Research Article Open Access
Early Adiposity Rebound and Small Dense Low-Density Lipoprotein in Childhood Obesity
| Arisaka O*, Ichikawa G, Koyama S, Shimura N, Imataka G, Kurosawa H and Nitta A | |
| Department of Pediatrics, Dokkyo Medical University School of Medicine Mibu, Shimotsuga-gun, Tochigi-ken, 321-0293 Japan | |
| *Corresponding Author : | Arisaka O Department of Pediatrics Dokkyo Medical University School of Medicine Mibu, Shimotsuga-gun, Tochigi-ken, 321-0293, Japan Fax: +81 (0) 282 86 7521 Tel: +81 (0)282 86 1111 E-mail: arisaka@dokkyomed.ac.jp |
| Received: February 16, 2016 Accepted: February 25, 2016 Published: February 29, 2016 | |
| Citation: Arisaka O, Ichikawa G, Koyama S, Shimura N, Imataka G et al. (2016) Early Adiposity Rebound and Small Dense Low-Density Lipoprotein in Childhood Obesity. J Obes Weight Loss Ther 6:301. doi:10.4172/2165-7904.1000301 | |
| Copyright: © 2016 Arisaka O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Aim: The adiposity rebound (AR) corresponds to the second rise in the body mass index (BMI) curve that occurs between ages 5 and 7 years. The goal of this study was to determine whether age at AR is related to the presence at 12 years old of small dense low-density lipoprotein (SDLDL), an atherogenic lipoprotein produced as a metabolic consequence of AR. Methods: A longitudinal population-based prospective study was performed in 215 children. Serial measurements of BMI were conducted at ages 1, 1.5, 2 and yearly thereafter until 12, based on which age at AR was calculated. The subjects were divided into 5 groups according to age at AR of ≤4, 5, 6, 7 and ≥8 years. Plasma lipids and SDLDL were measured at 12 years of age. SDLDL (LDL particle size <25.5 nm) was determined by nondenaturing 2-16% gradient gel electrophoresis. Results: The prevalences of SDLDL were 15.0% in children with age at AR ≤4 y, 8.1% in those with age at AR 5 y, and 0% in all other groups (AR at ≥6 years). An earlier AR was significantly associated with higher BMI, increased plasma triglyceride (p < 0.05), increased atherogenic index (p < 0.05), and decreased HDL-cholesterol (p < 0.05) at 12 years of age. Conclusion: Children with AR before 4 years old showed a high prevalence of atherogenic SDLDL, indicating a predisposition for future cardiovascular disease.
| Keywords |
| Childhood obesity; Adiposity rebound; Insulin resistance; Small dense LDL; Cardiovascular disease |
| Introduction |
| Low-density lipoprotein (LDL) particles are heterogeneous in density, size, lipid composition, and pathogenetic properties. Smaller and denser LDL particles have a stronger atherogenic impact, possibly because of low binding affinity of these particles to the LDL receptor, low resistance to oxidative stress, a prolonged half-life in plasma, and easier penetration of the arterial intima due to the small particle size [1,2]. LDL particles are reduced in diameter because of alteration in lipoprotein metabolism caused by insulin resistance, producing small dense LDL (SDLDL) particles of size ≤ 25.5 nm [3,4]. Many studies have shown that circulating SDLDL is associated with increased occurrence of atherogenic coronary artery events in adults [5,6]. Therefore, the presence of SDLDL in adolescence is a risk factor for future coronary heart disease [7,8]. |
| Excessive weight gain in early childhood is thought to lead to adult obesity-related morbidity and mortality [9]. Adiposity rebound (AR) is defined as the second rise in the body mass index (BMI) curve that normally occurs between ages 5 and 7 years; on average, a rapid increase in BMI occurs during the first year of life, subsequently declines and reaches a nadir at around 6 years of age, and then increases again throughout childhood [10]. Recent studies have shown that early AR (<4 years) is related to a risk for later obesity and metabolic consequences caused by insulin resistance [10-12]. These observations motivated us to examine the relationship between the timing of AR and production of SDLDL as a metabolic consequence of changes in adiposity during infancy. |
| Methods |
| Subjects |
| A longitudinal population-based study was performed in 215 children (114 boys and 101 girls) in a birth cohort in Tochigi Prefecture, Japan. All of the children were followed up in infant health checks at a health center during the preschool period, and data were stored at a regional health center. During the school-age period, children underwent an annual physical examination at school, and the resulting data were also kept at the regional health center. At 12 years of age, all the children underwent a blood examination at high school. Written informed consent was obtained from parents or guardians for the physical examinations. The study was approved by the ethics committee of Dokkyo Medical University. |
| Identification of age at adiposity rebound using BMI |
| Serial measurements of body weight and height were obtained yearly from ages 1 to 12 years, giving a total of 12 measurements in all subjects. Age at AR was defined as the age between 1 and 12 years at which the lowest BMI occurred before the second BMI rise. The subjects were then divided into 5 groups based on an age at AR ≤ 4 y, 5 y, 6 y, 7 y, and ≥8 y. |
| Measurements of lipids |
| Fasting blood sampling in the morning was performed at school. To obtain blood samples after an overnight fast, students were allowed to bring a packet box breakfast, which they ate after undergoing a health examination in the schoolroom. Blood samples collected in tubes containing 1 g/L EDTA were sent to the laboratory. Plasma was separated and stored at -80°C until measurement of LDL particle size. Levels of total cholesterol (TC) (Cholestest CHO, Daiichi Pure Chemicals Co. Ltd., Tokyo, Japan) and triglycerides (TG) (Aqua-auto TG-II, Kainos Laboratories, Inc., Tokyo, Japan) were determined using enzymatic methods. High-density lipoprotein cholesterol (HDL-C) was measured by precipitation of other lipoproteins with the direct method (Cholestest N HD, Daiichi Pure Chemicals Co. Ltd.). Lowdensity lipoprotein cholesterol (LDL-C) was determined using the Friedewald formula. The atherogenic index was calculated from the formula: [TC– HDL-C]/HDL-C [13]. |
| Determination of LDL particle size |
| The LDL particle diameter was determined using the method described by Krauss et al. [4,14] with 2.5 to 16% polyacrylamide gradient gel electrophoresis. Gels were equilibrated at 120 V for 20 min, and electrophoresis was then performed using serum samples diluted 1:2 with sample buffer (31% sucrose, 0.06% EDTA-2Na, and 0.01% BPP) to a volume of 20 mL. Each gel also contained thyroglobulin, apoferritin and latex beads, as reference standards of known diameter. The gels were electrophoresed at 120 V for 19 h, and then stained for lipids with Oil Red O with heating to 55°C for 24 h and for protein with Coomassie Brilliant Blue for 15 min. Gels were then decolorified with ethanol and immersion in acetic acid, scanned with an image scanner (Epson GT-6500; Seiko Epson Corp., Nagano, Japan), and analyzed using an image processing and analysis program for Macintosh (NIH Image 1.61; National Institutes of Health, United States). Migration distances were determined. The LDL particle diameter was calculated by comparing the mobility of the sample with the mobility of the three calibrated standards on each gel. SDLDL was defined as LDL with a particle diameter <25.5 nm, based on the criteria proposed by Austin et al. [15]. |
| Statistics |
| An unpaired Student t-test was used to compare parameters between the groups. Tests for trends and for the association of age at AR with BMI, LDL-C, HDL-C, TG and atherogenic index were examined by multivariate logistic regression analysis. P < 0.05 was considered to be significant. |
| Results |
| Serial changes in BMI from 4 months to 12 years according to age at AR in the 215 children are shown in Figure 1. Children with AR at an earlier age had a higher BMI at 12 years of age. Relationships between age at AR and BMI and serum lipids at 12 years of age are shown in Table 1. Earlier AR appeared to be associated with higher TG and atherogenic index, whereas there was an inverse relationship between age at AR and HDL-C levels, and no relationship between the timing of AR and plasma levels of TC and LDL-C. Prevalences of SDLDL at 12 years of age in relation to age at AR are shown in Table 2. Children with AR at an earlier age had an increased SDLDL, with prevalences of 15.0% and 8.1% in the AR ≤4 y and 5 y groups, respectively, but of 0% in the three higher age groups. |
| Discussion |
| In this study, children with early AR had higher BMI at 12 years of age, and circulating SDLDL was detected only in children with AR at <5 years. These children also exhibited atherogenic lipid profiles with elevated plasma levels of TG and atherogenic index and decreased plasma HDL-C, whereas TC and LDL-C, the metabolism of which is independent of insulin resistance [16], did not show significant changes related to the timing of AR. |
| A reduction in LDL particle size associated with an increase in TG and a decrease in HDL-C may result from alteration of lipoprotein metabolism caused by insulin resistance [1]. Metabolically, hypertriglyceridemia promotes TG transfer from very low-density lipoprotein (VLDL) to HDL. The TG-enriched HDL then transfers TG to LDL and removes cholesterol from LDL; thus, the cholesteroldepleted LDL then becomes smaller and denser. In the presence of hypertriglyceridemia, the cholesteryl ester transfer protein (CETP) allows cholesteryl esters to be transferred from LDL in exchange for a TG molecule from VLDL. TG is then hydrolyzed by hepatic triglyceride lipase (HTGL) or lipoprotein lipase to produce smaller, denser LDL particles [2-4]. The activities of CETP and HTGL are enhanced by increased adiposity or increased insulin resistance. Therefore, altered insulin secretion or insulin sensitivity caused by lifestyle-related dietary or physical activity may be metabolically responsible for the reduction in LDL particle size [16,17]. |
| The prevalence of SDLDL in children has been found to be 2-11% [18-20], and the overall prevalence of SDLDL in the current study was 6.9%. Genetic and environmental factors may be responsible for the varying prevalences of SDLDL [2,16]. |
| An association between early AR and presence of circulating SDLDL at 12 years of age was also shown in the present study. The mechanism underling the relationship of AR and SDLDL is complex, but metabolic programming leading to future insulin resistance may occur during early AR; changes of body composition associated with a BMI increase in this early period (<4 or 5 years of age) may play a pivotal role in development of insulin resistance, subsequently enhancing production of SDLDL [2,21]. Processes that regulate early body fat stores in early childhood have recently been shown to confer increased susceptibility to development of leptin resistance and insulin resistance [22-24]. |
| Childhood plasma lipids levels and lipoprotein phenotypes representative of insulin resistance are known to correlate strongly with values measured in middle age [25]. This indicates that atherogenic lipoprotein SDLDL produced in adolescence tracks to adulthood, thus increasing the prevalence of cardiovascular diseases such as cardiac infarction and stroke and serving as a risk factor for overall mortality. Therefore, we propose that the most effective method for prevention of a future insulin-resistant state is to prevent an increase of BMI during early childhood, especially before 4 years old. |
| A limitation of the current study is that we were unable to investigate the levels of blood glucose and insulin, which are directly related to insulin resistance. However, SDLDL is considered to be a surrogate marker of insulin resistance and is actually responsible for the development of atherosclerosis [3,7]. |
| Conclusion |
| In conclusion, the results of this longitudinal population-based cohort study indicate that children who exhibit AR before 4 years old have a high prevalence of atherogenic SDLDL, indicating a predisposition for future cardiovascular disease. Therefore, monitoring of AR may be effective for early identification of children at risk for cardiovascular disease in adulthood. |
| Acknowledgements |
| This study was supported by a grant from the Ministry of Health, Labour and Welfare (Pathophysiology, Diagnosis and Management of Metabolic Syndrome in Childhood). We thank Mrs. Yasuyo Kawai for her excellent technical assistance. |
References
- Arisaka O, Fujiwara S, Yabuta K, Mokuno H, Mitugi Y, et al. (1997) Characterization of low-density lipoprotein subclasses in children. Metabolism 46: 146-148.
- Stan S, Levy E, Delvin EE, Hanley JA, Lamarche B, et al. (2005) Distribution of LDL particle size in a population-based sample of children and adolescents and relationship with other cardiovascular risk factors. Clin Chem 51: 1192-1200.
- Mikhailidis DP, Elisaf M, Rizzo M, Berneis K, Griffin B, et al. (2011) European panel on low density lipoprotein (LDL) subclasses: a statement on the pathophysiology, atherogenicity and clinical significance of LDL subclasses: executive summary. CurrVascPharmacol 9: 531-532.
- Watabe Y, Arisaka O, Miyake N, Ichikawa G, Koyama S, et al. (2015) Estimation of LDL Particle Size Using Lipid Indices: A Population-Based Study of 1578 Schoolchildren. MetabSyndrRelatDisord 13: 465-469.
- Rizzo M, Pernice V, Frasheri A, Di Lorenzo G, Rini GB, et al. (2009) Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebro-vascular events in subjects with the metabolic syndrome. Clin Endocrinol (Oxf) 70: 870-875.
- Grundy SM (1997) Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation 95: 1-4.
- Toth PP (2014) Insulin resistance, small LDL particles, and risk for atherosclerotic disease. CurrVascPharmacol 12: 653-657.
- Kang HS, Gutin B, Barbeau P, Litaker MS, Allison J, et al. (2002) Low-density lipoprotein particle size, central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int J ObesRelatMetabDisord 26: 1030-1035.
- Koletzko B, Chourdakis M, Grote V, Hellmuth C, Prell C, et al. (2014) Regulation of early human growth: impact on long-term health. Ann NutrMetab 65: 101-109.
- Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F (2006) Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond) 30 Suppl 4: S11-17.
- González L, Corvalán C, Pereira A, Kain J, Garmendia ML, et al. (2014) Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int J Obes (Lond) 38: 1299-1304.
- Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, et al. (2014) Adiposity rebound and the development of metabolic syndrome. Pediatrics 133: e114-119.
- Castelli WP, Abbott RD, McNamara PM (1983) Summary estimates of cholesterol used to predict coronary heart disease. Circulation 67: 730-734.
- Krauss RM (1994) Heterogeneity of plasma low-density lipoproteins and atherosclerosis risk. CurrOpinLipidol 5: 339-349.
- Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, et al. (1988) Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 260: 1917-1921.
- Arisaka O, Kojima M, Yamazaki Y, Kanazawa S, Koyama S, et al. (2004) Relationship between the presence of small, dense low-density lipoprotein and plasma lipid phenotypes in Japanese children. J AtherosclerThromb 11: 220-223
- Arisaka O, Ichikawa G, Koyama S, Shimura N (2009) Relation between low-density lipoprotein particle size and insulin and diabetes mellitus. J Pediatr 155: 600.
- Shimabukuro T, Sunagawa M, Ohta T (2004) Low-density lipoprotein particle size and its regulatory factors in school children.J Clin EndocrinolMetab 89: 2923-2927.
- Steinbeck KS, Bermingham MA, Mahajan D, Baur LA (2001) Low-density lipoprotein subclasses in children under 10 years of age.J Paediatr Child Health 37: 550-553.
- Alabakovska S, Labudovic D, Tosheska K, Spiroski M, Todorova B (2004) Distribution of low density lipoprotein subclasses in Macedonian children. Med SciMonit 10: CR667-671.
- Slinger JD, van Breda E, Keizer H, Rump P, Hornstra G, et al. (2008) Insulin resistance, physical fitness, body composition and leptin concentration in 7-8 year-old children. J Sci Med Sport 11: 132-138.
- Boeke CE, Mantzoros CS, Hughes MD, L Rifas-Shiman S, Villamor E, et al. (2013) Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 21: 1430-1437.
- Sinaiko AR, Caprio S (2012) Insulin resistance. J Pediatr 161: 11-15.
- Gillman MW, Ludwig DS (2013) How early should obesity prevention start? N Engl J Med 369: 2173-2175.
- Juhola J, Magnussen CG, Viikari JS, Kähönen M, Hutri-Kähönen N, et al. (2011) Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr 159: 584-590.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
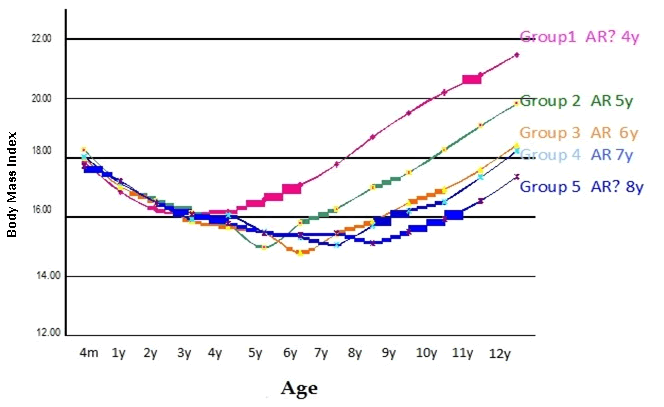 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 12029
- [From(publication date):
February-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11094
- PDF downloads : 935
