Dymista© Nasal Spray with Multifocal Analysis of its Impact on the Rhinitis Disease Experience
Received: 08-Aug-2014 / Accepted Date: 19-Sep-2014 / Published Date: 25-Sep-2014 DOI: 10.4172/2161-119X-1000173
Abstract
Background: Rhinitis is a prevalent condition both in primary care and in specialist centres. It has been shown to significantly impact on quality of life. Treatment is often best managed on combined therapies. Dymista© nasal spray is filling a niche for an ‘all in one’ treatment of allergic rhinitis. Method: The MSNOT-20 is a valid disease specific quality of life instrument for rhinitis and rhinosinusitis and it was used to evaluate the symptomatic response to treatment with Dymista nasal spray. Results: Dymista has been shown to improve all domains of the patient experience of rhinitis and rhinosinusitis with positive feedback by both patients and prescribing physicians. Conclusion: Dymista is effective at improving patient symptomatology and improves quality of life. These positive results have opened up further avenues for research to explore its efficiency and place as mode of treatment.
Background
Rhinitis means inflammation of the nasal mucous membrane and often precedes sinusitis (inflammation of the lining of the paranasal sinuses), it is rare for sinusitis to occur without coexisting rhinitis and as such the most appropriate term for both is rhinosinusitis [1,2].
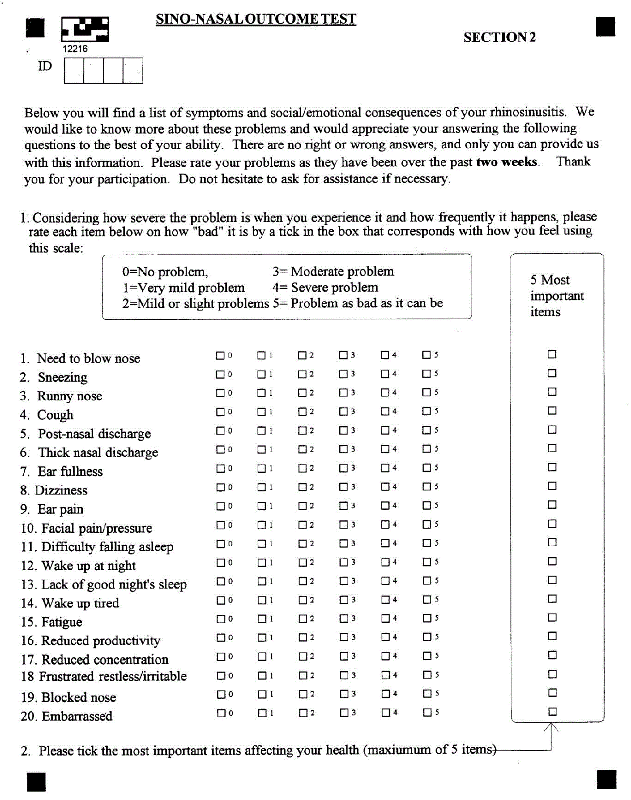
The MSNOT-20 questionnaire is a valid, disease specific quality of life instrument for Rhinitis/Rhinosinusitis (R/RS). The MSNOT-20 questionnaire has been shown to identify and discriminate between R/RS and non-disease and can evaluate the effect of treatment on symptomatology, based on severity scores, and on quality of life domains [3]. The MSNOT-20 consists of three sections; section one comprises of demographic details, section two is the disease specific section and section three is the quality of life section. The MSYP Questionnaire (MSYPQ), based on the MSNOT-20, is the equivalent tool to be used in young people (11-16 years old) and has proven to be able to identify and discriminate between R/RS and non-disease and to quantify severity [4-6]. This project shall use the MSNOT-20 to identify disease; it is validated for this condition and is disease specific as well as being technically quick and appropriate for the busy clinical setting.
Rhinitis affects the quality of life of sufferers and leads to deterioration in daily functioning related to study, work or profession. It is important to deal with the issues related to the disease and treat the cause in order to avoid physical, social and emotional issues which will help to optimize the efficiency and productivity of the educational and professional environment [7].
The MSNOT-20 questionnaire assesses symptomatology, which can be grouped into 7 different subgroups (Table 1), each one assessing a different domain affected by R/RS including paranasal, social and sleep domains [4].
| Subgroup | Questions within this sub group |
|---|---|
| Nasal | 1, 2, 3, 19 |
| Paranasal | 5, 6,7,8,9, 10 |
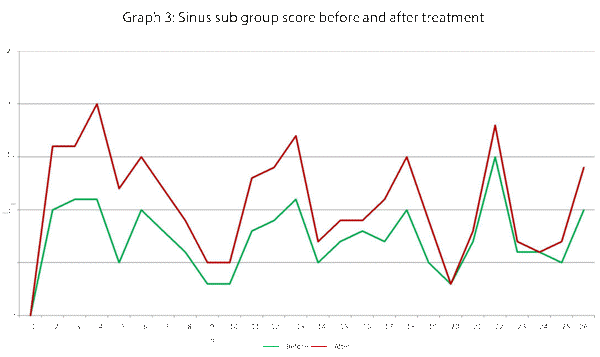
| Sinus | 5, 6, 10 |
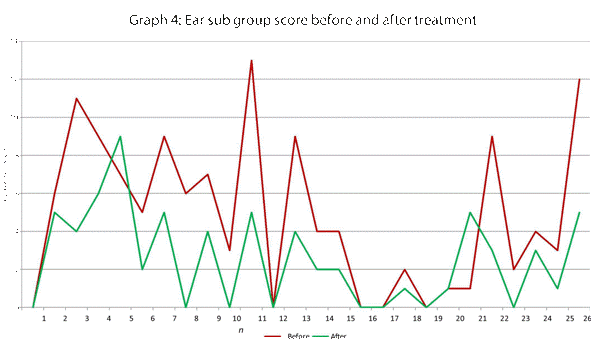
| Ear | 7, 8, 9 |
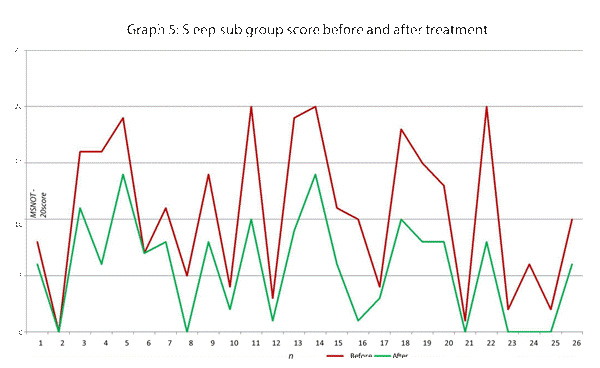
| Sleep | 11, 12, 13, 14 |
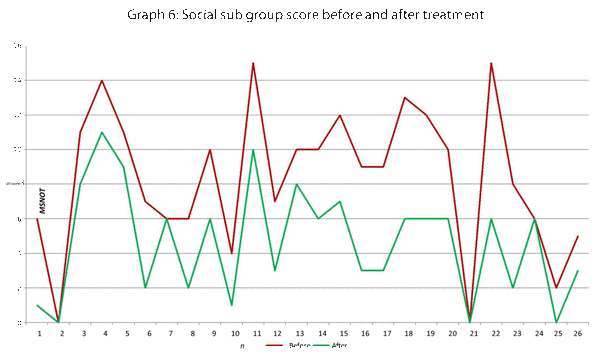
| Social | 15, 16, 17 |
| Emotional | 18, 20 |
Table 1: Breakdown of sub groups from section 2, MSNOT-20
Rhinitis and rhinosinusitis are some of the most frequent diseases in the population [1-8]. A large scale epidemiological study carried out in Farnborough, UK in 2002 quoted the prevalence of R/RS at 30% [4], which is slightly higher than a postal survey in the UK in 1991 which reported the prevalence of all forms of rhinitis to be 24%[9]. Seasonal allergic rhinitis has been shown to account for 16% in studies of 15-24 year olds [10,11].
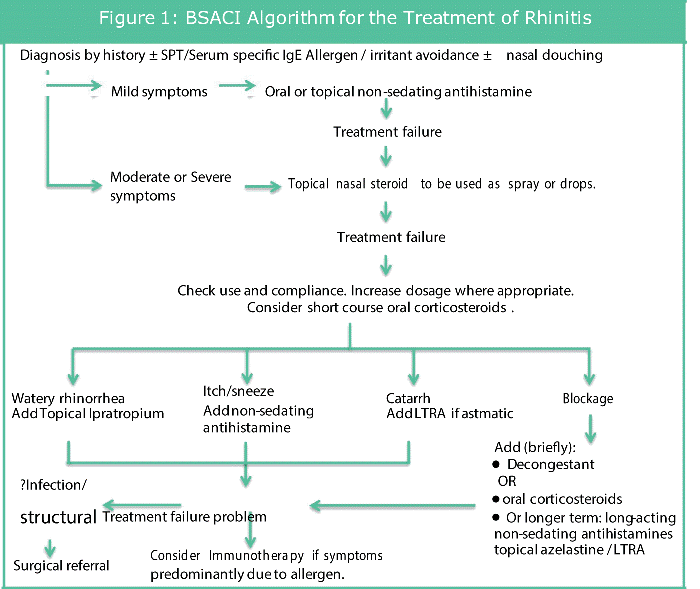
The British Society of Allergy and Clinical Immunology (BSACI) have put forward an algorithm for treatment of Rhinitis which takes into account the symptoms and its severity; this has been adapted and is shown in Figure [12]. A brief summary is such that mild cases would warrant oral or topical non-sedating anti-histamines used regularly with moderate to severe illness requiring additional intranasal corticosteroids. Further management would require a review of patient compliance, any dosage adjustments and even a modification of treatment dependent on symptomatology.
According to these guidelines, as above, and hence in clinical practice nasal steroids are frequently used as treatment for patients with nasal and sinus symptoms. Spraying steroids directly into the nose optimizes medication delivery to its target site, in this case the nasal mucosa, to maximize the wanted therapeutic effect; reducing inflammation which may be caused by an allergy or an infection [7,13]. There are many different nasal sprays available in the market, from over the counter to prescription only nasal sprays however most patients need to use the nasal spray for three to four days before they begin to notice any benefit. Multiple systematic reviews have proven intranasal corticosteroids to be effective at improving symptoms for allergic rhinitis [14,15] and it is currently endorsed by the BSACI guidelines for use in moderate to severe case (Figure 1).
Antihistamine nasal sprays are also used for nasal and sinus symptoms, they have been shown to improve rhinitic symptoms and quality of life [16]. Their function is to reduce swelling and relieve congestion by blocking the allergic cascade triggered by histamine. Effectiveness of these medications varies between individuals [17,18] such that where antihistamine may effectively relieve nasal and sinus symptoms in one patient it may prove not as effective in another. Antihistamines can be used either alone or in combination with steroid based nasal sprays. One study reported combination treatment (an antihistamine agent and a steroid agent) as being more efficacious in improving nasal symptoms than steroid, antihistamine and placebo treatment alone [19]. Combination therapy is also a strategy preferred, and more commonly used, by general practitioners [20]. Dymista nasal spray is a combination of azelastine hydrochloride (an antihistamine) and fluticasone propionate (a steroid agent) which will be assessed in this study.
The primary aim of this study was to assess the effect of Dymista nasal spray on patients suffering from rhinitis/rhinosinusitis symptomatology with a detailed analysis on effects in different symptom subgroups. Secondary aims are to look into the patient experience when using this medication.
Method
Dymista nasal spray was used in this study which evaluated the response of rhinitis/rhinosinusitis sufferers’ symptomatology to this medication. Section two of MSNOT-20 (Appendix 1) was used to assess patient symptomatology and symptom severity before treatment (values recorded when they first presented to clinic) and after treatment (the questionnaire was completed after 4 weeks of taking the medication). Treatment prescribed is defined as using the Dymista nasal spray at the recommended dose (see Dysmita information leaflet for more details). This project was carried out during the pollen season.
Patients considered for inclusion in this project were patients referred from general practice for assessment and treatment of their nasal and sinus symptoms as they were not responding to primary care treatment. Failure of primary care treatment is based upon BSACI treatment guidelines and equates to no significant response of symptomatology to oral or topical non-sedating anti-histamines used regularly then to additional intranasal corticosteroids. Such patients were assessed through history taking, clinical examination, skin prick test and Nasal Inspiratory Peak Flow (NIPF) at their first visit. The severity of the illness was quantified through the completion of section 2, MSNOT-20 which uses a Likert scale to represent increasing severity on a scale of 0-5, with 0 denoting no problem and 5 denoting that the symptom severity is as bad as it can be. Symptom severity was rechecked after four weeks of having started the treatment by, again, filling out section 2, MSNOT-20.
History taking and clinical analysis were used to determine suitability for involvement into the study and the data analysis presented in this study includes skin prick test results, nasal inspiratory peak flow results and data collected from section 2 of MSNOT-20 with a breakdown of symptomatology by grouping symptoms related to a particular pathology. These subgroups allow us to see how treatment affects each domain and are classified in Table 1. The overall result from the questionnaire was then analyzed for statistical significance.
Results
In total, data from 26 patients were found suitable for this research project. This study was initially planned to be larger however administrative and managerial restrictions limited the overall number of patients enrolled onto the study.
Allergy status and Nasal Inspiratory Peak Flow
Tables 2, 3 and 4 show the results of the skin prick test, common allergens and nasal inspiratory peak flow values, respectively.
| SPT | n |
|---|---|
| Positive | 19 |
| Negative | 6 |
| Not done as took antihistamine | 1 |
Table 2: Skin Prick Test results
| HDM | MGP | ALTERNIA | MTP | ASPERGILLUS | CAT | DOG | BIRCH POLLEN |
|---|---|---|---|---|---|---|---|
| 15 | 16 | 2 | 4 | 1 | 6 | 6 | 5 |
Table 3: The allergens patients are sensitive to; HMD= House Dust Mite, MGP= Mixed Grass Pollen, MTP=Mixed Tree Pollen.
| NIPF | n |
|---|---|
| Below 50 | 4 |
| 50-100 | 17 |
| 100-150 | 4 |
| 150-200 | 1 |
Table 4: Nasal Inspiratory Peak Flow values
Symptoms subgroup analysis
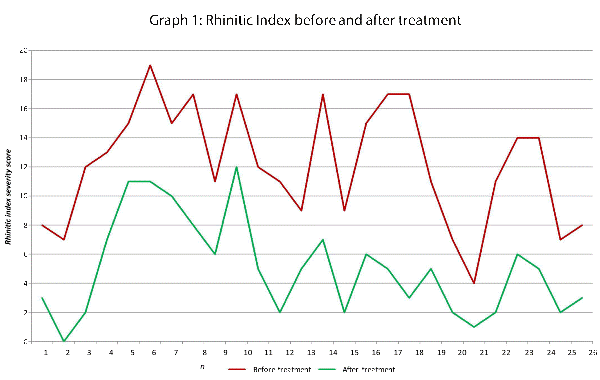
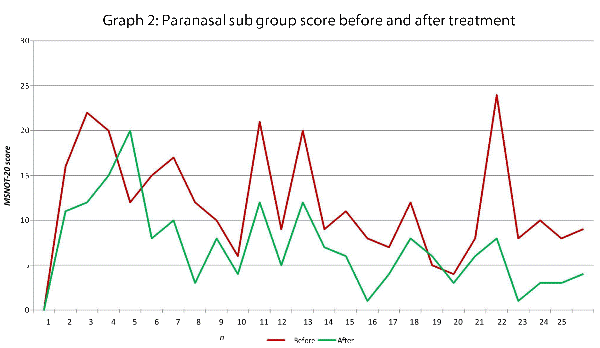
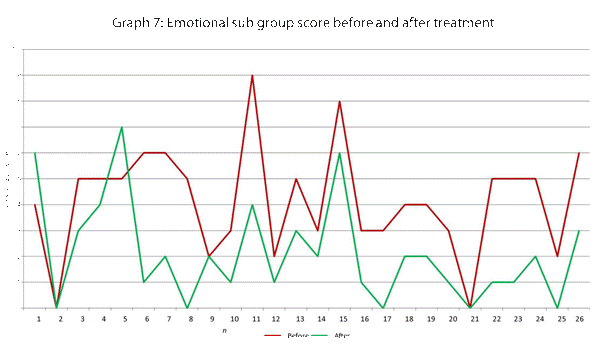
This data has been graphically represented (Graph 1-9) to show the differences in symptom severity before and after treatment with Dymista. Each sub group is individually represented, with ‘n’ signifying each individual patient and the MSNOT-20 signifying the severity of disease. The score is calculated using the quantitative severity (on the 0-5 scale, see above) as chosen by the patient on their completed section 2, MSNOT-20.
The Nasal subgroup score was analysed and is represented by a value called the ‘Rhinitic index’ (Graph 1), this value has been shown to be a good representative of symptoms associated with R/RS [3].
Overall MSNOT-20 score analysis
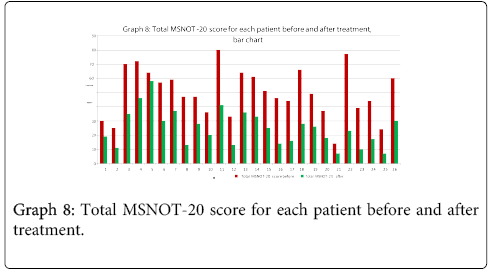
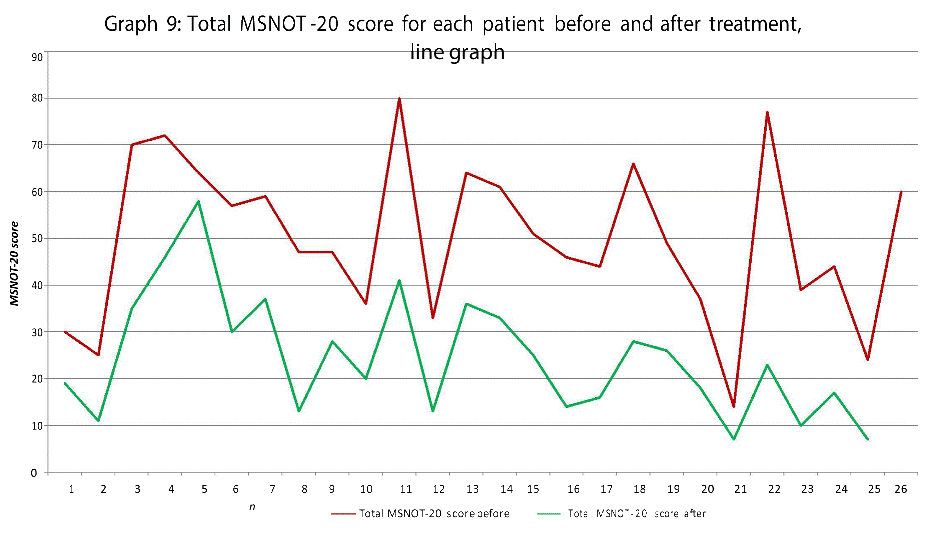
Graphs eight and nine show the total MSNOT-20 score (a result of the responses to all questions irrespective of sub group) before treatment with direct comparison to post treatment use score, this highlights the impact of treatment on patient symptomatology as a whole.
Statistical analysis
The statistical significance of change in symptomatology post treatment was calculated for each subgroup and the total MSNOT-20 score (this score was calculated by combining the data from each of the 20 items in Section 2 of the MSNOT-20). The statistical significance was calculated using Students T Test with the p value shown in Table 5.
Discussion
This study aimed to see the impact of the Dymista nasal spray on symptoms and symptom severity, as quantified by the validated disease specific quality of life questionnaire, MSNOT-20. This study further proved that the MSNOT-20 is a sensitive tool when analysing nasal and sinus disease and sensitive to changes in symptomatology following treatment.
This study was limited in number due to administrative and managerial issues; as such this shall be considered a pilot study with a larger project to be carried out in the future. These limitations also restricted the use of a control and comparison group. Having identified that Dymista has a positive impact on symptomatology and the patient experience (also see below) this opens the door to further projects which, on a larger scale, can use a greater sample with direct comparison to alternative treatment arms.
The majority of patients in this study had an allergic rhinitis with 73% of patients having a positive skin prick test (Table 2). The commonest allergies (Table 3) were to house dust mite and mixed grass pollen. This is significant as the project was carried out during the pollen season and so represented the effect of the medication with patients at their symptomatic peak.
In this pilot project we did not use a positive skin prick test as an inclusion criterion as we wanted to first gauge the response of symptoms related to R/RS to the medication and the patient experience. For future studies, as the majority of our patients suffered from allergic rhinitis, consideration can be given to using the skin prick test as such a criterion.
The Nasal Inspiratory Peak Flow (NIPF), a measure of nasal airway patency which is decreased by nasal obstruction, was less than normal in 15% of patients. It was not possible to repeat the NIPF at the 4 weeks symptom severity check due to issues with appointment allocations within the 4 week timeframe, the symptom check was unaffected by such problems as the questionnaire could be submitted or posted to the department within the 4 weeks. The NIPF has been shown to be an applicable tool to be used in monitoring response to treatment in allergic rhinitis patients [21] and this can be considered for future projects.
Analysis of all 7 subgroups (defined in Table 1), represented in (Graphs 1-7) showed that there is a significant improvement in symptomatology in each subgroup after using Dymista. The greatest benefit was seen in the nasal sub group (Graph 1, named rhinitis index) showing patients found greatest improvements in these symptoms. This was followed by improvement in sleep scores (Graph 5), closely followed by the positive impact in the paranasal subgroup (Graph 2).
There was a statistically significant decrease in symptom severity in all subgroups, including the quality of life domains (Graph 1-7), and in the overall MSNOT-20 score (Graph 8 and 9). These were all highly significant as all the calculated p values were equal to or lower than 0.01 [21] (Table 5).
Patient comments
Patients who used Dymista combination nasal spray (as opposed to the standard two individual sprays co-prescribed) found the combination spray more convenient and more effective to use. The below are a few remarks made by some patients, reproduced with permission:
“Within three days of using Dymista nasal spray I started noticing a difference”
“One bottle is really convenient to use, not a hassle at all!”
“Cheaper to buy one than two sprays”.
“Dymista nasal spray is more effective than all the other nasal sprays up until now”
Conclusion
This project reconfirms that the disease specific section of the MSNOT-20 is a sensitive instrument which can assess the changes in patients’ symptoms. This study also concludes that Dymista nasal spray can help improve the patients’ symptoms in all domains impacted by rhinitis and rhinosinusitis. The overall symptom burden and symptom severity is reduced and patients find this a satisfactory treatment method in terms of convenience and efficiency.
References
- Lanza DC, Kennedy DW (1997) Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 117: S1-7.
- Fokkens W, Lund V, Mullol J; European Position Paper on Rhinosinusitis and Nasal Polyps group (2007) European position paper on rhinosinusitis and nasal polyps 2007. RhinolSuppl : 1-136.
- Sami A (2010) Epidemiology of Rhinitis in secondary school children using MSYPQ and comparison with Modified SNOT-20 used in adult community based survey. European Academy of Allergy and Clinical Immunology.
- Sami AS, Scadding GK, Amjad M, Malik M (2013) "Rhinitis, sinusitis and ocular disease–2091. The MSYPQ: repeatability and applicability to rhinitis/rhinosinusitis." The World Allergy Organization Journal 6: 169.
- Sami, A; Scadding, G (2014) Rhinosinusitis in Secondary School Children-Part 1: Pilot of MSNOT-20 Young Persons Questionnaire (MSYPQ). Rhinology 52: 215.
- Sami A, Scadding, G. (2014) Rhinosinusitis in Secondary School Children-Part 2: Main project analysis of MSNOT-20 Young Persons Questionnaire (MSYPQ). Rhinology 52: 225.
- Sami A. Scadding G(2013) Management of Allergic Rhinitis in schools. British Journal of School Nursing 8: 119-123.
- Ghouri N, Hippisley-Cox J, Newton J, Sheikh A (2008) Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 101: 466-472.
- Sibbald B, Rink E (1991) Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax 46: 895-901.
- Strachan DP (1995) Epidemiology of hay fever: towards a community diagnosis. ClinExp Allergy 25: 296-303.
- Wüthrich B (1989) Epidemiology of the allergic diseases: are they really on the increase? Int Arch Allergy ApplImmunol 90 Suppl 1: 3-10.
- Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, et al. (2008) BSACI guidelines for the management of allergic and non-allergic rhinitis. ClinExp Allergy 38: 19-42.
- Masayuki Karaki, Kosuke Akiyama, Nozomu Mori (2001) Efficacy of intranasal steroid spray (mometasonefuroate) on treatment of patients with seasonal allergic rhinitis: Comparison with oral corticosteroids. Department of Otorhinolaryngology Head and Neck Surgery, Faculty of Medicine, Kagawa University, Japan. Ann Allergy Asthma Immunol 86: 28-35.
- Weiner J.M, Abramson M.J and Puy R.M (1998) Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. British Medical Journal 317: 1624-1629.
- Yanez A. and Rodrigo G.J (2002) Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Annals of Allergy, Asthma & Immunology 89: 479-484.
- Sheikh A, Singh Panesar S, Dhami S, Salvilla S (2007) Seasonal allergic rhinitis in adolescents and adults. ClinEvid (Online) 2007.
- Golden SJ, Craig TJ (1999) Efficacy and safety of azelastine nasal spray for the treatment of allergic rhinitis.PennState University College of Medicine, Hershey Medical Center, Hershey, Pa. 17033-0850, USA.J Am Osteopath assoc 99: 7S.
- Banov CH, Lieberman P (1999) Vasomotor Rhinitis Study Groups.Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial nonallergic) rhinitis. J Am Osteopath Assoc. 86: 28-35.
- Wolthers OD (2013) New patents of fixed combinations of nasal antihistamines and corticosteroids in allergic rhinitis. Recent Pat Inflamm Allergy Drug Discov 7: 223-228.
- Navarro A, Valero A, Rosales MJ, Mullol J (2011) Clinical use of oral antihistamines and intranasal corticosteroids in patients with allergic rhinitis. J InvestigAllergolClinImmunol 21: 363-369.
- de Souza Campos Fernandes S, Ribeiro de Andrade C, da Cunha Ibiapina C (2014) Application of Peak Nasal Inspiratory Flow reference values in the treatment of allergic rhinitis. Rhinology 52: 133-136.
- Harris M, Taylor G (2008) Medical statistics made easy, 2th edition. Oxfordshire: Scion
Citation: Sami AS, Ahmed N (2014) Dymista© Nasal Spray with Multifocal Analysis of its Impact on the Rhinitis Disease Experience. Otolaryngol (Sunnyvale) 4:173. DOI: 10.4172/2161-119X-1000173
Copyright: © 2014 Hutton J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17318
- [From(publication date): 10-2014 - Apr 18, 2025]
- Breakdown by view type
- HTML page views: 12711
- PDF downloads: 4607