Special Issue Article Open Access
Dynamics Phytoremediation of Zn and Diesel Fuel in Co-contaminated Soil using Biowastes
| Agamuthu P and Dadrasnia A* | |
| Institute of Biological Sciences, Faculty of Science, University of Malaya, Malaysia | |
| Corresponding Author : | Dadrasnia A Institute of Biological Sciences Faculty of Science, University of Malaya, Malaysia Tel: +603 79674631 Fax: +603 79674631 E-mail: are.dadrasnia@gmail.com |
| Received November 23, 2013; Accepted December 30, 2014; Published January 05, 2014 | |
| Citation: Agamuthu P, Dadrasnia A (2013) Dynamics Phytoremediation of Zn and Diesel Fuel in Co-contaminated Soil using Biowastes. J Bioremed Biodeg S4:006. doi:10.4172/2155-6199.S4-006 | |
| Copyright: © 2014 Agamuthu P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In phytoremediation of co-contaminated soil, developing strategies is a major challenge for simultaneous and efficient remediation of multiple pollutants. A lab-scale experiment was set up to investigate the efficiency of adding 5% (w/w) individually of three different organic waste amendments [tea leaves, soycake and potato skin] to enhance the phytoaccumulation of Zinc and diesel fuel co-contaminated soil by Dracaena reflexa for a period of 180 days. Soil contaminated with Zn (80 mg kg-1DW) was spiked with an initial concentration of diesel fuel (25000 mg kg-1DW). Application of biowastes demonstrates significant degradation of DF compared to control soil. The highest rate of oil degradation was recorded in co-contaminated soil planted with D. reflexa and amended with SC (85%) followed by PS and TL (60 and 53.3%). Although the plants did not accumulate any hydrocarbon from the contaminated soil but there was significant bioaccumulation of Zn in the root and stem of Dracaena plant which was observed. At the end of 180 days, 16.5 mg/kg and 12.2 mg/kg of Zn in the root and the steam of D. reflexa was recorded. Results indicated that the D. reflexa could effectively extract Zn from Zn-contaminated soils even in the presence of diesel fuel concentration.
| Keywords |
| Biowastes; Bioaccumulation; Diesel oil; Phytodegradation; Zinc |
| Abbreviations |
| TL: Tea Leaf; SC: Soy Cake; PS: Potato Skin; TPHs: Total Petroleum Hydrocarbons; DF: Diesel Fuel; DW: Dry Wight; MHs: Heavy Metals; °C: Degree Celsius; GC/MS: Gas Chromatography/ Mass Spectrometry; EPA: Environmental Protection Agency; BCF: Bioconcentration Factor; TF: Translocation Factor |
| Introduction |
| The two major sources of soil pollution are toxic metals and petroleum products. Millions of cubic meters of soil are polluted by these sources [1]. Total petroleum hydrocarbon (TPH) and Zn are persistent concerns due to their insolubility and presence in the environment. US Environmental Protection Agency (USEPA) (1999) reported that more than 40% of the national priority list sites are co-contaminated with both PAHs and heavy metals which are environmental concerns and must be reduced to acceptable levels [2]. Food web contamination is a one of the important pathways through which toxic pollutants enter into the human body. Consequently, heavy metals are often found to coexist in with PAHs, due to the same pollution sources. Metal-PAHs combination has been found in industrial and agricultural places where a strong relation between heavy metal and PAHs was observed [3]. Chemical and physical methods, as well as, a combination of them have been used to remediate co-contaminated soils. Information regarding the mechanisms, translocation, accumulation and the combined uptake of heavy metals and PAHs present in the soil, sediment and contamination are still under study. Due to rapid industrialization and urbanization in the world, soil pollution by PAHs and Cd has been considered during the last decade [4]. |
| Phytoremediation technology has been considered for restoration of co-contaminated soil by organic pollutants and toxic metal [5,6]. The main challenge to phytodegradation of co-contaminated lands is the concurrent removal of various pollutants. For phytodegradation of hydrocarbon compounds or metals, many hyper accumulating plants have been widely studied, as plant root exudates lead to increase in the population of native soil microbes [4,7]. Several researchers reported that some plants could forbid the loss of organic compounds in contaminated soil [8,9]. For instance, Sedum alfredii is a native plant in China and it has been demonstrated an efficient transport of Cd in the presence of pyrene or phenanthrene from Cd-contaminated soil into roots to shoots compared to other hyperaccumulators [10]. However, additional techniques are required to carry out simultaneous removal of PAHs and heavy metal from co-contaminated soils. The application of animal manures and organic fertilizers is a common approach to extract and enhance the PAHs and/or heavy metal from contaminated soil. It must be noted however this research is focused on organic waste (biowastes) as the supplements which are available cheap and easy to apply for remediation of soil and also replace fertilizers which are expensive. This could be an option for waste management especially in those areas that generate a high rate of food, vegetables and fruit wastes. However, the effects of biowastes on phytoextraction of heavy metals and PAHs in co-contaminated soil is not comprehensively studied, the objective of this study is to determine the feasibility of biowastes to remedy co-contaminated soil. |
| Materials and Methods |
| Collection of organic wastes, soil and diesel fuel |
| The garden soil was taken from a farm in Subang Jaya, Selangor, Malaysia. The organic wastes used in this study were collected from different locations; tea leaf (TL) and potato skin (PS) was collected from the IGS (Institute of Graduate Studies) canteen, University of Malaya, while the soycake (SC) was made in the laboratory. Physicochemical properties of organic wastes and soil employed were determined using standard methods. |
| Preparation of co-contaminated soil |
| According to the critical level of heavy metals pollution in Malaysian soil [11], it was decided to use Zn (80 mg kg-1). Plastic bags were tilled with 2 kg of soil and the desired amount of Zn was spiked with an aqueous solution of ZnCl2 as a source of Zn in 200 ml of distilled water. It was sprayed layer by layer, and the soil was completely mixed. Then, with the purpose of creating a balance between the different fractions of elements in the soil, the soil samples were put through an incubation period of one month. During this period, the moisture of the bags was kept at about (70% ± 10). At the end of the incubation period, soil samples were co-contaminated with 2.5 % w/w of diesel fuel and thoroughly mixed well. 5% (w/w) of different biowastes (TL, SC and PS) were also mixed individually with the oil contaminated soil. Then plants transfer to treatments. The experiments were carried out in three replicates at room temperature (30 ± 2°C). The plants were moderately watered with ordinary tap water every three days to prevent leaching from the plastic bags. The design of the experiment is shown in Table 1. |
| Sample collection |
| The pot experiment was monitored under laboratory conditions (Solid waste laboratory, University of Malaya) at a humidity of 75-85% and temperature 30-32 Oz. Fifteen grams of soil sample were collected from the phytoremediation experiments monthly and analyzed immediately across six months study duration. The soil samples were taken from the rhizosphere zone of the plants from each plastic bag monthly, and were homogenized for the analysis. |
| The total amount of diesel fuel loss in soil was determined by suspending ten grams of soil in 20 ml of acetone: hexane (1:1 v/v) in a 250 ml Erlenmeyer flask. After shaking for one hour in an orbital shaker, the oil-solvent mixture was filtered into a beaker of known weight and the solvent was completely evaporated by rotary evaporator (A1000S). The new weight of the beaker was recorded. Percentage degradation of oil was estimated using the follow formula [10]; |
| % biodegradation=[TPH control- TPH treatment / TPH control] × 100 (1) |
| Determination of biomass |
| The biomass of plants growing in clean soil and under diesel fuel and heavy metal stress (the control) was measured [12]. Prior to the analysis, plant samples were carefully washed with tap water and thoroughly rinsed with deionized water to remove any soil particles attached to the plant surfaces and wiped with filter paper to remove excess liquid before determining the fresh weight. Stem height and root length and also measured. Then the samples were oven-dried at 70°C until the weight was constant and the dry weight was recorded [13]. |
| Analysis of Zn and Diesel fuel |
| Plants were uprooted at the end of the experiment (180 days). The root tissue was extracted with a 1:1 hexane/acetone in a Soxhlet extractor for 10 hours to determine if the plant tissues had absorbed the hydrocarbon from the soil. To assess hydrocarbon content removal, the extracts were analyzed for hydrocarbons using GC (gas chromatography) with a mass-selective detector (QP2010A). The GC was equipped with cross-linked 5% phenyl methyl siloxane capillary column. Helium was used as carrier gas. The temperature was set at 40°C and raised by 10°C/min until 300°C, which was held for eight minutes. The amount of heavy metal in plant tissues was determinate by hot plate wet digestion method [14]. The Zn concentration in the soil was determined by the EPA method 3050B (acid extraction method). Briefly, soil was dried at 40°C and grounds with a laboratory blender (Waring model). 1 g samples were placed in a 250 ml flask for digestion. Then the samples were heated at the 95°C with 10 ml of 50% HNO3 without boiling. This was followed by the addition of 65% HNO3 until no brown fumes were given up by the samples. Then, gradually, 10 ml of 30% H2O2 and 37% HCL were added at the 95 OC for 15 minutes. The digested samples obtained were filtered (0.45 μm filter paper) and diluted to 100 ml with deionized water and analyzed by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). |
| Calculations of Translocation factor and Bioconcentration factor |
| The Translocation Factor (TF) index is described as the capability of heavy metals from the soil and transfer to the edible part of vegetables l |
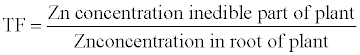 (2) (2) |
| For plants, the Bioconcentration factor (BCF) is as a measure of the metal accumulation efficiency. A BCF value of greater than one is an implication of plants potential to phytoextraction. BCF was calculated using the following formula, |
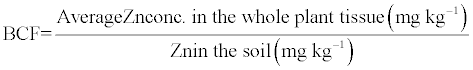 (3) (3) |
| Rate of metal uptake by plants |
| The rate of uptake of Zn by Dracaena was calculated using the first order kinetic model as follows: |
| k=- 1/t (ln M / Mo) (4) |
| Where; k=rate of metal uptake per month, t=time in month, M=mass of residual metal in the soil (ppm), Mo=initial mass of metal in the soil (ppm). |
| The data were analyzed for significant differences (p<0.05) between treatments using analyses of variance (ANOVA) with SPSS 18.0, and Duncan test comparison was used to determine the difference between treatments [15,16]. |
| Results and Discussion |
| The physicochemical properties of the investigated soils and biowastes used are presented in Table 2. The native soil had a natural pH (~7) with low N amount (0.24 %) and P content (0.08 %) (Table 2). |
| Loss of diesel fuel in co-contaminated soil |
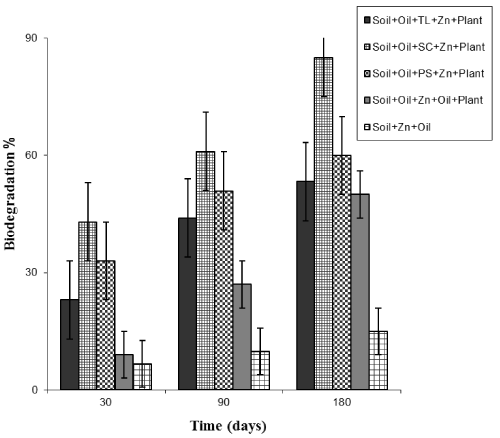
| The percentage of diesel degradation in all the amendments ranged from 9 to 85% in co-contaminated soil planted with D. reflexa (Figure 1). The percentage of biodegradation in contaminated soil containing D. reflexa without organic wastes recorded 50%, while in the control soil without the plant recorded a 15% oil loss during the period of study. |
| About 8% oil loss was recorded in autoclaved control soil without plant and polluted with Zn and 2.5% diesel fuel. The degradation of oil in autoclaved control soil (without plant) could be a result of some nonbiological factors such as volatilization or photodegradation which was recorded in poisoned treatments amended with oil and sodium azide. In addition, the highest rate of degradation was recorded in the soil amended with SC which is probably due to a higher amount of N and P, compared to other organic waste amendments. It was also reported by work that soil amended with SC, showed a higher rate of degradation [17,18]. Similarly, Dominguez-Rosado and Pichtel [19] recorded 67% engine oil loss in contaminated soil planted with mustard and sunflower plants. ANOVA result shows a significant difference between soil treated with different biowastes, unplanted contaminated soil and contaminated soil planted with D. reflexa at both concentrations (P < 0.05). This is similar to the finding of Vouillamoz and Milke [20] who indicated that addition of compost allowed diesel loss down from 1200 to 200 mg TPH kg-1 in contaminated soil planted with rye grass. Kim et al. [21] also recorded a significant reduction during the phytodegradation of diesel-contaminated soil at the end of 120 days. |
| Bioaccumulation of Zn by D. reflexa |
| Diesel fuel contained the lowest concentration of Zn compared to unplanted polluted soil. However, soil used for phytoremediation had 18.1 (mg/kg) Zn. After the soil was artificially polluted with 2.5% diesel oil and 80 (mg/kg) Zn, the amount of Zn reached 74.52 (mg/kg). Zn accumulation in treating soil amended with organic wastes recorded a range of between 32.5 mg/Kg and 41 mg/Kg, whereas in polluted and unamended treatment there was a higher quantity of Zn. At the end of 180 days, appreciable quantities of Zn (16.5, 12.2 and 6.3 ppm) were detected at D. reflexa roots, stem and leave (Table 3). The result is in conflict with the findings of Palmroth et al. [22] which demonstrated that contaminated soil with weathered hydrocarbons and amended with fertilizers (NPK) and biowaste composts; there is no accumulation of heavy metals in plant contextures. In general, from the results obtained and described above, D. reflexa obviously has the ability to accumulate heavy metals in tissues. Hence, the accumulation of Zn in D. reflexa plant amended with organic wastes was higher than with unamended control soil and treated with the D. reflexa without organic wastes. Organic waste amendments might provide a suitable condition to increase the bioavailability of metals in hydrocarbon polluted soil by enhancing the capability of the plant to uptake these metals in different plant tissues. The result is in line with the finding of Tan et al. [23] who reported that D. reflexa has the ability to accumulate Zn and Cd in different tissues in soil polluted with Zn and Cd, under greenhouse conditions. However, the result is in contrast to Clemente et al. [24] who reported that the mobility of heavy metals could be reduced in the case of fresh contamination by adding organic amendments and vegetation. |
| Plant uptake of diesel fuel |
| Gas chromatography mass spectrometry (GC/MS) analysis of the plant extract did not show the accumulation of hydrocarbons in any of the treatments. This is in contrast with the results of Palmroth et al. [25] who reported uptake of diesel oil by the root of the grass. The differences might be due to the different plants used in the studied; it might also be due to differences in the weather conditions as study by Palmroth et al. [25]. The result suggests that the mechanism of hydrocarbon removal by plants might be via phytovolitilization or rhizodegradation which has been well documented [26,27]. This is supported by the findings of different authors, who stated that flavonoids and other compounds released by roots can stimulate growth and activity of hydrocarbon degrading bacteria [25,28]. |
| Translocation, Bioconcentration factors and rate of Zn uptake by D. reflexa |
| The Bioconcentration factor was higher in those treatments amended with organic wastes compared with the unamended control soil. This might be because of available nutrients for the plant growth that produced high plant biomass, thereby causing bioaccumulation of the metals in the plant tissues more than those of the unamended control treatments. Statistical analysis shows that there is a significant difference between TF in stems and leaves (P < 0.05). The highest TF in leaves and steam was observed in the soil amended with SC (Table 4). |
| The highest BCF was observed in SC amended soil, while the lowest was, in the soil without any contamination (0.01) and plant. In terms of TF in stems and leaves, statistical analysis does not show significant differences between different treatments at P<0.05 (Table 4). The result is differed that of Adesodun et al. [29] who reported that TF of Zn in soil remediated with sunflower and contaminated with Zn was more than one. The differences in the results recorded in the two studies might be because of the use of different plants for phytoremediation. |
| Plant growth and biomass |
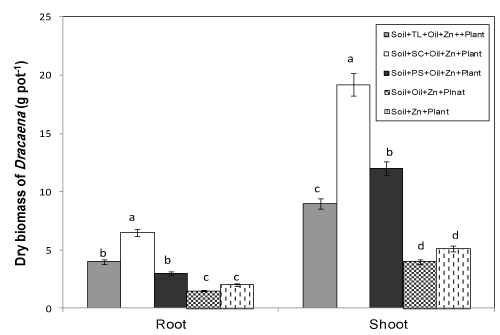
| After six months of growth, no plant death was recorded in cocontaminated soil; however, some of the plants showed signs of phytotoxicity such as stunted growth and yellowing of leaves compared with the control. The highest D. reflexa longitudinal growth was observed in the amendment with SC contaminated soils. The plant growth was significantly improved by application of organic wastes (Figure 2). |
| Conclusion |
| Within the 180 day period of study soils amended with biowastes recorded the highest rate of metal uptake than the unamended soil. However, soil amended with SC recorded a higher rate of uptake of Zn contamination, followed by the soil which was treated with PS and TL, but in the Zn contaminated soil the rate of uptake was as follows SC > TL > PS. This demonstrates that the potential of organic waste amendments to enhance phytoremediation of the oil and at the same time bioaccumulation of metals. The result is in line with the finding of the uptake rate in soil with D. reflexa plant. |
| Acknowledgement |
| This study was supported by the University of Malaya IPPP Grants FP014/2010A, PV055/2011B and PS300/ 2010B. The authors would like to thank Dr. Babak Motesharezadeh for his technical assistance. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13907
- [From(publication date):
specialissue-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9308
- PDF downloads : 4599
