Review Article Open Access
Eco-friendly and Cost-effective Use of Rice Straw in the Form of Fixed Bed Column to Remove Water Pollutants
Baljinder Singh*, Vasundhara Thakur, Garima Bhatia, Deepika Verma and Kashmir Singh
Department of Biotechnology, Panjab University, Chandigarh, India
- *Corresponding Author:
- Dr. Baljinder Singh
Assistant Professor, Department of Biotechnology
Panjab University, Chandigarh-160 014, India
Tel: +911722534085
Fax: +911722541409
E-mail: gilljwms2@gmail.com (or) sbaljinder@pu.ac.in
Received Date: July 20, 2016; Accepted Date: October 28, 2016; Published Date: October 31, 2016
Citation: Singh B, Thakur V, Bhatia G, Verma D, Singh K (2016) Eco-friendly and Cost-effective Use of Rice Straw in the Form of Fixed Bed Column to Remove Water Pollutants. J Bioremediat Biodegrad 7:374. doi: 10.4172/2155-6199.1000374
Copyright: © 2016 Singh B, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
We investigated the removal of three pollutants: methylene blue (MB), phosphorus, and nickel (Ni [II]) from water by using modified rice straw powder (RSP) and fixed-bed column adsorption technique. The experiments were conducted in single and binary solutions to study the effects of initial pollutant concentration and column bed depth on adsorption. It was observed that the maximum adsorption capacity of RSP for MB, phosphorus, and Ni (II) was 21.99, 4.22, and 4 mg/g, respectively. In the MB-phosphorus binary solution, the presence of one pollutant did not affect the adsorption of other pollutants. In the Ni (II)-MB binary solution, exhaustion time significantly decreased for Ni (II) adsorption; however, it increased for MB adsorption. The adsorption mechanism was analysed by using the Adams-Bohart, Thomas, and Yoon and Nelson models for describing the column's dynamic behaviour. The results indicated that the Thomas model was very suitable for RSP column design.
Keywords
RSP; Fixed bed column; Pollutants; Adsorption
Introduction
Safe drinking water is a necessity for the humankind. Unfortunately, more than 1 billion people worldwide do not have access to safe drinking water; among these, 800 million live in rural areas [1]. Effluents of many industries generate multi-component wastewater containing metals, dyes, and phosphorus leading to the need for complicated treatment processes. The elimination of effluents containing these three types of pollutants needs immediate attention because of their potential toxicity and aesthetic problems. Presently, over more than 100,000 types of dyes with 700,000 tons of dyestuffs are generated annually; further, based on their structure, these can be categorized as anionic and cationic. Methylene blue (MB) (3, 7-bis (dimethylamino)-phenothiazin-5- iumchloride), a thiazine cationic dye, is used in dying paper cottons, wools, silk, wood, and temporary hair colorant [2,3]. Initially, MB was not considered hazardous; however, a recent study has indicated several harmful effects [4].
Phosphorus plays a critical role in the growth and development of living organisms. However, high concentrations of phosphorus released from industries into receiving water bodies lead to phosphorus pollution, which is known as eutrophication. Therefore, the removal of phosphorus from water is necessary to avoid water deterioration.
The conventional methods used for removal of these pollutants (MB and phosphorus) are coagulation and flocculation, biological oxidation, chemical precipitation, and activated carbon adsorption. These technologies are expensive and can result in the generation of toxic sludge that poses another serious problem. Therefore, there is a growing interest in using low cost and commercially available materials (agricultural wastes) for pollutant adsorption [5-7]. Several research studies have demonstrated adsorption of MB and phosphorus by using agro-waste materials [8-10].
Rice (Oryza sativa L.) is the world’s largest cereal crop, and it produces large amounts of crop residues such as rice straw. Only about 20% of rice straw is used in the production of ethanol, paper, fertilizers, and fodders. Rice straw burning is a common post-harvest practice in many countries such as India, Egypt, Malaysia, Thailand, etc., which causes air pollution called the "Black Cloud" [11]. Rice straw mainly consists of cellulose, lignin, hemicellulose, and a small amount of mineral residues. It has many characteristics, which make it a potential adsorbent; it has binding sites that are able to remove metals from aqueous solutions.
Some efforts have been made to remove metals and dyes simultaneously [12]. Therefore, we have investigated the removal efficiencies for the two pollutants in co-pollutant solutions. The main objective of this study was to develop an innovative, cost-effective, and sustainable biosorbent for the removal of three pollutants (MB, phosphorus, and Ni [II]) using rice straw powder (RSP). The study was an extension of the previous studies where Ni (II) was removed using RSP [11]. We additionally investigated the adsorption efficiency of Ni (II) along with MB in single and binary solutions using RSP via columnbased studies. The influential factors (for example, initial concentration, and bed depth) were investigated for process optimization. The adsorption mechanisms based on fixed columns were further analysed using the Adams-Bohart, Thomas, and Yoon and Nelson kinetic models for describing the dynamic behaviour of the column.
Materials and Methods
RSP
The rice straw was collected from a crop field area of Patiala, India. It was thoroughly washed with de-ionized water and heated in oven at 50°C for 90 h to remove all the moisture present in the material. Ovendried straw was crushed, grounded, and sieved to the desired mesh size (300-500 μm) for use.
Modification of RSP
RSP was treated separately in two ways; first, with 0.1 M solution of sodium hydroxide (NaOH) and second, with solutions containing 0.1 M NaOH and 0.25 M ferric chloride (FeCl3). Each resulting mixture (RSP and treated solution) was taken in a beaker provided with a lid and stirred for 12 h. The resulting products were washed with deionized water and dried for 48 h at 60°C. These were named as treated RSP (TRSP) and iron loaded RSP (ILRSP), respectively.
Preparation of solutions
Stock solutions (1000 mg/L) of MB, phosphorus, and Ni (II) used in the study were prepared by dissolving an appropriate weight of dye, disodium hydrogen phosphate (Na2HPO4), and pure salt of NiSO4 in the desired volume of de-ionized water, respectively. The working solutions for MB (100 and 200 mg/L), phosphorus (20 and 40 mg/L), and Ni (II) (75 ppm) were prepared using stock solutions. The pH of all the solutions was adjusted to 7.0 by using 1 M HCl and 0.1 M NaOH solutions.
Fixed-bed column
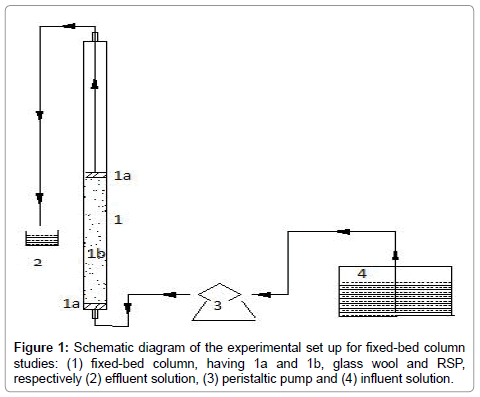
Continuous-flow sorption experiments were performed in a glass column designed with an internal diameter of 4 cm and length of 60 cm. At the bottom of the column, a 0.5-mm stainless sieve was attached followed by glass wool to ensure uniform liquid distribution (Figure 1).
Adsorption study with TRSP column: Column was packed with 1.5 g and 2 g of TRSP to obtain a particular bed height of the adsorbent (equal to 2, and 2.5 cm of bed depth, respectively) keeping the flow rate constant (10 mL/min). Five experiments were conducted. 1) In the first experiment, the varied influent (MB) concentration (100 and 200 mg/L) was pumped from the container to the fixed-bed column by a peristaltic pump. 2) In the second experiment, a fixed influent concentration of phosphate (20 mg/L) was loaded at a specific flow rate (10 mL/min) and bed depth (2 cm). 3) In the third experiment, binary solution with MB concentration of 100 mg/L and phosphate concentration of 20 mg/L were loaded at a specific flow rate (10 mL/min) and bed depth (2 cm). 4) In the fourth experiment, a fixed influent concentration of Ni (II) (75 mg/L) was loaded at a specific flow rate (10 mL/min) and bed depth (2 cm). 5) In the fifth experiment, binary solution with MB concentration of 100 mg/L and Ni (II) concentration of 75 mg/L were loaded at a specific flow rate (10 mL/min) and bed depth (2 cm). Effluents were collected at regular time intervals to determine the concentration of all the three pollutants. Flow to the column was continued until there was no further adsorption. The concentration of phosphorus was determined using modified molybdenum blue method [13]. Ni (II) concentration was analysed using atomic absorption spectrophotometry (AAS) using a GBC AVANTA GF 5000 model. MB was analysed at 667 nm by using UV-visible spectrophotometer (HITACHI U-2900). Analysis for Ni (II), phosphorus, and MB in binary solutions was performed by appropriate dilution.
Adsorption study with ILRSP column: A column was packed with a known amount of ILRSP (such as, 4 and 4.5 g) with different bed depths (such as, 2 and 2.5 cm). The first experiment was conducted using influent phosphate concentrations of 20 and 40 mg/L with a fixed flow rate (10 mL/min). In the second experiment, the influent concentration of MB (100 mg/L) was loaded at a fixed flow rate (10 mL/ min) and bed depth (2 cm). In the third experiment, a binary solution with MB concentration of 100 mg/L and concentration of phosphate (20 mg/L) was loaded at a flow rate of 10 mL/min and a bed depth of 2 cm.
Analysis of fixed-bed column data
Various calculations related to the column data were performed as described in the previous study [11]. Breakthrough point is the point that effluent concentration (Ct) reaches about 0.1% of the influent concentration (C0). The corresponding time is breakthrough time (tb). When the effluent concentration reaches 95% of the influent concentration, it is exhaustion point, and the time is exhaustion time (te).
Breakthrough curves
A successful design of column adsorption process/method mainly requires prediction of the breakthrough curve for the effluent [11]. Typically, a breakthrough curve is a plot of Ct/C0 as a function of time (t) or volume of effluent (Veff, mL), where Ct and C0 (mg/L) are the concentration of the adsorbate in the effluent and influent, respectively. The breakthrough point is reached when the bed becomes saturated with the adsorbate. The breakthrough point is defined as the time when Ct equals 5% of C0 . After reaching the breakthrough point the concentration of the adsorbate increases rapidly until it reaches the exhaustion point, where the column approaches complete saturation.
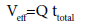 (1)
(1)
The value of the total mass of pollutants adsorbed, qtotal (mg):
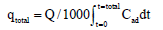 (2)
(2)
Equilibrium pollutants uptake or maximum capacity of the column:
qeq (mg/g), in the column is calculated as the following:
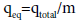 (3)
(3)
where m is the dry weight of adsorbent in the column (g), that is, 2 g (constant for all concentrations).
Total amount of metal ion entering column (mtotal) is calculated using the following equation:
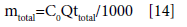 (4)
(4)
The removal percentage of pollutants can be obtained from Eq.
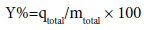 (5)
(5)
The removal efficiency of pollutants was calculated by:
Removal efficiency (%) = C0 - Ct / C0 × 100 (6)
where C0 and Ct are the original and residual pollutants concentrations in solution, respectively.
Modelling of the breakthrough curve
Predictive kinetic modelling of the effluent breakthrough curves is essential for the effective design of an adsorption column. This is because of the fact that the experimental evaluation of the performance of a column under different operating conditions is tedious and expensive. Several simple mathematical models have been developed for describing and analysing the lab-scale column studies for the purpose of industrial applications. Adams-Bohart, Thomas, and Yoon-Nelson models were performed to identify the best model for predicting the dynamic behaviour of the column.
Adams-Bohart model: Bohart and Adams [15] described the relationship between Ct /C0 and t in a continuous system. It is used for describing the initial part of the breakthrough curve. The expression is as follows:
ln(Ct/C0)=kABC0t-kABN0(Z/U0)
Where C0 and Ct are the influent and effluent concentrations (mg/L), kAB is the kinetic constant (L/mg/min), N0 is the saturation concentration (mg/L), Z is the bed depth of the fix-bed column (cm), and U0 is the superficial velocity (cm/min) defined as the ratio of the volumetric flow rate Q (cm3/min) to the cross-sectional area of the bed A (cm2).
Thomas model: The Thomas model [16] assumes plug flow behaviour in the bed. This is the most general and widely used model to describe the performance theory of the sorption process in fixedbed column. The linearized form of this model can be described by the following expression:
ln (C0/Ct-1)=kThq0m/Q-kthC0t
where kTh is the Thomas model constant (mL/min/mg), q0 is the adsorption capacity (mg/g), and t stands for total flow time (min).
The Yoon-Nelson model: It was used to describe the column adsorption data. Use of this model could minimize the error resulting from the use of the Thomas model, especially at lower or higher time periods of the breakthrough curve. The Yoon-Nelson model [17] is based on the assumption that the rate of decrease in the probability of adsorption for each adsorbate molecule is proportional to the probability of adsorbate adsorption and the probability of adsorbate breakthrough on the adsorbent. The linearized Yoon-Nelson model for a single component system can be expressed as follows:
ln(Ct/C0-Ct)=kYNt-τkYN
where kYN is the rate constant (min-1), and τ is the time required for 50% adsorbate breakthrough (min).
Results and Discussion
TRSP fixed-bed column
Adsorption of MB: Experiments with columns packed with TRSP (bed depth, 2 cm) were conducted under the following conditions: fixed influent concentration of MB, 100 mg/L; flow rate, 10 mL/min; and bed depth, 2 cm. The results are shown in Table 1. The maximum adsorption capacity (qeq) of RSP for MB was 21.99 mg/g, which is much higher than that of previous reported adsorbents in flask-level experiments, such as rice husk, wheat bran, guava seed, neem leaf powder, coconut coir, banana peel, spent rice biomass, and orange peel; however, it is less than some agricultural by-products such as pineapple stem, mango seed kernel, swede rape straw, pine cone, and coffee husk. These results have been thoroughly reviewed [18,19]. Moreover, the maximum adsorption capacity of MB was much higher than that of previous reported adsorbents such as cotton-alk (0.024 mg/g) [20]. Fourier transform infrared (FTIR) analysis of the biomass was conducted to identify possible interactions of pollutants and results of spectra are shown in Supplementary Figures 1 and 2.
| Sample | Column | C0 mg/L |
QmL/min | Z (cm) |
ttotal (min) |
Veff (mL) |
mtotal (mg) |
qtotal (mg) |
qeq (mg/g) |
Y(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| MethyleneBlue | TRSP | 200 | 10 | 2 | 60 | 600 | 120 | 116 | 29 | 96.67 |
| 100 | 10 | 2 | 90 | 900 | 90 | 87.96 | 21.99 | 97.73 | ||
| 100 | 10 | 2.5 | 110 | 1100 | 110 | 109 | 27.25 | 99.08 | ||
| ILRSP | 100 | 10 | 2 | 30 | 300 | 30 | 15.9 | 3.975 | 53 | |
| Phosphate | ILRSP | 40 | 10 | 2 | 50 | 500 | 20 | 18 | 4.5 | 90 |
| 20 | 10 | 2 | 90 | 900 | 18 | 16.88 | 4.22 | 93.79 | ||
| 20 | 10 | 2.5 | 100 | 1000 | 20 | 18.8 | 4.7 | 94 | ||
| TRSP | 20 | 10 | 2 | 30 | 300 | 6 | 3.15 | 0.7878 | 52.5 | |
| Nickel | TRSP | 75 | 10 | 2 | 50 | 500 | 37.5 | 16 | 4 | 42.66 |
Table 1: Parameters in fixed-bed column for MB, phosphorus, and Ni (II) adsorption in modified RSP. Co - the influent concentration, mg/L, Q - the volumetric flow rate, cm3/min, Z- the bed depth of the fix-bed, ttotal- the total flow time, min, Veff - the effluent volume, mL, mtotal - total amount of pollutants sent to column, g, qtotal- the total mass of pollutant adsorbed, mg, qo -equilibrium metal uptake or maximum capacity of the column, mg/g, Y-the removal per cent of Ni (II) ions, %.
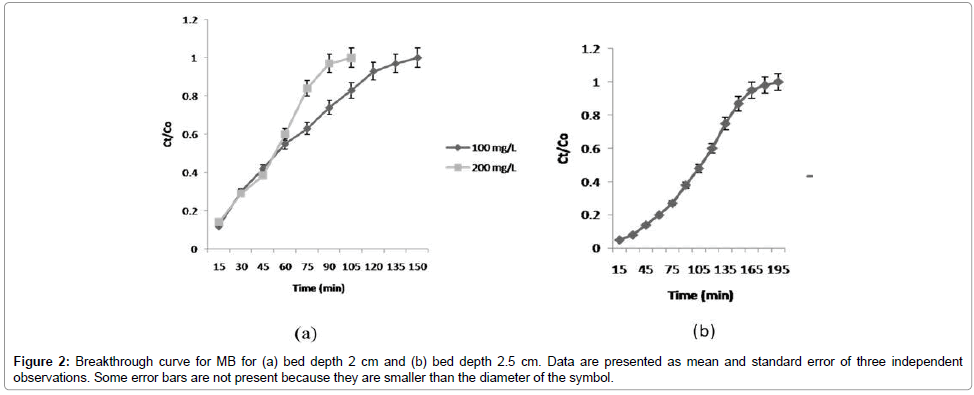
Effect of MB concentration on % Removal efficiency: The effect of initial MB concentration on breakthrough curves is shown in Figure 2a. The breakdown time and exhaustion time increased with decreasing initial MB concentration. Change in the initial MB concentration had a significant effect on the breakthrough curve. It was obvious that the breakthrough curves became sharper while the breakthrough time and exhaustion points became shorter with increasing concentration of MB. This can be explained by the fact that RSP is more saturated at initial high MB concentration; a lower concentration gradient caused a slower transport owing to a decrease in the diffusion coefficient or mass transfer coefficient. These results are similar to that reported in previous studies [19,21].
Effect of bed depth on % removal efficiency of MB: The effect of bed depth (2.0 and 2.5 cm) on the adsorption of MB in columns was investigated under a given condition. Results showed that adsorption quantities and capacities in both the columns increased with increasing bed depth. The increase in MB uptake capacity with increasing contact time in the fixed bed column is likely to be because of an increase in the surface area, which provides more binding sites for column adsorption [11]. Figure 2b shows that the % removal efficiency of MB in the column increases with the increase in the mass of adsorbent (rice straw). This is because of more adsorbent in the fixed-bed column, which provides more binding sites and larger surface area for the removal of phosphorus and MB.
Adsorption of phosphorus: The column was packed with TRSP, and a fixed influent concentration of phosphate (20 mg/L) was loaded at a specific flow rate (10 mL/min) and bed depth (2 cm). The results are shown in Table 1. Despite the differences in nature and chemical composition of MB and phosphorus, the results of the present study showed adsorption capacity (qeq) 0.7875 mg/g of TRSP towards phosphorus. These results showed that TRSP was not much efficient for remediating phosphorus from aqueous solutions. This might be because of the available sites on the adsorbent for the dye that led to ionic interactions and further resulted in colour removal of dye.
Adsorption of MB and phosphorus in binary solution: Owing to the differences in the chemical properties of the two pollutants, the results for binary solutions remained the same as for a TRSP column (Table 1). The results showed that the presence of one pollutant does not affect the removal efficiency of another pollutant or collision between pollutants and active sites of RSP. This might be due to different binding sites for the two pollutants with differences in their chemical nature. Therefore, TRSP can be used to remove low levels of phosphorus in industrial effluents containing both MB and phosphorus.
Adsorption of MB and Ni (II) in binary solution: To study the behaviour of co-pollutants (that is, MB and Ni) in a fixed-bed column, binary solutions containing two pollutants were prepared as influent solution under given conditions, that is, for Ni (II), 2 cm bed depth, 10 mL/min flow rate, 75 ppm concentration; and for MB, 2 cm bed depth, and 10 mL/min flow rate. It was observed that for Ni (II) adsorption in binary solutions on the column, the exhaustion time decreased from 50 to 32 min, and both adsorption quantities (qtotal from 16 to 10 mg) and capacities (qeq from 4 to 2.5 mg/g) also decreased. This might be due to more favourable adsorption of MB on TRSP than Ni (II), which was derived from ionic interaction between MB and RSP in a packed column. In contrast, the exhaustion time increased from 90 to 105 min for MB adsorption from binary solution on columns, and both adsorption quantities and capacities were also enhanced. This might be due to the presence of π-π conjugated electrons in the complex, which donates an electron to enhance the electron density to form stable complexes with the heavy metals [22].
ILRSP fixed-bed column
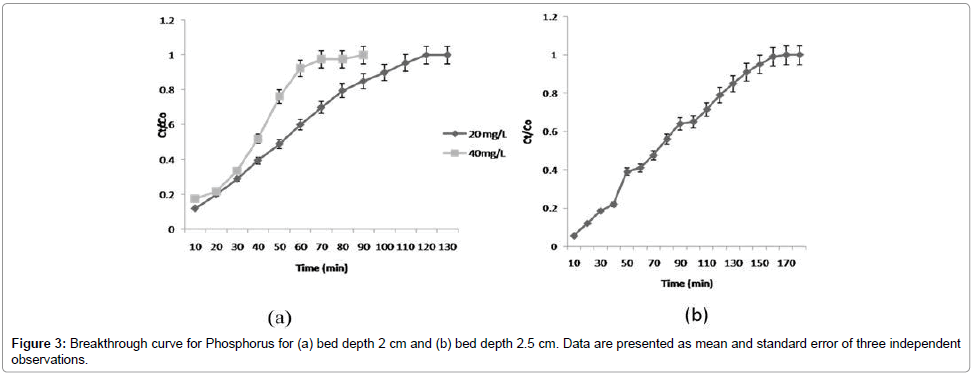
Effect of phosphorus concentration on % removal efficiency: The adsorption efficiency of columns was evaluated. Data are presented in Table 1. The maximum adsorption capacity of phosphorus was 4.22 mg/g (bed depth, 2 cm; with initial phosphorus concentration 20 mg/L). Meanwhile, the initial investigations showed that TRSP exhibited very low adsorption capacity (0.78 mg P/g (phosphorus per gram)). The presence of Fe (II) in the RSP played a decisive role in enhancing phosphorus capture. Treatment with FeCl3 considerably improved phosphorus retention of RSP. It was found that ILRSP was 5.41 times more efficient than TRSP in remediating phosphorus from aqueous solutions. Despite the differences in nature and chemical composition between RSP and okara, the results of the present study fitted well with the findings reported by Ngugen et al. [9] in a flask-level study. Figure 3a shows that the % adsorption decreased with increase in initial concentration of phosphorus. However, the uptake capacity increased with an increase in initial concentration, which might be due to the availability of a greater number of phosphate ions in solution.
Effect of bed depth on % removal efficiency of phosphorus: The effect of contact time on the removal of phosphate ions by ILRSP was studied by varying contact time for an initial concentration of 20 mg/L. Relationship between the contact time and removal efficiency of phosphate ions is shown in Figure 3b at a constant bed depth (2 cm and 2.5 cm). It is demonstrated that as time increases, concentration of effluent decreases, and removal efficiency increases [14]. This may be due to the increase in surface area, which provides more binding sites for column adsorption [11]. The increase in phosphate uptake capacity of ILRSP was observed as the bed depth increased with the highest bed capacity. This is due to increased RSP mass at higher bed depth that would provide a larger surface area and more anion fixations towards the active binding sites for the adsorption process leading to an increase in the volume of the treated effluent. These results revealed that the bed depth of 2 cm provided optimum % removal efficiency.
Generally, biosorbents do not show significant affinity towards phosphate ions because of the lack of available binding sites [23]. Therefore, to enhance the sorption capacity of phosphorus, researchers cationized biosorbents were impregnated with metal compounds [2,9]. The common metals used for this purpose include Fe (II, III), Zr (IV), La (III), Ce (III), and Zn (II). The base treatment of biosorbents is required for enhancing attachment of metals onto surface [8] because direct immersion of biosorbents into metal solutions usually does not have sufficient capacity for phosphate removal [23]. Many studies have confirmed the enhanced phosphorus removal of metal loaded biosorbents using agricultural by-products [6,8]. A summary of studies using metal loaded biosorbents for the decontamination of phosphorus was tabulated by Nguyen et al. [9].
Adsorption of MB in single solution and both MB and phosphorus in binary solution: Table 1 indicates that the maximum adsorption capacity (qeq) of MB was 3.975 mg/g in columns packed with ILRSP at fixed influent concentration of MB (100 mg/L), and a bed depth 2 cm. It means that ILRSP column was 5.53 times less efficient than TRSP column for remediating MB from aqueous solutions. This might be because of available sites on the adsorbent towards dye that leads to ionic interactions, which further results in the colour removal of dye.
Owing to the differences in chemical properties of the two pollutants, the results of binary solution remained same for ILRSP column (Table 1). The results showed that the presence of one pollutant did not affect the removal efficiency of another pollutant.
Breakthrough curve modelling
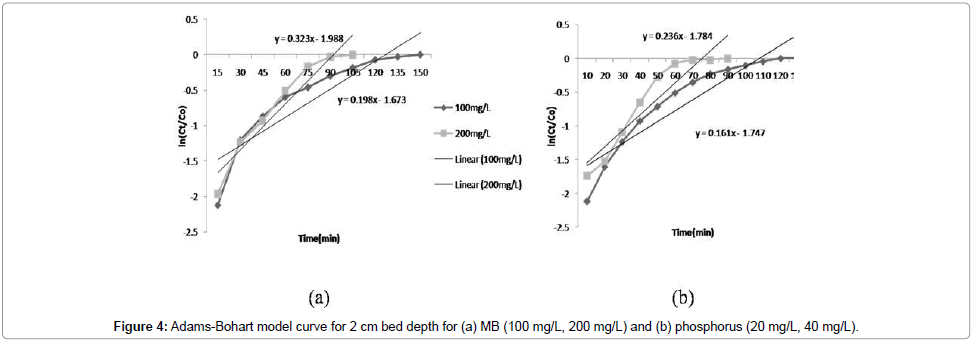
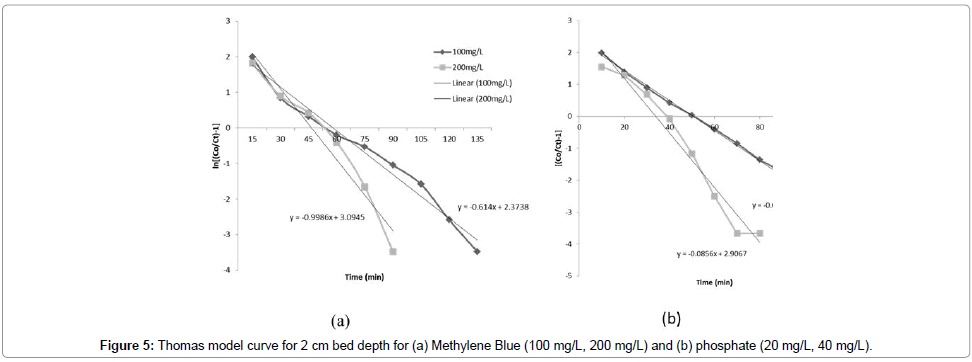
Adams-Bohart model: The constants kAB and N0 were calculated from the linear plot of ln(Ct/C0) against time (Figure 4a and 4b). Linear regression results and values of R2 for kinetic models are presented in Table 2. The values of kAB decreased with the increase in influent MB and phosphorus concentration; however, the values increased with increasing bed depth (Figure 3). It was indicated that the overall system kinetics was dominated by external mass transfer in the initial part of adsorption in the column [14]. Similar results were reported for phosphorus (Table 2). The value of R2 was more than 0.9 with initial influent MB concentration of 200 mg/L and bed depth 2 cm, which indicates that Adams-Bohart model describes the column data very well for MB.
| Breakthroughcurvemodels | Sample | C0(mg/L) | Q(ml/min) | Z(cm) | Parameters | ||
|---|---|---|---|---|---|---|---|
| Adam-Bohart | kAB(L/mg/min) | No(mg/L) | R2 | ||||
| MethyleneBlue | 100 | 10 | 2 | 1.98×10-4 | 2.78×103 | 0.811 | |
| 200 | 10 | 2 | 1.61×10-4 | 4.04×103 | 0.919 | ||
| 100 | 10 | 2.5 | 2.04×10-4 | 2.85×103 | 0.894 | ||
| Phosphate | 20 | 10 | 2 | 8.85×10-4 | 0.65×102 | 0.859 | |
| 40 | 10 | 2 | 5.90×10-4 | 0.99×102 | 0.879 | ||
| 20 | 10 | 2.5 | 6.99×10-4 | 0.78×102 | 0.81 | ||
| Thomas | kTH(mL/min/mg) | qo(mg/L) | R2 | ||||
| MethyleneBlue | 100 | 10 | 2 | 0.614×10-2 | 9.665×102 | 0.9775 | |
| 200 | 10 | 2 | 0.499×10-2 | 15.504×102 | 0.9528 | ||
| 100 | 10 | 2.5 | 0.583×10-2 | 16.217×102 | 0.9742 | ||
| Phosphate | 20 | 10 | 2 | 2.36×10-2 | 2.531×102 | 0.9949 | |
| 40 | 10 | 2 | 2.18×10-2 | 3.333×102 | 0.9654 | ||
| 20 | 10 | 2.5 | 1.95×10-2 | 3.710×102 | 0.9473 | ||
| Yoon-Nelson | kYN(min-1) | τ(min) | R2 | ||||
| MethyleneBlue | 100 | 10 | 2 | 0.614 | 3.8661 | 0.5842 | |
| 200 | 10 | 2 | 0.998 | 3.0988 | 0.5126 | ||
| 100 | 10 | 2.5 | 0.597 | 5.5384 | 0.5318 | ||
| Phosphate | 20 | 10 | 2 | 0.472 | 5.0651 | 0.4873 | |
| 40 | 10 | 2 | 0.586 | 3.3948 | 0.5278 | ||
| 20 | 10 | 2.5 | 0.391 | 7.4182 | 0.5953 | ||
Table 2: Parameters of Adam-Bohart model, Thomas model, and Yoon Nelson model under different condition. kAB - the kinetic constant, L/mg min, kTh - the Thomas model constant, mL/min mg, kYN - the rate constant, min-1, No - the saturation concentration, mg/L.
Thomas model: The values of kTh and q0 were determined from the linear plot of ln[(Co/Ct-1)] against t (Figure 5a and 5b). The Thomas model was applicable to the adsorption process, which indicates that the external and internal diffusions were not the limiting step [14,24]. It was observed that with an increase in the bed depth, the kTh values decreased (Table 2 and Figure 4). The values of kTh increased with increase in initial influent MB and phosphorus concentrations. Therefore, the higher influent concentration and bed depth would increase the adsorption of MB and phosphorus (single solution) using the TRSP and ILRSP columns, respectively. These model predictions agree well with the experimental results discussed earlier, which indicate that lower flow rates, higher influent concentrations, and higher bed heights enhance the adsorption ability of a fixed-bed column. Most values of R2 are more than 0.9, which indicates that the Thomas model describes the column data very well for both MB and phosphate. The Thomas model has been shown to be more suitable for cases where the adsorption process is not controlled by mass transfer [11].
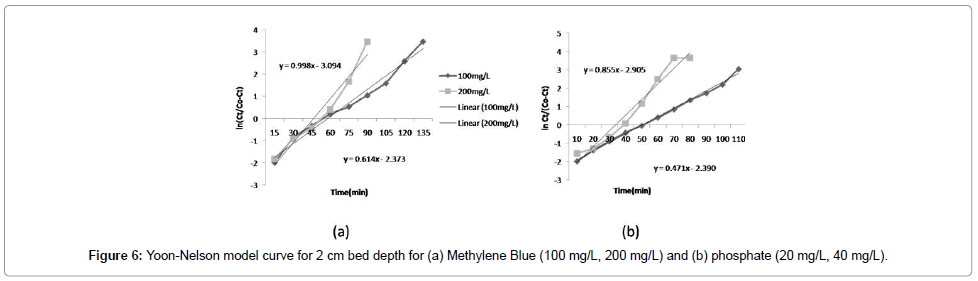
The Yoon-Nelson model: A linear plot of ln[Ct/(C0 -Ct)] against t determined the values of kYN and τ from the intercept and slope of the plot (as shown in the Figure 6a and 6b). Different statistical parameters of the Yoon-Nelson model were calculated and shown in Table 2. The kYN values decreased with increasing bed depth (Figure 5). Most values of R2 are more than 0.5, which indicate that compared to the Thomas model, the Yoon-Nelson model is not an appropriate for the breakthrough curves.
Comparison of all three models Adams-Bohart, Thomas, Yoon- Nelson model: From Figures 4-6, Table 1, and supplementary data, it was observed that the Thomas model is an appropriate model to describe a fixed-bed system (TRSP column for MB and ILRSP column for phosphorus). However, in the case of the Adams-Bohart and Yoon- Nelson models, low correlation coefficients (R2) were observed, which indicates that these models are not appropriate as predictors for the breakthrough curves. Thus, the Thomas model can be used to predict adsorption performance for the adsorption of both MB and phosphorus in a fixed-bed column.
Conclusion
In general, rice straw is an environment-friendly potential biosorbent for heavy metals. This work examined the efficiency of this sorbent for the removal of three important pollutants from single and binary solutions. The results indicate that several factors such as contact time, bed depth, and initial concentration affect the biosorption process. The physicochemical characteristics of wastewater from varying sources can be much more complex as compared to the aqueous metal solutions used in this study. Therefore, the effects of other components of wastewater on commercial pollutants' adsorption process should be determined. The two columns, TRSP and ILRSP, can be used in series to remove both these pollutants from the industrial effluents containing cationic dyes, heavy metals, and phosphorus. The saturated column was regenerated by 0.05 mol L-1 EDTA solution, and RSP could be reused in the removal of the three pollutants. Initial results showed the same removal efficiency after regeneration of column; however, further study under different conditions should be investigated. This work shows the possibility that rice straw may be a suitable material for the adsorption from aqueous solutions.
In this study, modified RSP was found to be an efficient biosorbent for sorption of MB, phosphorus, and Ni (II) in single and binary solutions. The adsorption capacity for a pollutant was increased with increasing bed height and initial dye concentration. It was found that Ni (II) adsorption was suppressed; however, MB uptake was enhanced in Ni (II)-MB binary solution in TRSP fixed-bed column. The column experimental data fitted well with the Thomas model; however, the Adams-Bohart and Yoon-Nelson models were found to be inapplicable.
Acknowledgements
The authors thank the Chairperson, Department of Biotechnology, Panjab University for providing necessary facilities.
References
- Sekhon GS, Singh B (2013) Estimation of heavy metals in the groundwater of Patiala district of Punjab, India. Earth Resources 1: 1-4.
- Han R, Wang Y, Zhao X, Wang Y, Xie F, et al. (2009) Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: experiments and prediction of breakthrough curves. Desalination 245: 284-297.
- Li Y, Du Q, Liu T, Peng X, Wang J, et al. (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chemical Engineering Research and Design 91: 361-368.
- Chen L, Ramadan A, LuÌÂ? L, Shao W, Luo F, et al. (2011) Biosorption of methylene blue from aqueous solution using lawny grass modified with citric acid. Journal of Chemical & Engineering Data 56: 3392-3399.
- Benyoucef S, Amrani M (2011) Removal of phosphorus from aqueous solutions using chemically modified sawdust of Aleppo pine (Pinus halepensis Miller): kinetics and isotherm studies. The Environmentalist 31: 200-207.
- Krishnan KA, Haridas A (2008) Removal of phosphate from aqueous solutions and sewage using natural and surface modified coir pith. Journal of Hazardous Materials, 152: 527-535.
- Riahi K, Thayer BB, Mammou AB, Ammar AB, Jaafoura MH (2009) Biosorption characteristics of phosphates from aqueous solution onto Phoenix dactylifera L. date palm fibers. Journal of hazardous materials 170: 511-519.
- Carvalho WS, Martins DF, Gomes FR, Leite IR, da Silva LG, et al. (2011) Phosphate adsorption on chemically modified sugarcane bagasse fibres. Biomass and Bioenergy 35: 3913-3919.
- Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, et al. (2013) Feasibility of iron loaded ‘okara’ for biosorption of phosphorus in aqueous solutions. Bioresource Technology 150:42-49.
- Xu X, Gao BY, Yue QY, Zhong QQ (2010) Preparation of agricultural by-product based anion exchanger and its utilization for nitrate and phosphate removal. Bioresource Technology 101: 8558-8564.
- Sharma R, Singh B (2013) Removal of Ni (II) ions from aqueous solutions using modified rice straw in a fixed bed column. Bioresource Technology 146: 519-524.
- Deng JH, Zhang XR, Zeng GM, Gong JL, Niu QY, et al. (2013) Simultaneous removal of Cd (II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chemical Engineering Journal 226: 189-200.
- Murphy JS, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. AnalyticaChimica Acta 27: 31-36.
- Chen S, Yue Q, Gao B, Li Q, Xu X, et al. (2012) Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. Bioresource Technology 113: 114-120.
- Bohart GS, Adams EQ (1920) Behaviour of charcoal towards chlorine.Journal of chemical Society 42: 523-529.
- Thomas HC (1944) Heterogeneous ion exchange in a flowing system.Journal of the American Chemical Society 66: 1466-1664.
- Yoon YH, Nelson JH (1984) Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. The American Industrial Hygiene Association Journal 45: 509-516.
- Mohammed MA, Shitu A, Ibrahim A (2014) Removal of methylene blue using low cost adsorbent: a review. Research Journal of Chemical Sciences 2231:491-102.
- Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Advances in Colloid and Interface Science 209: 172-184.
- Ding Z, Hu X, Zimmerman AR, Gao B (2014) Sorption and cosorption of lead (II) and methylene blue on chemically modified biomass. Bioresource Technology 167:569-573.
- Foo KY, Hameed BH (2012) Dynamic adsorption behaviour of methylene blue onto oil palm shell granular activated carbon prepared by microwave heating. Chemical Engineering Journal 203: 81-87.
- Gong JL, Zhang YL, Jiang Y, Zeng GM, Cui ZH, et al. (2015) Continuous adsorption of Pb (II) and methylene blue by engineered graphite oxide coated sand in fixed-bed column. Applied Surface Science 330: 148-156.
- Eberhardt TL, Min SH (2008) Biosorbents prepared from wood particles treated with anionic polymer and iron salt: Effect of particle size on phosphate adsorption. Bioresource Technology 99: 626-630.
- Banerjee K, Ramesh ST, Gandhimathi R, Nidheesh PV, Bharathi KS (2012) A novel agricultural waste adsorbent, watermelon shell for the removal of copper from aqueous solutions. Iranian Journal of Energy & Environment 3: 143-156.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15104
- [From(publication date):
November-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 13873
- PDF downloads : 1231