuscript, which describes many aspects of the adaptation of T. v. vittatus to extreme conditions, is that this sub-species is in danger of annihilation, especially due to its complex life under extreme conditions in habitats at the southern border of its distribution. In order to protect this species, terrestrial and aquatic habitats must be conserved. This paper emphasizes important parameters of T. v. vittatus adaptations.
References
- Litvinchuk SN, Zuiderwijk A, Borkin LJ, Rosanov JM (2005) Taxonomic status of triturus vittatus (amphibia: Salamandridae) in western Turkey: Trunk vertebrae count, genome size and allozyme data. Amphibia-Reptilia, 26: 305-323.
- Olgun K, Tok V, Arntzen JW, Turkozan O (1997) The taxonomic status of the banded newt (triturus vittatus) in southern Turkey. The Herpetol J 7: 169-171.
- Pearlson O, Bluestein L, Snir S, Goldberg D, Degani G (2010) Molecular variation in triturus vittatus vittatus (urodela) from breeding sites near the southern extremity of its distribution revealed by DNA sequencing of mitochondrial cytochrome b gene and control region. Current Herpetol 29: 11-22.
- Degani G, Mendelssohn H (1983) The habitats, distribution and life history of triturus vittatus vittatus (jenyns) in the Mount Meron area (Upper Galilee, Israel). British J of Herpetol 6: 317-319.
- Degani G, Kaplan D (1999) Distribution of amphibian larvae in Israeli habitats with changeable water availability. Hydrobiol 405: 49-56.
- Pearlson O, Degani G (2007) Molecular DNA variations among triturus vittatus vittatus (urodela) from different breeding sites at the southern limit of its distribution. Acta Herpetol 2: 69-77.
- Pearlson O, Degani G (2007) Triturus v. vittatus (urodela) larvae at various breeding sites in Israel. Progrese ?i Perspective in Medicina Veterinar? - Lucr?ri ?tiin?ifice 50: 214-226.
- Pearlson O, Degani G (2008) The life history of triturus v. vittatus (urodela) in various habitats. Asiatic Herpetol Research 11: 91-95.
- Goldberg T, Eviatar E, Degani G (2009) Sequence analysis of mitochondrial DNA in salamandra infraimmaculata larvae from populations in northern Israel. South American J of Herpetol 4: 268-274.
- Goldberg T, Nevol E, Degani G (2009) Breeding site selection according to suitability for amphibian larval growth under various ecological conditions in the semi-arid zone of northern Israel. Ecologia Mediterranea 35: 65-74.
- Warburg MR (1971) The water economy of Israel amphibians: The urodeles triturus vittatus (jenyns) and salamandra salamandra (l). Comparative Biochem and Physiol 40A: 1055-1063.
- Degani G (1982) Amphibian tadpole interaction in a winter pond. Hydrobiol 96: 3-8.
- Degani G (1982) The response to substrate moisture of triturus v. Vittatus (jenys) (amphibia, urodela). Biol. Behav 3: 215-220.
- Warburg MR (1988) Adaptation of amphibians to life in xeric habitats. In: Ecophysiology of desert vertebrates, Ghosh PK and Prakash I (Eds.). Scientific, Jodhpur, India p: 59-98.
- Degani G (2015) The effect of light and soil moisture on the environmental behavior of newts (triturus vittatus vittatus, urodela). Open J of Animal Sci 5: 411-417.
- Degani G (1986a) Growth and behavior of six species of amphibian larvae in a winter pond in Israel. Hydrobiolo 140: 5-10.
- Degani G (1986b). Plasma protein and morphology of salamandra salamandra in Israel. Amphibia-Reptilia 7: 105-114.
- Geffen ES, Gafny, Gasith A (1987) Contribution to the knowledge of the biology of the banded newt, triturus vittatus vittatus, in rainpools in Israel. Israel J of Zool 34: 213-223.
- Degani G (2013) Genetic variation among various populations of spadefoot toads (pelobates syriacus, boettger, 1869) at breeding sites in northern Israel. Adv in Bio Chem 3: 440-447.
- Degani GT, GoldbergT, Yom-Din S (2013) The ecology and variation in DNA of rana bedreagae from various breeding sites in northern Israel. Res Open J Anim Sci P: 1-14.
- Degani G, Pearlson O, Goldberg T (2013) Impermanent breeding site selection - fitness of gonadal cycle and larval growth of triturus vittatus vittatus (urodela) from the southern limit of its distribution. Amer Open Anim J 1: 16-30.
- Degani G, Goldberg T, Gazith A, Elorom E, Nevo E (2013) The DNA variation of pseudepidalea viridis (syn. Bufo viridis) from various habitats. Zool Studies 52: 1-15.
- Kutrup B, Yilmaz N (2005) Food of the banded newt, triturus vittatus ophryticus (berthold, 1846), at different sites in Trabzon. Turkish J of Zool 29: 83-89.
- Pearlson O, Degani G (2011) Water and ecological conditions of striped newt, triturus v. vittatus (urodela) breeding sites at various altitudes near the southern limit of its distribution. Herpetol Romanica 5: 27-42.
- Zajc I, Arntzen JW (2000) Evolutionary relationships among European newts (genus triturus) as inferred from two mtdna fragments. Pflügers Archiv: European J of Physiol 439: 21-22.
- Weisrock DW, Papenfuss TJ, Macey JR, Litvinchuk SN, Polymeni R, et al. (2006) A molecular assessment of phylogenetic relationships and lineage accumulation rates within the family salamandridae (amphibia, caudata). Molecular Phylogenetics and Evolution 41: 368-383.
- Wielstra B, Beukema W, Arntzen JW, Skidmore AK, Tosopes AG, Rase N (2012) Corresponding mitochondrial DNA and miche divergence for crested newt candidate species. PLos One 7: 1-5.
- Arntzen JW, BeukemaW, Galis F, Ivanovic A (2015) Vertebral number of highly evolabale in salamanders and newts (fire salamandrae) variably associated with climatic parameters. Contributions to Zool 84: 85-113.
- Tixera J, Marinex-Solno I, Bucklev D, Tarroso P, Garcia-Paris M, et al. (2015) Genealogy of the nuclear β-fibrinogen intron 7 in lissotriton boscae (caudata, salamandridae): Concordance with mtdna and implications for phylogeography and speciation. Contributions to Zool 84: 1993-2015.
- Veith M, Degani G, Seitz A (1992) High genetic homogeneity of salamandra salamandra (l.) in Israel Zoologischer Anzeiger 229: 63-72.
- Degani G, Jackson K, Dosoretz C, Plotzky Y (1999) Molecular DNA variation in salamandra infraimmaculata from different habitats. Israel J of Zool 44: 239-246.
- Goldberg T, Nevol E, Degani G (2011) Genetic diverseness and different ecological conditions in salamandra infraimmaculata larvae from various breeding sites. Animal Biology Journal, 2: 37-49.
- Goldberg T, Degani G, Gazith A, Elron E, Nevo E (2011) Sequence variation in the mitochondrial DNA of pseudepidalea viridis (syn. Bufo viridis) in Israel. Bulletin UASVM Animal Science and Biotechnol 51-57.
- Goldberg T, Pearlson O, Nevo E, Degani G (2007) Mitochondrial DNA analysis of salamandra infraimmaculata larvae from habitats in northern Israel.
- Goldberg T, Nevo E, Degani G (2010) Genetic variation in salamandra infraimmaculata from various breeding sites - a model for habitat selection. Asian Herpetological Research 1: 1-9.
- Wielstra B, Duijm E, Lagler P, Lammers Y, Meilink WRM, et al. (2014) Parallel tagged amplicon sequencing of transcriptome-based genetic markers for triturus newts with the ion torrent next-generation sequencing platform. Molecular Ecology Resources 14: 1080-1089.
- Mikulicek P, Pialek J (2003) Molecular identification of three crested newt species (triturus cristatus superspecies) by rapid markers. Amphibia-Reptilia 24: 201-207.
- Pearlson O, Jackson K, Degani G (2007) The gonadal cycle in males and females of triturus vittatus vittatus (urodela) from the southern limit of its distribution. Progressses and Perspective in Veterinary Medicine p: 227-233.
- Degani G (2017) Ecological, biological and genetic adaptation to xeric habitats of salamandra infraimmaculata on the southern border of its distribution. Open J of Animal Sci 7: 70-92.
- Degani G (1996) The salamander at the southern limit of its distribution. Israel: Laser Pages Publishing.
- Warburg MR (2007) Longevity in salamandra infraimmaculata from Israel with a partial review of life expectancy in urodeles. Salamandra 43: 21-34.
- Bar-David SO, Segev N, Peleg N, Hill AR, Templeton C, et al. (2007) Long distance movements by fire salamanders (salamandra salamandra infraimmaculata) and implications for habitat fragmentation. Israel J of Ecol and Evol 53: 179-196.
- Eiselt J (1958) Der feuersalamander, salamandra salamandra ge zubreit (l.) einer taxonomischen synthese. Abh Ber Naturkde Vorgesch Magdeburg 10: 77-154.
- Degani GT, Goldberg T, Nevo E (2014) Genetic variation in salamandra infraimmaculata from different habitats using amplified fragment length polymorphism. J Biophys Chemist 5: 54-66.
- Degani G, Nagar R, Yom-Din S (2012) Molecular DNA variation in hyla felixarabica, Herpetol. Romanica 64: 651-667.
- Pearlson O (2011) Ecology and genetic variance of the banded newt triturus vittatus vittatus in northern Israel. Department of Evolutionary and Environmental Biology, University of Haifa 1-127.
- Caccone A, Milinkovitch MC, Sbordoni V, Powell JR (1997) Mitochondrial DNA rates and biogeography in European newts (genus euproctus). Molecular Systems Biol 46: 126-144.
- Garcia-Prais M, Aramo B, Herreo P (2001) Molecular characterization of the contact zone between triturus pygmaeus and t. Marmoratus (caudata: Salamandridae) in central Spain and their taxonomic assessment. Rev Esp Herp 15: 115-126.
- Progrese ?i Perspective in Medicina Veterinar?”- Lucr?ri ?tiin?ifice 23-31.
- Halliday T (1990) The evolution of courtship behavior in newts and salamanders. Advances in the Study of Behavior 19: 137-169.
- Pearlson O (2012) Ecology and genetic variance of the banded newt triturus vittatus vittatus in northern Israel. University of Haifa 1-135.
- Staniszewski MS (1995) The spectacular male of the south-eastern Turkish triturus vittatus cilicensis from the adana region of south east Turkey. Amphibians in Captivity 3: 1-4.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 4654
- [From(publication date):
April-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 3830
- PDF downloads : 824