Dysostosis Multiplex (Gm-1 Gangliosidosis: Type II)
Received: 24-Dec-2015 / Accepted Date: 05-Jan-2016 / Published Date: 11-Jan-2016
Abstract
GM1 gangliosidosis is an autosomal recessive lysosomal storage disorder characterized by the generalized accumulation of GM1 ganglioside, oligosaccharides, and the mucopolysaccharide keratan sulfate (and their derivatives). Deficiency of the lysosomal hydrolase, acid β -galactosidase, causes GM1 gangliosidosis. GM1 gangliosidosis is a rare disorder, and the estimated incidence is 1:100,000- 200,000 live births. GM1 gangliosidosis is found in all races, although specific alleles can be identified in certain ethnic groups. A high frequency of GM1 gangliosidosis has been reported from Southern Brazil, and a large number of Japanese patients with the adult form have been reported. All 3 types of GM1 gangliosidosis are inherited as autosomal recessive traits and have equal sex distributions.
Keywords: Mucopolysachroid; Keratan sulphate; Dermal melanocytosis; Autosomal recessive
77744Introduction
GM1 gangliosidosis is an autosomal recessive lysosomal storage disorder characterized by the generalized accumulation of GM1 ganglioside, oligosaccharides, and the mucopolysaccharidekeratan sulfate (and their derivatives). Deficiency of the lysosomal hydrolase, acid β-galactosidase, causes GM1 gangliosidosis [1]. GM1 gangliosidosis is a rare disorder, and the estimated incidence is 1:100,000-200,000 live births [2]. GM1 gangliosidosis is found in all races, although specific alleles can be identified in certain ethnic groups. A high frequency of GM1 gangliosidosis has been reported from Southern Brazil, and a large number of Japanese patients with the adult form have been reported [3]. All 3 types of GM1 gangliosidosis are inherited as autosomal recessive traits and have equal sex distributions.
Case Report
5 years old boy with normal birth history born to a nonconsanginous parents, presented with mild developmental delay, gait difficulties and stiffness of limbs since 4 year of age. Initially parents noticed child had tiptoe walking, later he had stiffness of both upper and lower limbs which is gradually progressive. Child is still able to walk but unable to run. There is history of febrile seizures at 1.5 year of age. Younger sibling also having similar complaints
On examination
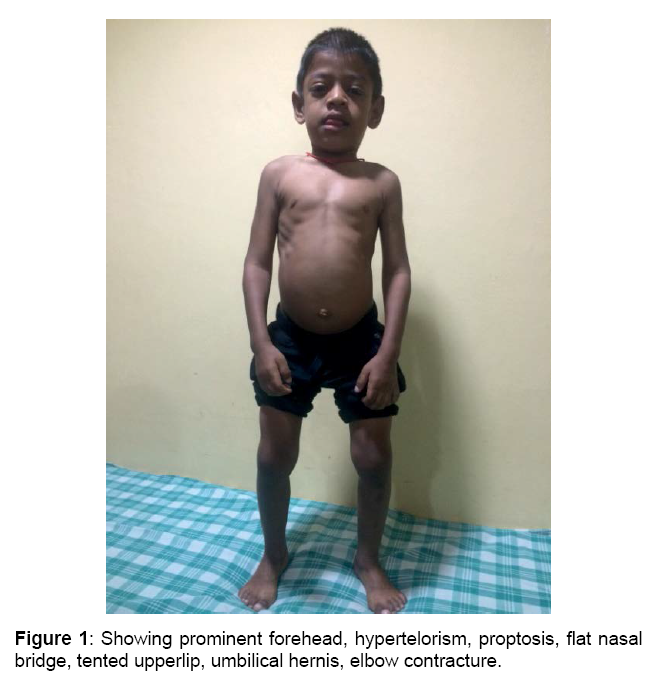
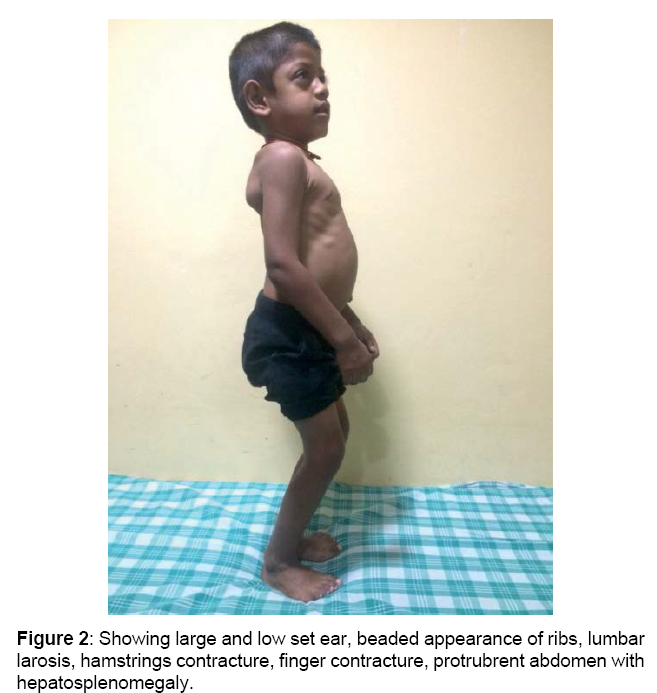
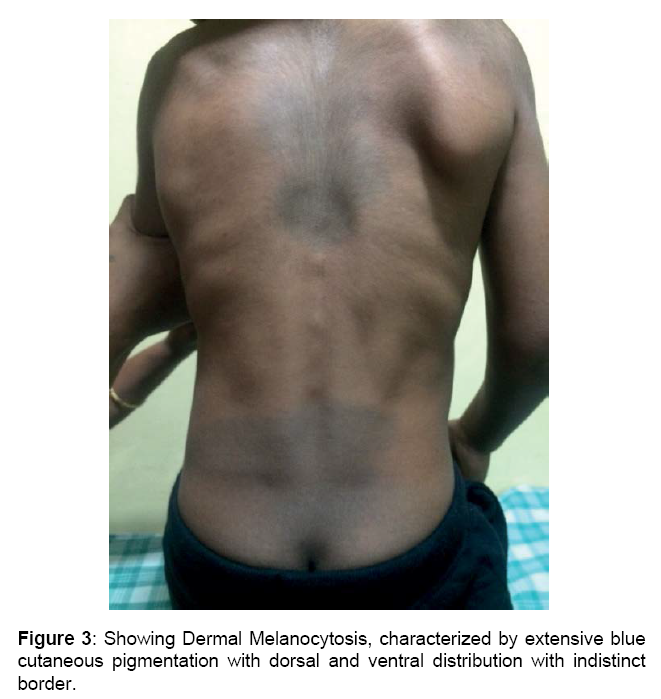
Boy is alert, cooperative GPE revealed, Coarse facial features, proptosis, prominent forehead, tented upper lip, hepatosplenomegaly, melanotic patches over back, elbow, finger & hamstrings contracture, protrubrent abdomen with umbilical hernia, brisk reflexes, power 5/5 and spastic gait.
Investigations
CBC showed normocytic hypochromic with mild relative monocytosis, LFT’ & RFT’s were normal, Urine test positive for MPS, Serum Hexosaminidase. A enzyme levels raised above normal limits, Aryl sulfatase negative, Beta galactosidase enzyme levels were reduced.
Cardiac evaluation
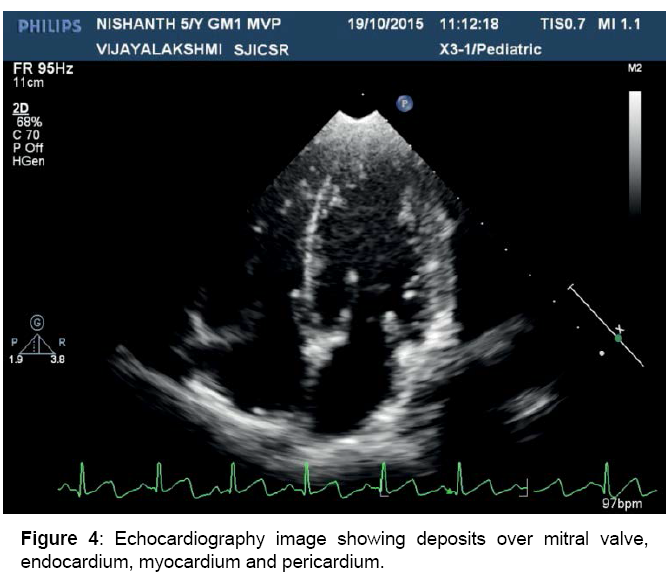
Mild MR, MVP, dilated LV, pericardial thickening, depositions over heart valves, chordae, endocardium and pericardium
X-ray
Thick calavaria, J shaped sella, Beaked vertebra
MRI brain
Prominent elongated perivascular spaces were noted in bilateral posterior
and lobar white matter, as well as corpus callosum and centrum semiovale. T2 FLAIR white matter signal changes are also noted in these regions (sparing the corpus callosum [4]). Sella appears J shaped (Figures 1-4)
Figure 4: Echocardiography image showing deposits over mitral valve, endocardium, myocardium and pericardium.
Discussion
Acid β -galactosidase is a lysosomal hydrolase that catalyzes the removal of the terminal β-linked galactose from glycoconjugates (eg, GM1 ganglioside), generating GM2 ganglioside. It also functions to degrade other β-galactose–containing glycoconjugates, such as keratan sulfate.
Enzyme activity is markedly reduced in patients with GM1 gangliosidosis. Deficiency of acid β-galactosidase results in the accumulation of glycoconjugates in body tissues and their excretion in urine. GM1 ganglioside and its derivative asialo-GM1ganglioside (GA1), glycoprotein-derived oligosaccharides, and keratan sulfate are found at elevated intracellular concentrations[1,5-8]. The gene has been isolated and is located on chromosome band 3p21.33. Various types of mutations have been identified in the acid β-galactosidase gene, including missense/nonsense, duplication/insertion, and splice site abnormalities [9].
Three clinical subtypes
There are of GM1 gangliosidosis are recognized.
The infantile form (Type 1): Typically presents between birth and age 6 months with progressive organomegaly, dysostosis multiplex, facial coarsening, and rapid neurologic deterioration withi the first year of life. Death usually occurs during the second year of life because of infection (usually due to pneumonia that results from recurrent aspiration) and cardiopulmonary failure [10]
The juvenile form (Type 2): Typically presents at age 1-2 years with progressive psychomotor retardation. Little visceromegaly and milder skeletal disease are present compared to the infantile form. Death usually occurs before the second decade of life.
The adult form (Type 3): Typically presents during childhood or adolescence as a slowly progressive dementia with prominent parkinsonian features and extrapyramidal disease, particularly dystonia. Marked phenotypic variability may occur. Age at death may widely vary.
Other clinical features of GM1 Ganglosiadosis are
Neurological features: Developmental delay [11], arrest, and regression, Generalized hypotonia initially, developing into spasticity, Exaggerated startle response, Hyperreflexia, Seizures, Extrapyramidal disease, Generalized dystonia (adult subtype) [12], Ataxia, Dementia, Speech and swallowing disturbance [13].
Opthalmaologic features: Macular cherry-red spots, Optic atrophy, Corneal clouding
Dysmorphic features: Frontal bossing, Depressed nasal bridge and broad nasal tip, Large low-set ears, Long philtrum, Gingival hypertrophy and macroglossia [1], Coarse skin, Hirsutism, Cardiovascular - Dilated and/or hypertrophic cardiomyopathy, valvulopathy.
Per abdomen: Hepatosplenomegaly, Inguinal hernia.
Skeletal abnormalities: Lumbar gibbus deformity and kyphoscoliosis, Dysostosis multiplex, Broad hands and feet, Brachydactyly, Joint contractures, Prominent dermal melanocytosis [14-17].
Diagnosis
Diagnosis of G M1 gangliosidosis can be confirmed by measurement of acid β-galactosidase activity in peripheral blood leukocytes. Patients with the infantile form have almost no enzyme activity, whereas patients with the adult form may have residual activity of 5-10% of reference values.
Urine
Galactose-containing oligosaccharides are excreted in the urine. Their presence may be used as an ancillary diagnostic test, and the concentration of the metabolites is proportional to disease severity.
CBC count
Vacuolation of lymphocytes may be present in patients with GM1 gangliosidosis but is a nonspecific.
Dried blood spots
Diagnosis of GM1 gangliosidosis has been made based on dried blood spots from newborn screening filter paper, even after 15 months in storage [18].
Molecular analysis
Molecular analysis of the β-1 galactosidase gene (GLB1) is clinically available [9].
Prenatal diagnosis
Prenatal diagnosis has been performed successfully by assay of β-galactosidase activity in cultured amniocytes or amniotic chorionic villi [1]. Mutation identification allows prenatal or preimplantation genetic diagnosis [19,20].
Treatment
Currently, no effective medical treatment is available for the underlying disorder in patients with GM1 gangliosidosis. Bone marrow transplantation was successful in an individual with infantile/juvenile GM1 gangliosidosis; however, no long-term benefit was reported [21]. Presymptomatic cord-blood hematopoietic stem-cell transplantation has been advocated by some as a possible treatment because of success in other lysosomal storage disorders [22]. Symptomatic treatment for some neurologic sequelae is available but does not significantly alter the clinical course. Active research in the areas of enzyme replacement and gene therapyfor GM1 gangliosidosis is ongoing but has not advanced to human trials [2].
References
- Suzuki Y,Oshima A,Nanba E,Scriver CR, Sly WS,et al.(2002)The Metabolic and Molecular Bases of Inherited Disease.Biochemistry (Moscow) 67: 611-612.
- Brunetti-Pierri N, Scaglia F(2008) GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab94:391-396.
- Severini MH,Silva CD, Sopelsa A (1999) High frequency of type 1 GM1 gangliosidosis in southern Brazil.Clin Genet 56: 168-169.
- Takenouchi T, Kosaki R, Nakabayashi K, Hata K, Takahashi T, et al.(2014) Paramagnetic Signals in the Globus Pallidus as Late Radiographic Sign of Juvenile-Onset GM1 Gangliosidosis. PediatrNeuro 52:226-229.
- CeltikçiB,Aydin HI,Sivri S,Sönmez M,Topçu M, et al. (2012) Four novel mutations in the ß-galactosidase gene identified in infantile type of GM1 gangliosidosis. ClinBiochem 45: 571-574.
- OhtoU,Usui K,Ochi T,Yuki K,SatowY,Shimizu T,et al. (2012)Crystal structure of human ß-galactosidase: structural basis of GM1 gangliosidosis and morquio B diseases. J BiolChem 287: 1801-1812.
- King JE, Dexter A, Gadi I, Zvereff V, Martin M, et al. (2014) Maternal uniparentalisodisomy causing autosomal recessive GM1 gangliosidosis: a clinical report. J Genet Couns 23: 734-741.
- Zhong L, Zhang Z, Lu X, Liu S, Chen CY, et al. (2013) NSOM/QD-based visualization of GM1 serving as platforms for TCR/CD3 mediated T-cell activation.BioMed Research International.
- Suzuki Y,Sakuraba H,Oshima A,Yoshida K,Shimmoto M, et al (1991)Clinical and molecular heterogeneity in hereditary beta-galactosidase deficiency. DevNeurosci13: 299-303.
- Lenicker HM,Vassallo A P,Young EP,Attard MSP (1997) Infantile generalized GM1 gangliosidosis: high incidence in the Maltese Islands. J Inherit Metab Dis 20: 723-724.
- Suzuki K (1991) Neuropathology of late onset gangliosidoses. A review DevNeurosci 13: 205-210
- Roze E, Paschke E, Lopez N (2005) Dystonia and parkinsonism in GM1 type 3 gangliosidosis. MovDisord 20: 1366-1369.
- Muthane U, Chickabasaviah Y, Kaneski C (2004) Clinical features of adult GM1 gangliosidosis: report of three Indian patients and review of 40 cases. MovDisord 19: 1334-1341.
- Dweikat I, Libdeh BA, Murrar H, Khalil S, Maraqa N, et al. (2011) GM1 gangliosidosis associated with neonatal-onset of diffuse ecchymoses and mongolian spots. Indian J Dermatol 56: 98-100.
- Hanson M, Lupski JR, Hicks J,Metry D (2003) Association of dermal melanocytosis with lysosomal storage disease: clinical features and hypotheses regarding pathogenesis. Arch Dermatol 139: 916-920.
- Snow TM (2005) Mongolian spots in the newborn: do they mean anything?. Neonatal Netw 24: 31-33.
- Armstrong JA,Chu CJ (2014) Child Neurology: Exaggerated dermal melanocytosis in a hypotonic infant: A harbinger of GM1 gangliosidosis. Neurology 83:166-168.
- Chamoles NA,Blanco MB,Iorcansky S, GaggioliD,SpecolaN, et al. (2001) Retrospective diagnosis of GM1 gangliosidosis by use of a newborn-screening card. ClinChem 47: 2068.
- Morrone A, Bardelli T, Donati MA, GiorgiM,Di Rocco et al. (2000) Beta-galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum Mutat15: 354-366
- Cunningham M, Cox EO (2003) Hearing assessment in infants and children: recommendations beyond neonatal screening. Pediatrics 111:436-440.
- Shield JP,StoneJ,StewardCG (2005) Bone marrow transplantation correcting beta-galactosidase activity does not influence neurological outcome in juvenile GM1-gangliosidosis. J Inherit Metab Dis 28:797-798.
- Wynn RF, Wraith JE, Mercer J (2009)Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr154:609-611.
Citation: Madhu KJ, Vijyalakshmi IB, Narsimhan C, Manjunath CN (2016) Dysostosis Multiplex (Gm-1 Gangliosidosis: Type II). Cardiovasc Ther 1: 102.
Copyright: © 2016 Madhu KJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 13412
- [From(publication date): 2-2016 - Dec 05, 2024]
- Breakdown by view type
- HTML page views: 12653
- PDF downloads: 759