Research Article Open Access
Early Contact with Palliative Care Services: A Randomized Trial in Patients with Newly Detected Incurable Metastatic Cancer
Martin HN Tattersall1*, Andrew Martin2, Rhonda Devine3, Joan Ryan RN4, Jesse Jansen5, Lucy Hastings6, Michael Boyer7, Paul Glare8, Martin Stockler9 and Phyllis Butow10
1Professor of Cancer Medicine, Sydney Medical School, Blackburn Building, DO6, University of Sydney, Australia
2Statistician, NHMRC Clinical Trials Centre, University of Sydney, Australia
3Research Nurse, Department of Medical Oncology, Royal Prince Alfred Hospital (RPAH), Missenden Road, Camperdown, NSW 2050, Australia
4Palliative Care nurse, Department of Palliative Care, RPAH, Australia
5Research Psychologist, Department of Public Health, University of Sydney, Australia
6Central Clinical School, University of Sydney, Australia
7Clinical Professor, Department of Medical Oncology, RPAH, Australia
8Associate Professor and Head of Palliative, RPAH, Australia
9Associate Professor, Clinical Trials Centre, University of Sydney, Australia
10Professor of Psychology, University of Sydney, Australia
- *Corresponding Author:
- Martin HN Tattersall
Professor of Cancer Medicine, Sydney Medical School
Blackburn Building, DO6, University of Sydney
NSW 2006, Australia
Tel: +61-29-660-7362
E-mail: martin.tattersall@sydney.edu.au
Received date December 27, 2013; Accepted date February 20, 2014; Published date February 27, 2014
Citation: Tattersall MHN, Martin A, Devine R, Joan Ryan RN, Jansen J, et al. (2014) Early Contact with Palliative Care Services: A Randomized Trial in Patients with Newly Detected Incurable Metastatic Cancer. J Palliat Care Med 4: 170. doi:10.4172/2165-7386.1000170
Copyright: © 2014 Tattersall MHN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Background: It is not known when in the course of incurable cancer referral to a specialist palliative care service should optimally be made.
Methods: We randomly assigned patients with newly detected incurable metastatic cancer with an estimated survival of less than 12 months to receive either (1) standard oncologic care plus contact from a palliative care nurse who served as a link to palliative care services in the hospital and community (PC) or (2) standard oncologic care alone. Quality of life (QoL) measures were assessed at baseline and monthly thereafter. The primary endpoint was quality of life over time measured by the McGill QOL total score.
Findings: 120 patients were randomized, 60 to each group. Forty four patients had gastrointestinal cancer, 23 lung cancer, 19 gynaecological cancer and 17 breast cancer. The mean time since initial cancer diagnosis was 34 months in the standard care group and 29 months in the early palliative care contact group. There was no evidence that early PC nurse contact reduced symptoms or improved quality of life. If anything, there was a trend towards the opposite. There were non-significant trends for the place of death of early contact PC patients to be other than in an acute hospital, and for greater PC input during their final acute hospital admission. Early contact with palliative care was not found to influence the number of lines of chemotherapy received.
Interpretation: The study did not demonstrate a QoL benefit for early contact with a PC nurse.
Keywords
Incurable cancer; Chemotherapy; Palliative care contact; Quality of life; Symptoms; End of life
Introduction
In cancer patients receiving chemotherapy, 35% experience distress [1] 70% experience nausea [2] while up to 33% experience severe diarrhoea [3]. A recent systematic review concluded that over half of people at the end stage of cancer have distress, pain, dyspnoea, and fatigue [4]. A large proportion (30-40%) of cancer patients and carers reports significant unmet need for information, symptom relief and support [5,6].
Health professionals often delay discussion of end of life (EOL) issues until only days before death [7]. For example, the US SUPPORT study documenting care for over 9000 seriously ill hospitalised adults [8] reported that only 47% of physicians knew whether their patients preferred to avoid cardiopulmonary resuscitation (CPR), and 46% of do-not-resuscitate (DNR) orders were written within 2 days of death. Wright et al. [9] found that EOL discussions conducted a median of 4.4 months before death were associated with less aggressive medical care near death and earlier hospice referrals. More aggressive medical care has been found to be associated with worse quality of life and no survival benefit.
Early contact with palliative care services has the potential to overcome some of these distressing outcomes. The proportion of terminal cancer patients currently referred to palliative care services varies in Australia, and internationally. Moreover there is wide variation in the time course of advanced cancer when patients are referred to palliative care for the first time. A study from Boston randomized 151 patients with newly diagnosed metastatic non-small cell lung cancer to receive either palliative care integrated with standard oncologic care or standard oncologic care alone [10]. Patients assigned to early palliative care had a better quality of life at 12 weeks and fewer depressive symptoms than did patients assigned to standard care.
We report the results of a randomised trial of early contact with palliative care services in patients with newly detected incurable metastatic cancer. We hypothesized that early contact with palliative care services would improve patients’ EOL experiences through better symptom control and quality of life; addressing patients’ supportive care needs; reducing the lines of chemotherapy delivered; and reducing the likelihood of dying in the acute hospital setting. It was anticipated that meeting and talking with a palliative care nurse at the time of recruitment would subsequently provide a pathway for patients to contact the palliative care service independent of the oncologist. It was hoped this facilitated access to the PC service would have the benefit of improved symptom control.
Methods
Study design and intervention
Between April 2003 to January 2005 ambulatory patients with newly detected incurable metastatic cancer attending a medical oncology clinic with a life expectancy of less than 12 months were invited to take part in a randomized controlled trial of early contact with a palliative care nurse consultant with ongoing oncologist care or oncologist care alone. For allocation of the participants, a computer-generated list of random numbers was used, and allocation was concealed using sequentially numbered, opaque sealed envelopes.No stratification was made for oncologist or cancer diagnosis. A sample size of 150 patients was sought to provide over 80% power to detect an effect size of 0.50 (standard deviations) at the two-sided 5% level of significance based on a two-sample t-test; with even greater precision achievable using analyses incorporating the baseline score as a covariate [11].
Patients assigned to the early palliative care group met with a palliative care nurse consultant (PC nurse) member of the hospital palliative care team. She outlined available palliative care services including advice about symptom control, and she offered to arrange review by a palliative care physician, and provided contact details for the palliative care service. The PC nurse offered to telephone the patient monthly to check on their well-being, or, if the patient preferred, provided her contact details. Standard oncologic care was given in line with the oncologist’s recommendation. Control patients were referred to the palliative care service when recommended by the oncologist.
Assessments and endpoints
At baseline, oncologists documented their estimate of the patient’s life expectancy [12].
Symptom severity, feeling supported and overall QoL were prespecified as being the key outcome measures. The severity of symptoms and overall quality of life were measured using the McGill quality of life (MQOL) questionnaire [13] and the Rotterdam Symptom Checklist (RSC) [14]. Construct validity of the MQOL has been demonstrated through its correlations with the Spitzer Quality of Life scale [15]. Cronbach’s alpha for subscales was moderate to high (0.462-0.858) and test-retest reliability (Spearman’s r(s) ranged from 0.512-0.861 [16]. Validity has been demonstrated through correlations with a range of related scales [17].
The degree of perceived support was measured using the Supportive Care Needs – Short Form questionnaire (SCNS-short) [18]. Content and face validity has been found to be high and construct validity is supported by a robust factor structure [19]. Patients were requested to complete the MQOL and RSC at monthly intervals and the SCNS-short every 4 months.
Other secondary endpoints collected from hospital medical records included end of lifeexperiences, number of lines of chemotherapy, and place of death.
Analysis methods
All analyses were performed in accordance with the intentionto- treat principle. Repeated measures analyses were undertaken on the longitudinal RSC, MQOL, and SCNS assessments using a mixed modeling approach with the baseline measure as a covariate and treatment group, assessment time point, and a treatment group-bytime point interaction as factors. The interaction term allowed the model to evaluate the difference between groups at each time point. Imputation of missing data was not required as the two groups was compared using a log-rank test and presented graphically using Kaplan-Meier plots. Cox proportional hazards regression models were used to estimate hazard ratios for the treatment effect with and without adjustment for other predictors of survival.
Results
Baseline characteristics
One hundred and twenty of the 141 patients approached consented to take part and were randomized to either the early referral group (N=60) or standard care (N=60). Recruitment to the study was halted at this point due to resource constraints. Forty four patients had a gastrointestinal primary cancer, 23 lung cancer, 19 gynecological cancers, 17 breast cancer 2 prostate cancer and 15 other primary sites, or unknown. Most baseline characteristics were adequately balanced across the two study groups (Table 1), however there were differences between the groups in the time since initial cancer diagnosis (mean of 29versus 34 months in the early referral and standard care groups respectively), and the oncologists’ estimate of likely survival (e.g. 11 versus 20 patients with estimates of >12 months likely survival in the early referral and standard care groups respectively). Therefore these variables were controlled for in subsequent analyses. There were no remarkable baseline differences between the groups on the patient reported outcome measures.
| Early Palliative Care Contact group N=60 |
Standard care group N=60 |
|
|---|---|---|
| Age in years: Mean(SD) | 63 (11.2) | 64 (11.1) |
| Female sex: N (%) | 28 (47%) | 34 (57%) |
| Partner: N (%) | 40 (67%) | 41 (68%) |
| EducationYear 10 or less: N (%) | 23 (38%) | 32 (53%) |
| Australian born: N (%) | 29 (48%) | 36 (60%) |
| Months since initial cancer diagnosis Mean (SD) (5 missing values) |
29 (40.0) | 34 (53.0) |
| Cancer diagnosis: N (%) | ||
| Gastrointestinal | 20 (33%) | 24 (40%) |
| Lung | 12 (20%) | 11 (18%) |
| Prostate | 0 (0%) | 2 (3%) |
| Breast | 5 (8%) | 12 (20%) |
| Gynaecologic | 11 (18%) | 8 (13%) |
| Other/Unknown primary | 12 (20%) | 3 (5%) |
| Oncologist estimate of patient’s likely survival time: N (%) | ||
| 4-12 weeks | 1 (2%) | 0 (0%) |
| 3-6 months | 9 (15%) | 6 (10%) |
| 6-12 months | 33 (55%) | 30 (50%) |
| >12 months | 11 (18%) | 20 (33%) |
| Not stated | 6 (10%) | 4 (7%) |
Table 1: Baseline Demographics and Clinical Characteristics of Study Patients.
Contact with palliative care services
Initial contact: Patients in the early palliative care contact group had at least one meeting with the PC nurse, with the median time to first contact being 2 weeks after randomization. Further contact with the PC nurse was more frequent when the initial contact with the PC nurse was face to face. Most patients were happy for the PC nurse to explore the role of palliative care, and for records of their response to be kept. Several patients stated they thought they were not ready for palliative care but were happy to have it explained. Many patients reported feeling better having discussed palliative and end of life care options even though they were receiving anticancer treatments.
Subsequent contact: Many patients preferred to contact the PC nurse when they needed assistance rather than receiving monthly telephone contact, and many made contact during clinic attendance. Twenty eight patients had 1 subsequent telephone contact with the PC nurse, 3 had 2-3 telephone contacts and 5 had more than 3. Two patients preferred to telephone the PC nurse and did so. 20 patients had no subsequent contact with the palliative care nurse. The average number of telephone contacts with the PC nurse in the early contact group was 3.
Fifty one patients in the early palliative care contact group were seen at least once by a palliative care physician consultant compared to 8 patients in the control group. In the intervention group 25 patients were seen by a palliative care physician in the last few weeks of life (16 in the last month of life compared to 6 in the control group).
Quality of life and unmet needs
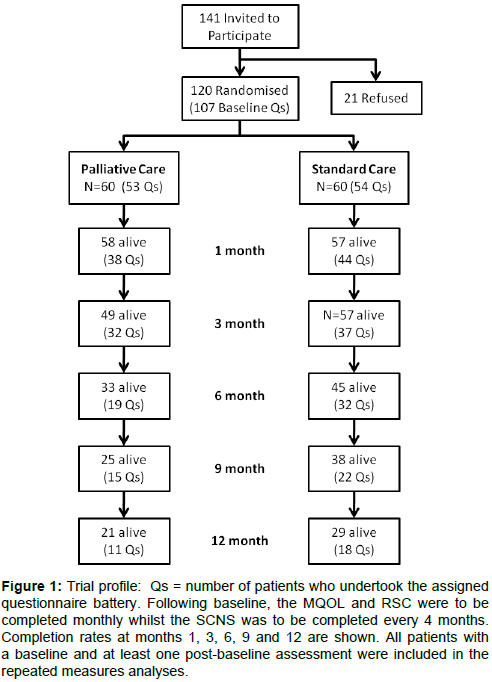
Data on patient-reported outcome measures were available on 107 of the 120 patients randomized at baseline (Figure 1). This number declined to 51of 79 alive at 6 months, 29 of 52 alive at 12 months, and 7 of 36 alive at 18 months. The median duration of follow-up on the self-reported outcome measures was 4.8 versus 8.1 months for the early referral and standard care groups respectively (p-value = 0.13).
Figure 1: Trial profile: Qs = number of patients who undertook the assigned questionnaire battery. Following baseline, the MQOL and RSC were to be completed monthly whilst the SCNS was to be completed every 4 months. Completion rates at months 1, 3, 6, 9 and 12 are shown. All patients with a baseline and at least one post-baseline assessment were included in the repeated measures analyses.
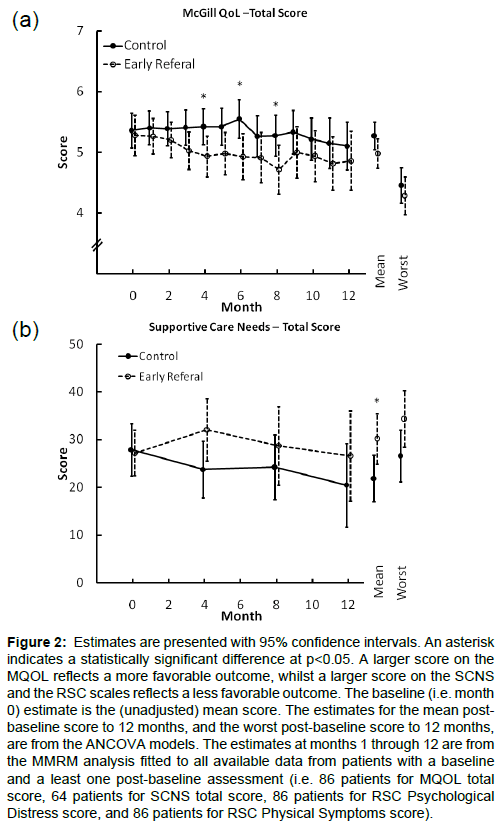
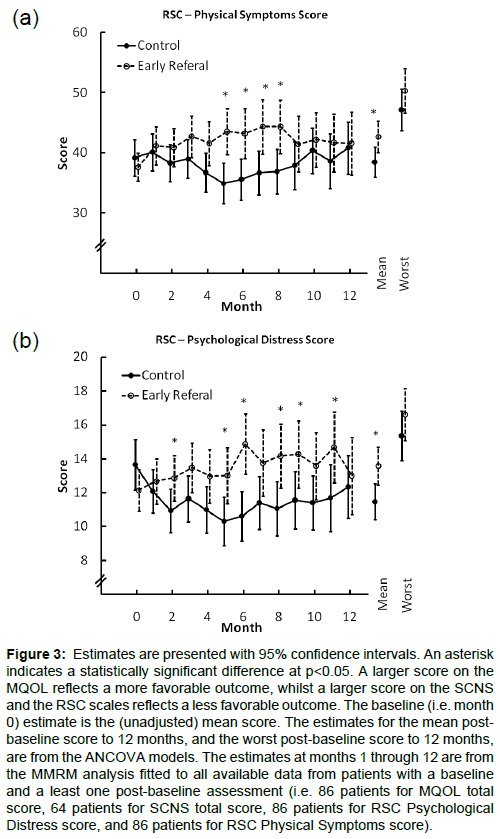
There was no evidence that early PC intervention was superior to the control on patient reported measures. Figures 2 and 3 summarize the estimates for the two groups over the first 12 months of followupon the MQOL total score, the RSCL physical symptom scale and the RSCL psychological distress scale. Across these measures there were consistent post baseline trends of modest magnitude favoring the control arm with differences that occasionally reached statistical significance. These results were not materially changed when adjusted for the oncologist’s baseline estimate of likely survival, diagnosis, months since diagnosis, and gender were included as covariates (results presented in e-supplement).
Figure 2: Estimates are presented with 95% confidence intervals. An asterisk indicates a statistically significant difference at p<0.05. A larger score on the MQOL reflects a more favorable outcome, whilst a larger score on the SCNS and the RSC scales reflects a less favorable outcome. The baseline (i.e. month 0) estimate is the (unadjusted) mean score. The estimates for the mean postbaseline score to 12 months, and the worst post-baseline score to 12 months, are from the ANCOVA models. The estimates at months 1 through 12 are from the MMRM analysis fitted to all available data from patients with a baseline and a least one post-baseline assessment (i.e. 86 patients for MQOL total score, 64 patients for SCNS total score, 86 patients for RSC Psychological Distress score, and 86 patients for RSC Physical Symptoms score).
Figure 3: Estimates are presented with 95% confidence intervals. An asterisk indicates a statistically significant difference at p<0.05. A larger score on the MQOL reflects a more favorable outcome, whilst a larger score on the SCNS and the RSC scales reflects a less favorable outcome. The baseline (i.e. month 0) estimate is the (unadjusted) mean score. The estimates for the mean postbaseline score to 12 months, and the worst post-baseline score to 12 months, are from the ANCOVA models. The estimates at months 1 through 12 are from the MMRM analysis fitted to all available data from patients with a baseline and a least one post-baseline assessment (i.e. 86 patients for MQOL total score, 64 patients for SCNS total score, 86 patients for RSC Psychological Distress score, and 86 patients for RSC Physical Symptoms score).
Table 2 presents a summary of the symptoms ever reported on the MQoLas severe (i.e. assigned a score >5) by the 47 patients who completed an assessment within the three months prior to death. Unexpectedly, somewhat more patients in the early PC intervention group than in the control group had severe scores for pain and poor appetite.
| Troublesome Symptom* | Randomisation Group | Total | |||||
|---|---|---|---|---|---|---|---|
| Intervention N=24 |
Control N=23 |
p-value** | |||||
| Pain | 12 | (50%) | 8 | (35%) | 0.38 | 20 | (43%) |
| Tired | 8 | (33%) | 7 | (30%) | 1.00 | 15 | (32%) |
| Appetite | 9 | (38%) | 2 | (9%) | 0.04 | 11 | (23%) |
| Other | 8 | (33%) | 3 | (13%) | 0.17 | 11 | (23%) |
| Weakness | 8 | (33%) | 3 | (13%) | 0.17 | 11 | (23%) |
| Breathing | 5 | (21%) | 3 | (13%) | 0.70 | 8 | (17%) |
| Sleep | 3 | (13%) | 4 | (17%) | 0.70 | 7 | (15%) |
| Constipation | 4 | (17%) | 2 | (9%) | 0.67 | 6 | (13%) |
| Nausea | 2 | (8%) | 2 | (9%) | 1.00 | 4 | (9%) |
| Diarrhoea | 1 | (4%) | 1 | (4%) | 1.00 | 2 | (4%) |
*Symptoms reported by subjects on multiple occasions appear only once in this able. Infrequently reported symptoms have been coded to other category
**Fisher’s Exact Test
Table 2: Severe symptoms (i.e. ever assigned a score > 5) reported on the MQoL within the three months prior to death.
Cancer treatment and PC input during the final acute hospital
Patients from both groups received an average of 1.8 lines of chemotherapy. Forty-two patients (42/4986%) randomized to the early referral group received palliative care contact during the last acute hospital admission compared to 29 patients (29/37 78%) in the control group (p=0.37).
Survival
The median survival of the early PC contact group was 7.0months (95% CI: 5.2-9.8) compared to 11.7months (95% CI: 9.8-18.8) for the standard care group (log rank p=0.014) (Figure 3). The estimated hazard ratio was 1.6 (95% CI:1.1 to 2.3; p=0.015). This estimate changed to 1.5 (95% CI 0.99 to 2.2; p=0.06) when adjusted for the oncologist’s baseline estimate of likely survival, diagnosis, months since diagnosis, and gender.
Place of death
There was little evidence of a difference between randomization groups in terms of place of death (Table 3). Only 2 patients (both early palliative care contact group) were admitted to ICU during their final acute hospital admission. One patient had attempted cardiopulmonary resuscitation (early palliative care contact group), and 2 had mechanical ventilation (1 in each group).
| Early Palliative Care Contact group |
Standard care group |
P-value | |
|---|---|---|---|
| Place of death | 0.46 | ||
| Home | 13 (22%) | 8 (15%) | |
| Hospice/NursingHome | 11 (19%) | 8 (15%) | |
| Acute Hospital | 34 (59%) | 37(70%) | |
| Total | 58 | 53 | |
| Palliative carecontact at lastadmission | 0.37 | ||
| Yes | 42 (86%) | 29 (78%) | |
| No | 7 (14%) | 8 (22%) | |
| Total | 49 | 37 |
Table 3: Place of death, and palliative care contact during last admission.
Discussion
This trial provides no evidence that early PC contact with a specialist PC nurse –who outlined available palliative care services, and offered to arrange review by a palliative care physician ultimately improved patient symptoms or quality of life. If anything, there was a trend towards the opposite. There were non-significant trends for the place of death of early contact PC patients to be other than in an acute hospital, and for greater PC input during their final acute hospital admission.
There was a trend for control patients’ scores for overall QoL, pain and appetite to be better than the early intervention group. The trend might simply be due to chance imbalances between the treatment groups. Early PC contact patients may have felt more comfortable reporting symptoms because of PC contact – less trying to “please the doctor”. These factors also may have contributed to the greater mean scores in the early PC contact group in the RSCL physical symptoms and psychological distress scales, and the pattern of scores in the MQOL. Alternatively patients in the standard care group may have experienced a protective effect of denial. Lung cancer patients who displayed a moderate or increasing level of denial over time reported better quality of life compared with those who displayed low levels of denial (p<0.0001) [20].
There was a statistically significant difference in survival time favoring the control arm. The estimated effect was smaller and of marginal statistical significance when adjusted for gender, diagnosis, months since initial diagnosis and the oncologist’s baseline estimate of likely survival time. The observed survival differences are likely due to chance imbalances between the groups.
Our results differ from those reported by others [10]. Differences in the eligibility of patients may have played a role in that our trial included patients with a range of cancer types, most of whom had recurrent cancer. The Boston trial recruited advanced lung cancer patients within 8 weeks of first cancer diagnosis. The nature and ‘dose’ of the palliative care intervention also differed between our study and that reported from Boston. In Boston, PC contact involved the patient meeting with a member of the PC team which consisted of 5 palliative care physicians and one advanced practice nurse, and 55% of the consultations were conducted by physicians, and 44% by the advanced practice nurse. The median time for the first palliative care outpatient visit was 55 minutes [21]. Subsequent contact in Boston was at least monthly in the outpatient setting.
Our results also differ from those obtained in Project Enable (Educate, Nurture, Advise, BeforeLife Ends) [22], where the intervention involved not only specialist palliative care nurse educators, but also nurse practitioners, and palliative care physicians. It used a case management, educational approach with monthly shared medical appointments to encourage patient activation, empowerment, and self-management. There was higher quality of life (P<0.02), lower symptom intensity (P<0.06), and lower depressed mood (P<0.02) in Project Enable patients.
Several strengths and limitations of our study deserve mention. In spite of randomization, there were differences between the groups in the numbers of patients with breast cancer, the time since initial cancer diagnosis, and the treating oncologist’s prediction of expected survival. Findings from a single institution and palliative care nurse consultant may not be generalizable. The ‘dose’ of the palliative care contact and intervention in our study was substantially less than in the other studies, and it seems that our intervention was not adequate to facilitate access to the PC service with the anticipated benefit of improved symptom control. A larger, stratified multicentre trial is needed to reduce the likelihood of important imbalances, and to improve the generalisability of the results.
A strong case can be made for further, rigorously-designed, large randomized trials in differing health care settings of better-defined ‘early’ PC intervention in cancer patients with limited survival expectations, to determine effects on quality of life, quality of death, and survival. Future studies should specify the issues that were addressed during the PC “consultation” such as pain management, symptom control, psychosocial and spiritual issues, prognosis, burdens and benefits of different treatment options, advanced care planning, and preferences for place of care. Similarly the endpoints of new trials should include repeated measures of quality of life, and measures of hope and denial. Studies also need to distinguish between patients whose palliative care needs are straightforward and manageable by the oncologist versus more complex presentations.
Author Contribution
Martin Tattersall (MHNT), Michael Boyer (MB), Paul Glare (PG), Martin Stockler (MS) and Phyllis Butow (PB) developed the research proposal and conducted the trial. MHNT and PG performed the literature search. Rhonda Devine (RD) & Joan Ryan (JR) recruited patients, and assisted with gathering base line information & QoL measures. JR was the Palliative Care (PC) nurse involved in the early PC contact intervention. Lucy Hastings (LH) reviewed the patients’ medical records to identify patients’ end of life experiences. Andrew Martin (AM), Jesse Jansen (JJ) and PB performed the data analysis, and all authors were involved in data interpretation. MHNT & AM wrote the first draft of the manuscript, and all authors had input in preparing the final version.
The trial was supported by a National Health & Medical Research Council Strategic Palliative Care research grant. MHNT had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
Supported by an NHMRC strategic palliative care research grant no: 219141. Trial Registration: ACTRN12611001137987.
References
- Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S (2001) The prevalence of psychological distress by cancer site. Psychooncology 10: 19-28.
- Booth CM, Clemons M, Dranitsaris G, Joy A, Young S, et al. (2007) Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol 5: 374-380.
- Maroun JA, Anthony LB, Blais N, Burkes R, Dowden SD, et al. (2007) Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. CurrOncol 14: 13-20.
- Solano JP, Gomes B, Higginson IJ (2006) A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 31: 58-69.
- Sanson-Fisher R, Girgis A, Boyes A, Bonevski B, Burton L, et al. (2000) The unmet supportive care needs of patients with cancer. Supportive Care Review Group. Cancer 88: 226-237.
- Cancer Institute NSW (2010) NSW Cancer Patient Satisfaction Survey. Catalogue number: CF-2010-01 SHPN: (CI) 1000282010.
- Koedoot CG, Oort FJ, de Haan RJ, Bakker PJ, de Graeff A, et al. (2004) The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are. Palliative chemotherapy and watchful-waiting. Europ J Cancer 40: 225-235.
- (1995) A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 274: 1591-1598.
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, et al. (2008) Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300: 1665-1673.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, et al. (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363: 733-742.
- Borm GF, Fransen J, Lemmens WA (2007) A simple sample size formula for analysis of covariance in randomized clinical trials. J ClinEpidemiol 60: 1234-1238.
- Stockler MR, Tattersall MH, Boyer MJ, Clarke SJ, Beale PJ, et al. (2006) Disarming the guarded prognosis: predicting survival in newly referred patients with incurable cancer. Br J Cancer 94: 208-212.
- Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, et al. (1997) Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 11: 3-20.
- deHaes JC, van Knippenberg FC, Neijt JP (1990) Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer 62: 1034-1038.
- Cohen SR, Mount BM, Strobel MG, Bui F (1995) The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 9: 207-219.
- Lua PL, Salek S, Finlay I, Lloyd-Richards C (2005) The feasibility, reliability and validity of the McGill Quality of Life Questionnaire-Cardiff Short Form (MQOL-CSF) in palliative care population. Qual Life Res 14: 1669-1681.
- De Haes H (1996) Measuring the quality of life of cancer patients with the Rotterdam Symptom Checklist: A manual. Northern Centre for Healthcare Research (NCH), University of Groningen.
- Boyes A, Girgis A, Lecathelinais C (2009) Brief assessment of adult cancer patients' perceived needs: development and validation of the 34-item Supportive Care Needs Survey (SCNS-SF34). J EvalClinPract 15: 602-606.
- Bonevski B, Sanson-Fisher R, Girgis A, Burton L, Cook P, et al. (2000) Evaluation of an instrument to assess the needs of patients with cancer. Supportive Care Review Group. Cancer 88: 217-225.
- Vos MS, Putter H, van Houwelingen HC, deHaes HC (2011) Denial and social and emotional outcomes in lung cancer patients: the protective effect of denial. Lung Cancer 72: 119-124.
- Jacobsen J, Jackson V, Dahlin C, Greer J, Perez-Cruz P, et al. (2011) Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 14: 459-464.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, et al. (2 009) Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 302: 741-749.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 16079
- [From(publication date):
February-2014 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 11180
- PDF downloads : 4899