Dynamics of Swallowing Tablets during the Recovery Period following Surgery for Tongue Cancer
Received: 30-Oct-2015 / Accepted Date: 09-Dec-2015 / Published Date: 15-Dec-2015 DOI: 10.4172/2161-119X.1000218
Abstract
1.1. Objective: Medicinal tablets are sometimes difficult to swallow, even for healthy individuals. Accordingly, it is likely more difficult for patients to swallow tablets after oral surgery for tongue tumors. In this study, we aimed to investigate the dynamics of swallowing tablets in the recovery period following surgery for tongue tumors.
1.2. Methods: Two experiments were conducted (Experiment 1 and Experiment 2). In Experiment 1, 20 tongue cancer patients swallowed simulated tablets and underwent videofluoroscopic (VF) examination of swallowing before and after surgery. The ability or inability to pass the tablet to the esophagus and the number of swallowing attempts required to ingest the tablet were evaluated. In Experiment 2, 48 similar subjects swallowed thickened barium and simulated tablets and underwent VF examination of swallowing after surgery. The ability or inability to pass the tablet to the esophagus, the number of swallows required to ingest the tablet, the tablet position after the initial and the final swallowing reflexes, and the oral transit time and pharyngeal transit time for swallowing the thickened barium solution and simulated tablets were evaluated.
1.3. Results: After subtotal glossectomy, more subjects were unable to pass the tablet to the esophagus after surgery rather than before surgery. However, after surgery, patients needed more numbers of swallowing reflex attempts in order to successfully swallow tablets. Also, the tablets remained not only in the mouth, but also in the epiglottic vallecula and pyriform sinus. In the patients who could pass the tablet, the oral transit time of the thickened barium solution was shorter than in the patients who could not.
1.4. Conclusion: In cases of subtotal glossectomy, tablet intake should be avoided, particularly in the recovery phase, and VF or endoscopic evaluation of swallowing should be performed when tablets are prescribed. After tongue cancer surgery, patients should be recommended to make multiple swallowing attempts when swallowing tablets.
Keywords: Deglutition, Deglutition disorders, Medical tablets, Tongue cancer, Videofluoroscopic examination of swallowing, Glossectomy, Mandibulectomy, Neck dissection
251263Introduction
Compared to foods, medicinal tablets are difficult to swallow: even 10-20% of healthy subjects have trouble swallowing tablets [1,2]. Compared to healthy subjects, patients with dysphagia reportedly experience more difficulty swallowing tablets and require an increased volume of water for tablet ingestion, a longer ingestion time, and an increase in the number of swallowing attempts [2-4]. In order to improve compliance with taking medication, investigations into the dosage forms and physical properties of tablets that can be easy to ingest have been conducted in the past [5,6]. The larger the tablets, the harder they are to ingest; conversely, if they are too small, they can be difficult to handle. A tablet size of 7-8 mm in diameter is considered the easiest for Japanese people to swallow [4,7,8].
Immediately after surgery for tongue tumors, dysphagia occurs. The mobility of the tongue is reduced due to glossectomy, and mastication and passage of food from the oral cavity to the pharynx become difficult. Furthermore, since the range of movement of the root of the tongue is restricted, pharyngeal constriction becomes dysfunctional. When neck dissection is performed to prevent metastasis of the tumor to the lymph nodes, dysfunctions of raising and closing the larynx and opening the entry to the esophagus occur. When the range of the tumor excision is enlarged, dysphagia can be severe [9-14]. In particular, when the root of the tongue is excised, dysphagia is more likely to occur [15-17]. For patients in an advanced tumor stage, and especially for those who need subtotal glossectomy, an increase in the bolus passage time occurs preoperatively [18,19]. Therefore, in patients who have undergone tongue cancer surgery, retention of tablets in the oral cavity or pharynx may occur due to dysphagia, and so the decision to prescribe tablets can be difficult. Moreover, retention of tablets can cause ulcers in the esophageal mucosa, and similar caution is relevant for the mucous membranes of the oral cavity and pharynx [20].
To date, reports on the dynamics of swallowing tablets are limited, and in particular, investigations in patients after tongue cancer surgery have not been conducted. In this study, the aim was to investigate the dynamics of swallowing tablets during the recovery period after surgery, a period in which dysphagia can readily occur.
Materials and Methods
Tongue cancer patients who underwent tumor excision followed by immediate reconstruction and neck dissection at the Tokyo Medical and Dental University Hospital Faculty of Dentistry between May 2010 and October 2012 were enrolled in the present study.
Patient images were collected from the VF image database and analyzed and investigated using a personal computer (FMVA53CW, Fujitsu, Tokyo, Japan). For VF examinations, an X‐ray fluoroscopic table (Medix-900DR, Hitachi Medical Corporation, Tokyo, Japan; 30 frames/sec) was used. For image recording and time measurement, a digital video recorder (GV-D1000, Sony, Tokyo, Japan) and video timer (VTG-33, FOR-A, Tokyo, Japan) were used. The simulated tablet was manufactured using barium (baritogensol, baritogen HD, Fushimi Pharmaceutical, Kagawa, Japan), and had a cylindrical shape with a diameter of 8 mm and a length of 4 mm. The swallowing of the simulated tablet was performed in a sitting position in the following manner: the patients put the tablet on the dorsum of the tongue by themselves; then, after instruction from the investigator, swallowed the tablet while drinking a cup of water thickened with a thickening agent and prepared as a purée, containing no barium. The viscosity was adjusted using a thickening agent (Toromi Up Perfect, Nisshin Oillio, Tokyo, Japan). Cases where the tablet did not pass the upper esophageal sphincter (UES) 30 seconds after the instruction to swallow were deemed ‘unable to pass’. Subjects who had problems with arousal or dementia, and thus had difficulties with communication, were excluded from the study.
Experiment 1: Pre- and postoperative comparison
A total of 20 patients who underwent swallowing assessment pre and postoperatively by VF using a simulated tablet were investigated in this study arm.
VF was performed an average of 2.6 ± 1.5 (range, 1-19) days before surgery and an average of 17.3 ± 5.7 (9-29) days after surgery. The patients were 15 men and 5 women with a mean age of 56 ± 15 (36-77) years. The assessment criteria were the ability or inability to ingest the tablet, and the number of swallowing attempts required to ingest the tablet.
Experiment 2: Assessment of postoperative swallowing dynamics
Simultaneously with Experiment 1, for postoperative VF investigation of tongue cancer patients who underwent tumor excision/immediate reconstruction and neck dissection, 48 patients were enrolled and underwent assessment of both the swallowing of 4 mL of a thick barium solution and a simulated tablet.
VF was performed 20.3 ± 7.1 (9-29) days postoperatively. The patients were 38 men and 10 women, and the mean age was 58 ± 14 (20-82) years.
Regarding the barium solution with a thickener added, a thickening agent was added to a 50% barium solution and then prepared into a purée form. The barium solution with thickener was applied to the dorsum of the tongue of the subjects by the investigator using a syringe. The solution was swallowed after instruction from the investigator.
The assessment criteria were as follows: ability or inability to pass the tablet to the esophagus; the number of swallows required to ingest the tablet; the tablet position after the initial and the final swallowing reflex (after initial swallowing reflex, after final swallowing reflex); and the oral transit time (OTT) and pharyngeal transit time (PTT) for swallowing the barium solution with thickener and the simulated tablet. The time measurements were performed based on the following standards: OTT was measured from the initiation of tongue movement for passing the bolus to when the tail end of the bolus passed the inferior border of the mandible, and the PTT was defined as the time from when the leading end of the bolus passed the inferior border of the mandible until the tail end of the bolus passed the UES.
Statistical analyses were performed using SPSS 11.0J for Windows (IBM, New York, NY). The Mann-Whitney test was used for comparisons of OTT and PTT, and the chi-square and Fisher’s exact tests were used for the comparison of the ability or inability to pass the tablet. Significance levels were set at <5%.
This study was performed with the approval of the Tokyo Medical and Dental University Faculty of Dentistry ethics committee (receipt number 962).
Results
Experiment 1: Pre- and postoperative comparison
Experiment 1 data of patients is shown in Table 1. Based on the VF performed before surgery, all subjects were able to pass the tablet to the esophagus. In cases of subtotal glossectomy, many subjects were unable to pass the tablet to the esophagus after surgery (Fisher’s exact test, p=0.02) (Table 2). In cases of hemiglossectomy, many patients were able to pass the tablet after surgery, and no significant differences were observed between pre- and postoperative states (Table 3).
| Case # | Sex | Age | Primary site | TNM stage | Glossectomy | Mandibulectomy | Reconstruction | Neck dissection |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 37 | Left | 4 | SG | SM | FRAF and Plate | Both |
| 2 | M | 43 | Right | 4 | SG | SM | FRAF and Plate | Left |
| 3 | F | 77 | Right | 4 | SG | SM | FRAF and Plate | Both |
| 4 | F | 42 | Left | 4 | SG | MM | FRAF | Left |
| 5 | M | 74 | Right | 3 | SG | FRAF | Right | |
| 6 | F | 39 | Right | 3 | SG | FFF | Both | |
| 7 | M | 50 | Right | 2 | SG | FFF | Right | |
| 8 | M | 58 | Left | 4 | HG | FFF | Left | |
| 9 | M | 69 | Right | 4 | HG | FFF | Right | |
| 10 | M | 76 | Left | 3 | HG | FFF | Left | |
| 11 | F | 55 | Left | 3 | HG | FFF | Left | |
| 12 | M | 36 | Left | 3 | HG | ALTF | Left | |
| 13 | M | 40 | Left | 2 | HG | FFF | Left | |
| 14 | M | 39 | Left | 2 | HG | FFF | Left | |
| 15 | M | 61 | Right | 2 | HG | FFF | Right | |
| 16 | M | 68 | Right | 2 | HG | FFF | Right | |
| 17 | M | 45 | Left | 2 | HG | FFF | Right | |
| 18 | M | 73 | Right | 2 | HG | FFF | Right | |
| 19 | M | 62 | Right | 2 | HG | FFF | Right | |
| 20 | M | 76 | Right | 1 | HG | FFF | Right |
SG: subtotal glossectomy; HG: hemiglossectomy; SM: segmental mandibulectomy; MM: marginal mandibulectomy;FRAF: free rectus abdominis flap; Plate: mandibular plate reconstruction; FFF: free forearm flap; ALTF: anterolateral thigh flap.
Table 1: Experiment1 data of patients.
| Able to pass | Unable to pass | |
|---|---|---|
| Preoperative | 7 | 0 |
| Postoperative | 2 | 5 |
* p = 0.02
Fisher's exact test
Table 2: Tablet passage ability or inability in subtotal glossectomy cases (n =7).
| Able to pass | Unable to pass | |
|---|---|---|
| Preoperative | 13 | 0 |
| Postoperative | 10 | 3 |
p = 0.59
Fisher's exact test.
Table 3: Tablet passage ability or inability in hemiglossectomy cases (n = 13).
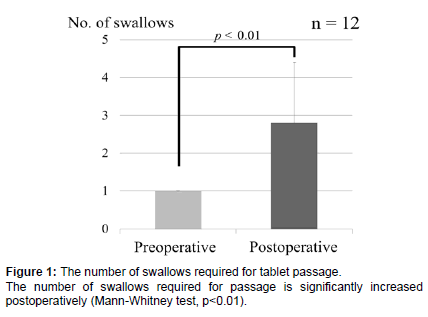
In the 12 cases where the tablet could be passed to the esophagus, the number of swallowing reflex actions required for passage was investigated. The number of swallows required to pass the tablet to the esophagus was 1 for all cases preoperatively, and an average of 2.8 ± 1.6 (1-6) swallows postoperatively. Even in the cases where passage was possible, the number of swallowing times was significantly increased postoperatively (Mann-Whitney test, p<0.01) (Figure 1).
Experiment 2: Assessment of postoperative swallowing dynamics
Experiment 2 data of patients is shown in Table 4. Based on postoperative VF, 22 of 48 (45.8%) cases could not pass the tablet to the esophagus. According to TNM stage classifications, the cases where the tablet could not be passed to the esophagus were: 2 cases in Stage I (66.7%); 5 cases in Stage II (31.3%); 7 cases in Stage III (58.3%); and 8 cases in Stage IV (47.1%). A significant difference was not observed in the ability or inability to pass the tablet (chi-square test, p=0.26) based on the TNM stage classification.
For the extent of glossectomy, the numbers of subjects who could not pass the tablet to the esophagus were: 3 cases for partial glossectomy (42.9%); 9 cases for hemiglossectomy (36.0%); and 10 cases for subtotal glossectomy (62.5%). A significant difference was not observed in the ability or inability to pass the tablet (chi-square test, p=0.16) based on the extent of glossectomy.
Regarding mandibulectomy, the numbers of subjects who could not pass the tablet to the esophagus were: 6 cases for segmental mandibulectomy (75.0%); 2 cases for marginal mandibulectomy (33.3%); and 14 cases for no excision of the mandible (41.2%). A significant difference was not observed in the ability or inability to pass the tablet (chi-square test, p=0.15) according to the extent of mandibulectomy.
From the method of reconstruction, the numbers of subjects who could not pass the tablet to the esophagus were: 12 cases for free forearm flap (38.7%); 4 cases for free rectus abdominis flap (50.0%); 5 cases for free rectus abdominis flap and mandibular plate reconstruction (83.3%); 1 case for vascularized osteocutaneous scapular flap (50.0%); and no cases for anterolateral thigh flap. A significant difference was not observed in the ability or inability to pass the tablet (chi-square test, p=0.23) regarding the reconstruction.
| Case # | Sex | Age | Primary site | TNM stage | Glossectomy | Mandibulectomy | Reconstruction | Neck dissection |
|---|---|---|---|---|---|---|---|---|
| I | F | 77 | Right | 4 | SG | SM | FRAF and Plate | Both |
| 2 | M | 46 | Right | 4 | SG | SM | FRAF and Plate | Both |
| 3 | F | 37 | Left | 4 | SG | SM | FRAF and Plate | Both |
| 4 | M | 49 | Right | 4 | SG | SM | FRAF and Plate | Both |
| 5 | M | 49 | Right | 4 | SG | SM | FRAF and Plate | Both |
| 6 | M | 43 | Right | 4 | SG | SM | FRAF and Plate | Left |
| 7 | M | 82 | Left | 4 | SG | FRAF | Both | |
| 8 | M | 55 | Right | 4 | SG | FRAF | Both | |
| 9 | F | 42 | Left | 4 | SG | MM | FRAF | Left |
| 10 | M | 66 | Right | 4 | SG | FRAF | Right | |
| 11 | M | 65 | Left | 3 | SG | MM | FRAF | Left |
| 12 | M | 57 | Left | 3 | SG | MM | FRAF | Left |
| 13 | M | 74 | Right | 3 | SG | FRAF | Right | |
| 14 | F | 39 | Right | 3 | SG | FFF | Both | |
| 15 | M | 41 | Right | 2 | SG | FRAF | Right | |
| 16 | M | 50 | Right | 2 | SG | FFF | Right | |
| 17 | M | 68 | Left | 4 | HG | SM | VOSF | Left |
| 18 | M | 59 | Left | 4 | HG | FFF | Left | |
| 19 | M | 58 | Left | 4 | HG | FFF | Left | |
| 20 | M | 69 | Right | 4 | HG | FFF | Right | |
| 21 | F | 76 | Right | 3 | HG | FFF | Both | |
| 22 | M | 76 | Left | 3 | HG | FFF | Left | |
| 23 | F | 55 | Left | 3 | HG | FFF | Left | |
| 24 | M | 62 | Left | 3 | HG | FFF | Left | |
| 25 | M | 20 | Right | 3 | HG | FFF | Left | |
| 26 | M | 64 | Left | 3 | HG | FFF | Left | |
| 27 | M | 75 | Right | 3 | HG | FFF | Right | |
| 28 | M | 36 | Left | 3 | HG | ALTF | Left | |
| 29 | F | 48 | Left | 2 | HG | MM | FFF | Left |
| 30 | M | 40 | Left | 2 | HG | FFF | Left | |
| 31 | M | 58 | Left | 2 | HG | FFF | Left | |
| 32 | M | 39 | Left | 2 | HG | FFF | Left | |
| 33 | F | 55 | Right | 2 | HG | FFF | Right | |
| 34 | M | 61 | Right | 2 | HG | FFF | Right | |
| 35 | M | 61 | Right | 2 | HG | FFF | Right | |
| 36 | M | 68 | Right | 2 | HG | FFF | Right | |
| 37 | M | 45 | Left | 2 | HG | FFF | Right | |
| 38 | M | 65 | Right | 2 | HG | FFF | Right | |
| 39 | M | 73 | Right | 2 | HG | FFF | Right | |
| 40 | M | 62 | Right | 2 | HG | FFF | Right | |
| 41 | M | 76 | Right | 1 | HG | FFF | Right | |
| 42 | F | 62 | Left | 4 | PG | FFF | Both | |
| 43 | M | 63 | Left | 4 | PG | MM | FFF | Both |
| 44 | F | 67 | Left | 4 | PG | FFF | Both | |
| 45 | M | 53 | Right | 2 | PG | FFF | Right | |
| 46 | M | 65 | Right | 2 | PG | FFF | Right | |
| 47 | M | 52 | Right | 1 | PG | SM | VOSF | Both |
| 48 | M | 63 | Left | 1 | PG | MM | FFF | Left |
SG: subtotal glossectomy; HG: hemiglossectomy; PG: partial glossectomy; SM: segmental mandibulectomy; MM: marginal mandibulectomy; FRAF: free rectus abdominis flap; Plate: mandibular plate reconstruction; FFF: free forearm flap; VOSF: vascularized osteocutaneous scapular flap; ALTF: anterolateral thigh flap.
Table 4: Experiment 2 data of patients.
The number of swallows required to pass the tablet from the oral cavity to the esophagus was 2.8 ± 1.7 (1-6) times in the 26 cases (of the 48 investigated) where passage was possible.
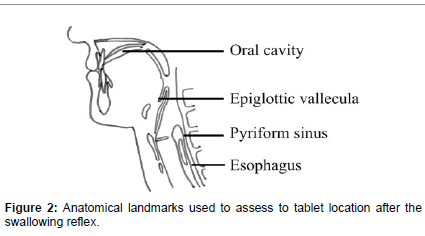
Of the 48 subjects, the tablet location after the initial swallowing reflex was in the: oral cavity in 29 cases; epiglottic vallecula in 7 cases; pyriform sinus in 5 cases; and esophagus in 7 cases. The tablet location after the final swallowing attempt was in the: oral cavity in 16 cases; epiglottic vallecula in 4 cases; pyriform sinus in 2 cases; and esophagus in 26 cases (Figure 2 and Table 5).
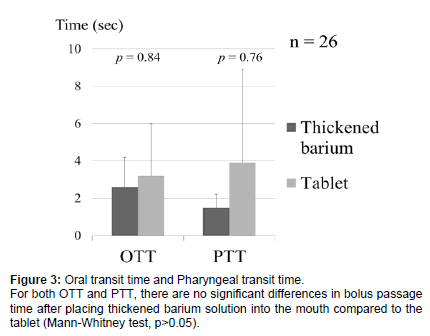
Of the 26 cases where tablet passage was possible, the OTT and PTT were measured for the thickened barium solution and the tablets. For the thickened barium solution, the OTT was 2.6 ± 1.6 seconds, and the PTT was 1.5 ± 0.7 seconds. For the tablets, the OTT was 3.2 ± 2.8 seconds, and the PTT was 3.9 ± 5.0 seconds. For both the OTT and PTT, there were no significant differences in bolus passage time between the thickened barium solution and the tablets (Mann-Whitney test, p>0.05) (Figure 3).
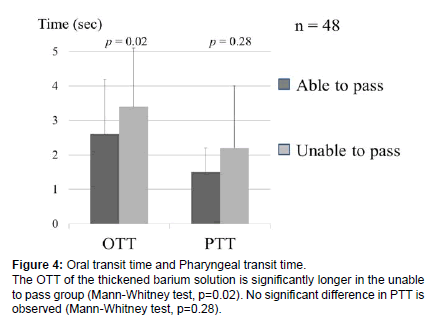
Comparison of the OTT and PTT of the thickened barium solution between cases that could pass the tablet (able to pass group) and that could not pass the tablet (unable to pass group) revealed that, in the unable to pass group, the OTT of the thickened barium solution was 3.4 ± 1.7 seconds, and the PTT was 2.2 ± 1.8 seconds. The OTT of the thickened barium solution was significantly longer in the unable to pass group (Mann-Whitney test, p=0.02), but no significant difference was observed in the PTT (Mann-Whitney test, p=0.28) (Figure 4).
Discussion
When comparing the passage of tablets based on the extent of glossectomy, there was no significant difference. However, a high proportion of patients were unable to pass the tablets after subtotal glossectomy, and when comparing the pre- and postoperative findings, a significant increase was observed in the proportion of those who could not pass the tablets after surgery. When the extent of glossectomy increases, swallowing function is known to decrease [9,11,15] and subtotal glossectomy in particular results in difficulties with passing a bolus and an increase in mealtime duration [19,21]. Moreover, when compared with subtotal glossectomy, partial glossectomy and hemiglossectomy result in a more mild form of dysphagia, which is known to improve within a few months to 1 year postoperatively [19,22-24]. Therefore, in patients who undergo subtotal glossectomy, it is considered that tablet intake should be avoided, particularly in the recovery phase. Over time, resolution of dysphagia can be expected; however, assessments of the ability to ingest tablets should be performed using VF or VE while confirming food intake status. With surgical procedures, no significant differences were observed in the pass ability. However, patients undergoing segmental mandibulectomy and accompanying rectus abdominis flap/plate reconstruction had an increased tendency to be unable to pass.
| Initial | Final | |
|---|---|---|
| Oral cavity | 29 | 16 |
| Epiglotticvallecula | 7 | 4 |
| Pyriform sinus | 5 | 2 |
| Esophagus | 7 | 26 |
Table 5: Tablet location after swallowing reflex (n= 48).
Moreover, there was no significant difference in the proportion of individuals able or unable to pass according to the TNM classification. Further investigation based not on the stage of the tumor but on the site of excision, occurrence of neck dissection, and the extent of dissection is required. Regarding retention in the oral cavity, the entry of the tablet into the dead space was the cause; therefore, avoiding the creation of dead space with the use of a flap and of dentures may be important [25].
Preoperatively, all cases required only one swallow to ingest the tablet. However, when only considering postoperative cases where passage was possible, an average of 2.8 (median 2) swallows were required. Mann et al. reported that outpatients with dysphagia require a median of 3 swallows to ingest a tablet [3]. Furthermore, the location of tablet retention was predominantly in the oral cavity due to the effect of glossectomy; however, there were also retentions in the epiglottic vallecula and pyriform sinus. Therefore, even in cases where there is no tablet retention in the oral cavity after ingestion, the possibility of retention in the pharynx must be considered, and a confirmation of the sensation of retention within the pharynx and additional swallowing should be performed.
The normal OTT and PTT values for liquids in healthy individuals are 1-1.5 and 0.38-0.48 seconds, respectively [26-31]. In the current study, a thickened barium solution resulted in an OTT of 2.6 ± 1.6 seconds. A lengthening of the OTT is known to occur in tongue tumor patients [32,33]. Pauloski et al. reported that the OTT of thick solutions 1 month post-surgery is 2.33 seconds, which is similar to the present findings [34]. Regarding PTT, a lengthening compared to normal healthy individuals was seen for both the thickened solution and tablets, and the tablets in particular showed an increase in the transit time through the pharynx. However, a longer transit time through the pharynx occurred in cases of tablet retention in the pharynx, and there was large variability in the values. In cases where the tablet passage was not possible, the OTT of the thickened solution was longer than in cases where passage was possible. Therefore, in patients where the passage of a thickened solution is difficult, the passage of tablets is also likely to be difficult. In institutions where assessments using simulated tablets are not performed, an extrapolation of the ability or inability to pass may be made from the passage of thickened solutions.
Conclusion
After subtotal glossectomy, many patients are unable to swallow tablets, and confirmation using VF or VE should be performed when prescribing tablets. For the swallowing of tablets after tongue cancer surgery, patients should be instructed to attempt multiple swallows. Even in cases where tablet retention in the pharynx is not seen in the oral cavity, investigation of the sensation of retention should be performed.
Acknowledgements
Funding: This research was carried out with a research grant from the Japanese Society of Dysphagia Rehabilitation, Japan (2010) from August 1st, 2010 to July 31st, 2011.
References
- Kottke MK, Stetsko G, Rosenbaum SE, Rhodes CT (1990) Problems encountered by the elderly in the use of conventional dosage forms. J Geriatr Drug Ther 5: 77-92.
- Roy N,Stemple J, Merrill RM, Thomas L (2007) Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann OtolRhinolLaryngol 116: 858-865.
- Carnaby-Mann G,Crary M (2005) Pill swallowing by adults with dysphagia. Arch Otolaryngol Head Neck Surg 131: 970-975.
- Miura H, Kariyasu M (2007) Effect of size of tablets on easiness of swallowing and handling among the frail elderly. Nihon Ronen IgakkaiZasshi 44: 627-633.
- Overgaard AB,Højsted J, Hansen R, Møller-Sonnergaard J, Christrup LL (2001) Patients' evaluation of shape, size and colour of solid dosage forms. Pharm World Sci 23: 185-188.
- Hey H, Jørgensen F, Sørensen K, Hasselbalch H, Wamberg T (1982) Oesophageal transit of six commonly used tablets and capsules. Br Med J (Clin Res Ed) 285(6356): 1717-1719.
- Oshima T, Hori S, Maida C, Miyamoto E (2006) Effect of size and shape of tablets and capsules on ease of grasping and swallowing (1): Comparison between elderly and students. Jpn J Pharm Health Care Sci 32: 842-848.
- Sugihara M, Hidaka M, Saito A (1986) Discriminatory features of dosage form and package. Jpn J Hosp Pharm 12: 322-328.
- Hosoda M, Koshima I, Rata T, Deguchi H, Katayama Y (1998) Assessment of swallowing function following reconstruction of base of tongue and/or lateral pharyngeal wall with microvascular tissue transfer. Jpn J Head Neck Cancer 24: 352-357.
- Hirano M,Kuroiwa Y, Tanaka S, Matsuoka H, Sato K, et al. (1992) Dysphagia following various degrees of surgical resection for oral cancer. Ann OtolRhinolLaryngol 101: 138-141.
- Fujimoto Y, Matsuura H, Kawabata K, Takahashi K, Tayama N (1997) [Assessment of Swallowing Ability Scale for oral and oropharyngeal cancer patients]. Nihon JibiinkokaGakkaiKaiho 100: 1401-1407.
- Schliephake H,Jamil MU (2002) Prospective evaluation of quality of life after oncologic surgery for oral cancer. Int J Oral MaxillofacSurg 31: 427-433.
- Diz Dios P,FernándezFeijoo J, Castro Ferreiro M, Alvarez Alvarez J (1994) Functional consequences of partial glossectomy. J Oral MaxillofacSurg 52: 12-14.
- Nicoletti G,Soutar DS, Jackson MS, Wrench AA, Robertson G (2004) Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. PlastReconstrSurg 114: 329-338.
- Kuroiwa Y (1992) Dysphagia following various degrees of surgical resection for oral and oropharyngeal cancer. JibiToRinsho 38: 812-824.
- Sato K, Kuroiwa Y, Matsuoka H, Yoshida T, Hirano M (1993) [Dysphagia following extensive oral cancer surgery.] JibiToRinsho 39: 326-328.
- Joo YH, Hwang SH, Park JO, Cho KJ, Kim MS (2013) Functional outcome after partial glossectomy with reconstruction using radial forearm free flap. AurisNasus Larynx 40: 303-307.
- Pauloski BR,Rademaker AW, Logemann JA, Stein D, Beery Q, et al. (2000) Pretreatment swallowing function in patients with head and neck cancer. Head Neck 22: 474-482.
- Matsunaga K, Oobu K, Ohishi M (2002) [The clinical study on pre and postoperative swallowing function in tongue cancer patients.] Jpn J Dysphagia Rehab 6: 53-64.
- Bailey RT Jr, Bonavina L, McChesney L, Spires KJ, Muilenburg MI, et al. (1987) Factors influencing the transit of a gelatin capsule in the esophagus. Drug IntellClin Pharm 21: 282-285.
- Yanai C, Kikutani T, Adachi M, Thoren H, Suzuki M (2008) Functional outcome after total and subtotal glossectomy with free flap reconstruction. Head Neck 30(7): 909-918.
- Hsiao HT,Leu YS, Chang SH, Lee JT (2003) Swallowing function in patients who underwent hemiglossectomy: comparison of primary closure and free radial forearm flap reconstruction with videofluoroscopy. Ann PlastSurg 50: 450-455.
- Uwiera T,Seikaly H, Rieger J, Chau J, Harris JR (2004) Functional outcomes after hemiglossectomy and reconstruction with a bilobed radial forearm free flap. J Otolaryngol 33: 356-359.
- Panchal J, Potterton AJ, Scanlon E, McLean NR (1996) An objective assessment of speech and swallowing following free flap reconstruction for oral cavity cancers. Br J PlastSurg 49: 363-369.
- Okuno K, Nohara K, Tanaka N, Sasao Y, Sakai T (2014) The efficacy of a lingual augmentation prosthesis for swallowing after a glossectomy: a clinical report. J Prosthet Dent 111: 342-345.
- Mandelstam P, Lieber A (1970) Cineradiographic evaluation of the esophagus in normal adults. A study of 146 subjects ranging in age from 21 to 90 years. Gastroenterology 58: 32-39.
- Blonsky ER, Logemann JA, Boshes B, Fisher HB (1975) Comparison of speech and swallowing function in patients with tremor disorders and in normal geriatric patients: a cinefluorographic study. J Gerontol 30: 299-303.
- Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, et al. (1989) Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia 4: 90-94.
- Lof GL, Robbins J (1990) Test-retest variability in normal swallowing. Dysphagia 4: 236-242.
- Robbins J, Hamilton JW, Lof GL, Kempster GB (1992) Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 103: 823-829.
- Rademaker AW,Pauloski BR, Logemann JA, Shanahan TK (1994) Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res 37: 314-325.
- Logemann JA, Bytell DE (1979) Swallowing disorders in three types of head and neck surgical patients. Cancer 44: 1095-1105.
- Zu Y, Narayanan SS, Kim YC, Nayak K, Bronson-Lowe C, et al. (2013) Evaluation of swallow function after tongue cancer treatment using real-time magnetic resonance imaging: a pilot study. JAMA Otolaryngol Head Neck Surg 139: 1312-1319.
- Pauloski BR, Logemann JA, Rademaker AW, McConnel FM, Heiser MA, et al. (1993) Speech and swallowing function after anterior tongue and floor of mouth resection with distal flap reconstruction. Speech Hear Res 36: 267-276.
Citation: Yoshizumi Y, Mikushi S, Nakane A, Tohara H, Minakuchi S (2016) Dynamics of Swallowing Tablets during the Recovery Period following Surgery for Tongue Cancer. Otolaryngology 6:218. DOI: 10.4172/2161-119X.1000218
Copyright: © 2016 Yoshizumi Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14343
- [From(publication date): 2-2016 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 13416
- PDF downloads: 927