Duration of COVID-19 and Predictors for Long COVID: A Retrospective US Healthcare Database Analysis
Received: 28-Jan-2023 / Manuscript No. JIDT-23-88097 / Editor assigned: 01-Feb-2023 / PreQC No. JIDT-23-88097(PQ) / Reviewed: 15-Feb-2023 / QC No. JIDT-23-88097 / Revised: 22-Feb-2023 / Manuscript No. JIDT-23-88097(R) / Published Date: 02-Mar-2023 DOI: 10.4173/2332-0877.23.S1.001
Abstract
Background: Risks of Ongoing Symptomatic COVID-19 (OSC) and Post-acute COVID-19 Syndrome (PCS), following COVID-19 infection, are not well documented. Our study evaluated those risks based on patient and COVID-19 Signs and Symptoms (CSS).
Methods: Patients with COVID-19 (first date=index) from April 1, 2020, to June 30, 2021, and ≥ 6 months of continuous enrollment pre- and post-index, in IBM® MarketScan® Commercial and Medicare Supplemental databases, were identified and stratified by severity during the disease’s acute phase, using mild, moderate and Severe/Critical (SC) algorithms defined by the Janssen COVID-19 vaccine Phase 3 Trial. Variables included demographics, comorbidities, and individual CSS. Patients with OSC (COVID-19 disease duration >4 weeks) or PCS (COVID-19 disease duration >12 weeks) were identified. Logistic regression analyses were conducted to identify risk factors for PCS across all COVID-19 patients and within each severity group.
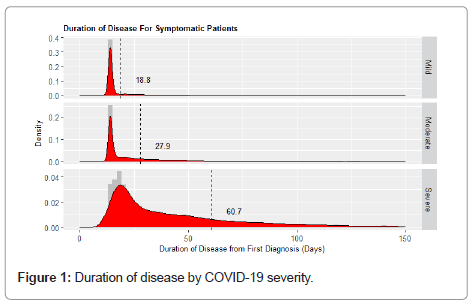
Results: 383,883 patients (160,326 mild (DoD: 18.8 days (standard deviation (SD): 15.3)); 189,240 moderate (DoD: 27.9 days (SD: 30.8)); 34,317 SC cases (DoD: 60.7 days (SD: 67.0)))were included. The percentage of patients with OSC and PCS in the mild, moderate and severe cohort was as follows: 11.8% and 0.6%, 25.2% and 3.6%, and 41.4% and 19.5%, respectively. Patients with PCS, suffered mostly from continued shortness of breath and malaise (24% and 16%, respectively). In the overall COVID-19 population, the main risk factors for PCS was severity at time of acute disease (OR for PCS in severe vs. mild population: 3.113, (95%CI: 2.336-4.149), OR for PCS in moderate vs. mild population: 1.911 (95%CI: 1.838-1.988)); CSS associated with increased risk of PCS, included somnolence: OR: 1.911 (95%CI: 1.838-1.988) and thrombosis: OR: 1.787 (95%CI: 1.717-1.860).
Conclusions: PCS affected nearly one in 5 patients with severe or critical COVID-19 at time of acute infection.
Keywords: COVID-19; Signs and symptoms; Disease predictors
Abbreviations
CSS: COVID-19 Signs and Symptoms; PCS: Post- acute COVID-19 Syndrome; OSC: Ongoing Symptomatic COVID-19; OR: Odds Ratio; DoD: Duration of Disease; SD: Standard Deviation
Introduction
The Coronavirus disease (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents different clinical manifestations, ranging from complete absence of symptoms to critical illness. The US National Institutes of Health (NIH) defines the following categories of severity among adults with COVID-19: asymptomatic, mild, moderate, severe, and critical Illness. Comparable categorization of COVID-19 severity have also been developed and used in the Janssen Phase 3 ENSEMBLE vaccine clinical trials [1].
The average course of COVID-19 has been reported between 2 and 6 weeks. Some patients, however, have been shown to exhibit symptoms lasting for weeks or months following the acute stage of the illness. This phenomenon has been observed in both, patients with severe acute disease as well as patients that did not require hospitalization and only suffered mild COVID-19 [2]. The National Institute for Health and Care Excellence defines long COVID as: Ongoing Symptomatic COVID-19 (OSC), in patients with symptoms that extend from 4 to 12 weeks, and Post-COVID-19 Syndrome (PCS), for patients with ongoing symptoms for more than 12 weeks [3].
The magnitude of OSC and PCS in both occurrence and persistence is not well known. The primary findings from a nationally representative survey from the United Kingdom Office for National Statistics has shown that approximately 1 in 10 participants with COVID-19 may have persistent symptoms for 12 weeks or more [4]. Other studies, conducted in the United States and Switzerland show that approximately a third of patients with COVID-19 had not recovered their initial state of health at 3 to 6 weeks after receiving the diagnosis [5,6]. Children may also be affected by OSC and PCS, although less frequently. In such cases, symptoms are similar to those of adults, including fatigue, headache, insomnia, lack of concentration, muscle and joint pain, and cough [7].
Predictors for PCS are currently investigated. In patients 65 years and older, an association was observed between the count of distinct signs and symptoms present during the acute stage of COVID-19 and the risk for PCS. This study also found that fatigue during the acute phase was associated with PCS [8]. In other studies, severity at index was the main reported risk factor for PCS. [9,10] Another study in China also suggested differences in gender, with a higher likelihood of women vs. men experiencing fatigue and anxiety/depression at 6 months follow- up, similar to patients who survived SARS [10-12]. Other underlying conditions, including diabetes, obesity, chronic cardiovascular and kidney disease, cancer and organ transplantation, already associated with more severe forms of COVID-19, may be potential predictors of PCS [13,14].
To further understand prevalence of and risks for, OSC and PCS, we conducted an analysis to accurately evaluate the duration of COVID-19 and used predictive analytical methods to evaluate risk factors for OSC and PCS for each group of patients (mild, moderate vs. severe) separately. Our study contributes to the current body of evidence as patients’ demographics, comorbidities, COVID-19 signs and symptoms, as well as treatments for COVID-19, are assessed in order to identify risk factors for OSC or PCS. Our study also separately analyzed patients with mild, moderate severe/critical acute disease to identify potential risk factors specific to each population.
Materials and Methods
Data sources
The IBM® MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Supplemental databases were used. The IBM® MarketScan® databases comprise enrollment information, demographics, and adjudicated inpatient medical, outpatient medical, and outpatient pharmacy claims data collected from over 300 large, self- insured U.S. employers and over 25 U.S. health plans. The Commercial database includes information for individuals who are under the age of 65 and are the primary insured or a spouse or dependent thereof.
Study population
Patients with a COVID-19 diagnosis (U071-U072-B34.2, B97.29, J12.81) from April 1, 2020, to June 30, 2021, were identified in the database. For each patient, the date of earliest diagnosis was defined as “index date” and the location of the medical encounter that was associated with the index was defined as the index site of care (in case of multiple diagnoses of COVID, the first instance was considered) and categorized as inpatient, outpatient or emergency room. All patients in the study also required at least 6 months of continuous medical enrollment pre-index.
Variable creation
Patient demographics (age, gender, and geographic location within the United States) at index date were captured. Thirty-one patient comorbidities as defined by Elixhauser, as well as the Elixhauser Comorbidity Index (ECI), were captured for all patient [15,16]. The ECI was selected instead of the Charlson Comorbidity Index (CCI) as recent research suggests that the ECI might be more predictive of frailty and mortality than the CCI which could have relevance on course of COVID-19 [17,18]. In addition, the following disease states were identified for all patients: Renal disease (mild, moderate and severe), psychosis, osteoporosis, liver disease (mild, moderate and severe), visual and hearing impairment, dementia, degenerative disc disease, anxiety, angina and asthma. Pregnancy status (with date of first pregnancy condition less than 280 days prior and up to index date) was also flagged. All comorbidities were identified between 365 and 15 days prior to index date (to avoid including morbidities related to the COVID-19 disease).
Duration of disease, ongoing symptomatic COVID-19 and Post-COVID-19 Syndrome
Duration of disease was determined based on the presence of any one COVID-19 Sign or Symptom (CSS). A complete list of CSSs, and corresponding diagnosis codes, are shown in Supplemental Table 1. For each patient with COVID-19, CSS diagnoses were identified starting 5 days before the COVID-19 code. Diagnoses listed prior to the index date were used because many patients presented with CSSs before being tested for, and given a diagnosis of, COVID-19. (For many mild and moderate cases, the majority of healthcare use happened prior to index). COVID-19 was considered ongoing as long as the gap between distinct CSS diagnoses was less than 35 days. When a new diagnosis was identified after a 35-day gap, it was assumed that this was a new condition not related to the COVID index event. This rule was used as most CSSs are very generic in nature (e.g., cough, headache) and potential association to index COVID-19 may thus decline as the time gap in diagnosis dates increases. The decision to use a 35-day gap (versus a shorter or longer gap) was based on review of histograms of time gaps for CSS conditions, for all patients. For patients with mild or moderate severity with no healthcare events in the database after their COVID-19 index code, it was assumed that they would have duration of disease of 9 days following the last index COVID-19 diagnosis. The minimum duration of any symptomatic patient, in our study, was 14 days. This was based on prior publications of disease duration [6,19]. Patients with no CSS diagnoses in the 5 days prior, and up to 35 days post index, were considered “asymptomatic” and excluded from the study. Ongoing Symptomatic COVID-19 (OSC) was defined as duration of disease from 4 to 12 weeks. Post COVID-19 Syndrome (PCS) was defined as duration of disease greater than 12 weeks.
COVID-19 signs and symptoms
For each CSS as listed in Supplemental Table 1, three variables were created, based on whether the CSS was observed: 1) anytime during the duration of disease; 2) during the PCS phase, after 12 weeks from index, or 3) during the initial phases of the disease (first 28 days, prior to OSC status). The presence of CSSs during the initial phases of the disease were used to determine severity during the acute phase of the disease, and to identify early signs and symptoms associated with PCS, using logistic regression and predictive modeling approaches.
Disease severity
The classification of mild, moderate and severe/critical was made based on the Janssen trial definitions and the presence of CSS diagnoses. Supplemental Table 2A shows the criteria for each severity classification [20,21]. Mild COVID-19 patients were characterized as having only one symptom. Patients with moderate disease presented with either 2 symptoms or 1 symptom indicative of pneumonia or lower respiratory disease. Severe/critical COVID-19 patients experienced at minimum, severe respiratory conditions such as acute respiratory distress or failure, along with other symptoms. All hospitalized patients had either moderate or severe/critical disease. All patients admitted to the Intensive Care Unit (ICU) or on ventilators were categorized as having severe/critical form of a disease.
Statistical analyses
Descriptive statistics was reported for all study variables, duration of disease and prevalence of OSC and PCS, based on severity of disease- means and standard deviations for continuous variables, frequencies and percentages for categorical variables. Logistic regression analyses were conducted to identify risk factors for PCS across all COVID-19 patients and within each severity group. These analyses were conducted in R version 4.1.
Results
The study included 383,883 patients with a COVID-19 diagnosis, including 160,326 mild, 189,240 moderate, and 34,317 Severe/Critical (SC) cases. Demographic and comorbid data of patients categorized by severity of disease are presented in Supplemental Tables 3 and 4. The mean (Standard Deviation (SD)) age of patients with mild, moderate, and severe severity was 37.71 (SD: 17.13), 42.70 (SD: 16.49), and 55.58 (SD: 14.87) respectively. The proportion of female population was 55% for mild, 57% for moderate and 44% for severe patients. Inpatient admissions were observed for 1% mild, 7% moderate and 96% severe/ critical cases. One third of the severe/critical COVID-19 patients were admitted to the Intensive Care unit (ICU), and 5% required mechanical ventilation. The Elixhauser comorbidity scores for patients with mild, moderate and SC severity were 0.68 (SD: 1.15), 1.04 (SD: 1.49), and 2.16 (SD: 2.35) respectively, as shown in Supplemental Table 4. For each of the 31 chronic diseases described by the Elixhauser, the rate of chronic conditions in the population increased with increasing COVID-19 severity. For example, diabetes uncomplicated (Elixhauser 11) was observed in 5.4% of the “mild” population, but affected 8.6% of the moderate and 22.2% of the severe/critical cases.
Supplemental Table 5 shows the main CSSs (affecting more than 5% patients) reported by severity group. A diagnosis of cough was the most prevalent CSS in patients with mild and moderate disease. For mild disease, sore throat, runny nose and fever affected 23%, 11%, and 11% patients, respectively. In the moderate category, shortness of breath was the 2nd most common diagnosis (43% patients), followed by fever, sore throat and malaise (30%, 28% and 25%, respectively). For severe COVID-19, shortness of breath, pneumonia and acute respiratory failure were the main CSSs (91%, 82% and 78%, respectively).
The mean (SD) duration of disease in the mild, moderate and severe cohorts was as follows: 18.8 (15.3) days, 27.94 (30.80) days, and 60.7 (67.0) days, as shown in Figure 1. The percentage of patients with OSC and PCS was 11.8% and 0.6% in the mild, 25.2% and 3.6% in the moderate and 41.4% and 19.5% in the severe/critical cohorts, respectively, as shown in Figure 2.
Demographics and comorbidity tables specifically focusing on patients with OSC and PCS are shown in Tables 1 to 3. A total of 288,709 patients had disease duration <4 weeks, whereas 80,873 patients experienced OSC, and 14,301 patients had PCS. As shown in Table 1, duration of disease increased with increasing age. For each duration category, the percentage of patients with initial mild, moderate and severe disease is shown. Whereas only 0.6% of mild cases developed PCS, of the final PCS cohort, 6.2% initially had mild disease. The final PCS cohort had the same proportion ( ~ 47%) of patients with moderate and severe/critical disease at time of index. Due to the high proportion of patients with index severe/critical disease, PCS patients also had greater proportion of ICU and mechanical ventilator use cases. Table 2 shows baseline comorbidities of patients with COVID-19, at the time of their acute disease, stratified by eventual duration of disease. Duration of disease also increased with rates of all reported comorbidities. The CSSs experienced during the acute COVID-19 disease are shown in Table 3. Rates for most CSSs increased significantly between the <4 weeks, OSC and PCS cohorts. None of the CSSs were observed exclusively in one or the other cohort, but some CSSs were rare in the <4 week cohort, such as acute respiratory distress syndrome (<4 week cohort: 0.6%).
| <4 weeks | OSC | PCS | |
|---|---|---|---|
| N | 2,88,709 | 80,873 | 14,301 |
| Age-Mean (SD) | 40.02 (17.06) | 46.01 (16.83) | 53.05 (17.29) |
| Gender: Female-n (%) | 155,424 (53.8%) | 47,325 (58.5%) | 8,002 (56.0%) |
| Payer: Medicare -n (%)* | 10,715 (3.7%) | 6,501 (8.0%) | 2,729 (19.1%) |
| Severity of COVID-19 at index acute disease | |||
| Mild | 140,452 (48.6%) | 18,991 (23.5%) | 883 (6.2%) |
| Moderate | 134,837 (46.7%) | 47,674 (58.9%) | 6,729 (47.1%) |
| Severe/Critical | 13,420 (4.6%) | 14,208 (17.6%) | 6,689 (46.8%) |
| Duration of Disease in Days-Mean (SD) | 15.7 (3.3) | 44.5 (13.8) | 157.7 (81.9) |
| ICU Admission | 3,814 (1.3%) | 5,008 (6.2%) | 3,110 (21.7%) |
| Mechanical ventilator use | 304 (0.1%) | 701 (0.9%) | 884 (6.2%) |
Note: OSC: Ongoing COVID-19 Symptomatic COVID-19 (4-12 weeks); PCS: Post-COVID-19 Syndrome (>12 weeks)
Table 1: Baseline demographic characteristics of patients with COVID-19, stratified by duration of disease.
| <4 weeks | OSC | PCS | |
|---|---|---|---|
| Elixhauser comorbidity score | |||
| Mean (SD) | 0.82 (1.31) | 1.34 (1.74) | 2.38 (2.55) |
| Elixhauser score category | |||
| 0 | 169,547 (58.7%) | 35,291 (43.6%) | 3,987 (27.9%) |
| 1-2 | 89,637 (31.0%) | 29,981 (37.1%) | 4,968 (34.7%) |
| 3-4 | 23,082 (8.0%) | 10,962 (13.6%) | 2,889 (20.2%) |
| 5+ | 6,443 (2.2%) | 4,639 (5.7%) | 2,457 (17.2%) |
| Comorbidities, n (%) | |||
| Elixhauser 1: Congestive heart failure | 3,177 (1.1%) | 2,160 (2.7%) | 1,168 (8.2%) |
| Elixhauser 2: Cardiac Arrhythmia | 10,178 (3.5%) | 5,447 (6.7%) | 2,065 (14.4%) |
| Elixhauser 3: Valvular disease | 3,935 (1.4%) | 2,236 (2.8%) | 859 (6.0%) |
| Elixhauser 4: Pulmonary circulation disorders | 1,154 (0.4%) | 769 (1.0%) | 445 (3.1%) |
| Elixhauser 5: Peripheral vascular disorders | 3,451 (1.2%) | 2,217 (2.7%) | 1,074 (7.5%) |
| Elixhauser 6: Hypertension Uncomplicated | 54,456 (18.9%) | 23,121 (28.6%) | 6,107 (42.7%) |
| Elixhauser 7: Hypertension Complicated | 4,463 (1.5%) | 2,870 (3.5%) | 1,383 (9.7%) |
| Elixhauser 8: Paralysis | 360 (0.1%) | 261 (0.3%) | 173 (1.2%) |
| Elixhauser 9: Other neurological disorders | 3,403 (1.2%) | 1,869 (2.3%) | 891 (6.2%) |
| Elixhauser 10: Chronic pulmonary disease | 16,884 (5.8%) | 7,943 (9.8%) | 2,576 (18.0%) |
| Elixhauser 11: Diabetes Uncomplicated | 20,496 (7.1%) | 9,234 (11.4%) | 2,703 (18.9%) |
| Elixhauser 12: Diabetes Complicated | 14,399 (5.0%) | 6,835 (8.5%) | 2,270 (15.9%) |
| Elixhauser 13: Hypothyroidism | 17,482 (6.1%) | 7,289 (9.0%) | 1,802 (12.6%) |
| Elixhauser 14: Renal failure | 4,298 (1.5%) | 2,755 (3.4%) | 1,384 (9.7%) |
| Elixhauser 15: Liver disease | 5,018 (1.7%) | 2,474 (3.1%) | 726 (5.1%) |
| Elixhauser 16: Peptic ulcer disease excluding bleeding | 574 (0.2%) | 303 (0.4%) | 108 (0.8%) |
| Elixhauser 17: AIDS/HIV | 792 (0.3%) | 234 (0.3%) | 39 (0.3%) |
| Elixhauser 18: Lymphoma | 550 (0.2%) | 327 (0.4%) | 162 (1.1%) |
| Elixhauser 19: Metastatic cancer | 723 (0.3%) | 463 (0.6%) | 232 (1.6%) |
| Elixhauser 20: Solid tumor without Metastasis | 5,077 (1.8%) | 2,396 (3.0%) | 805 (5.6%) |
| Elixhauser 21: Rheumatoid Arthritis/collagen | 6,264 (2.2%) | 2,989 (3.7%) | 881 (6.2%) |
| Elixhauser 22: Coagulopathy | 2,029 (0.7%) | 1,143 (1.4%) | 481 (3.4%) |
| Elixhauser 23: Obesity | 27,001 (9.4%) | 11,070 (13.7%) | 2,744 (19.2%) |
| Elixhauser 24: Weight loss | 1,502 (0.5%) | 878 (1.1%) | 447 (3.1%) |
| Elixhauser 25: Fluid and electrolyte disorders | 5,026 (1.7%) | 2,911 (3.6%) | 1,407 (9.8%) |
| Elixhauser 26: Blood loss anemia | 1,034 (0.4%) | 543 (0.7%) | 198 (1.4%) |
| Elixhauser 27: Deficiency anemia | 5,881 (2.0%) | 3,074 (3.8%) | 962 (6.7%) |
| Elixhauser 28: Alcohol abuse | 1,711 (0.6%) | 657 (0.8%) | 190 (1.3%) |
| Elixhauser 29: Drug abuse | 2,167 (0.8%) | 776 (1.0%) | 296 (2.1%) |
| Elixhauser 30: Psychoses | 478 (0.2%) | 273 (0.3%) | 112 (0.8%) |
| Elixhauser 31: Depression | 25,468 (8.8%) | 9,733 (12.0%) | 2,372 (16.6%) |
Note: OSC: Ongoing COVID-19 Symptomatic COVID-19 (4-12 weeks); PCS: Post-COVID-19 Syndrome (>12 weeks)
Table 2: Baseline comorbidities of COVID-19 patients at time of acute infection, stratified by duration of disease.
| Symptom | <4 weeks | OSC | PCS |
|---|---|---|---|
| Shortness of Breath | 51,505 (17.8%) | 33,579 (41.5%) | 9,771 (68.3%) |
| Pneumonia | 34,110 (11.8%) | 25,257 (31.2%) | 8,191 (57.3%) |
| Malaise | 43,669 (15.1%) | 25,170 (31.1%) | 7,809 (54.6%) |
| Cough | 119,013 (41.2%) | 37,198 (46.0%) | 7,107 (49.7%) |
| Chest Pain | 31,369 (10.9%) | 23,801 (29.4%) | 6,778 (47.4%) |
| Acute Respiratory Failure | 10,471 (3.6%) | 11,649 (14.4%) | 5,836 (40.8%) |
| Hypoxemia | 8,525 (3.0%) | 8,657 (10.7%) | 4,454 (31.1%) |
| Fatigue | 32,348 (11.2%) | 17,099 (21.1%) | 4,356 (30.5%) |
| Fever | 63,304 (21.9%) | 16,816 (20.8%) | 3,973 (27.8%) |
| Confusion | 6,909 (2.4%) | 7,155 (8.8%) | 3,711 (25.9%) |
| Tachycardia | 7,283 (2.5%) | 7,222 (8.9%) | 3,414 (23.9%) |
| Sore Throat | 38,471 (13.3%) | 11,246 (13.9%) | 2,904 (20.3%) |
| Renal Failure | 3,544 (1.2%) | 4,225 (5.2%) | 2,631 (18.4%) |
| Sepsis | 4,009 (1.4%) | 4,510 (5.6%) | 2,617 (18.3%) |
| Diarrhea | 13,168 (4.6%) | 6,807 (8.4%) | 2,067 (14.5%) |
| Myalgia | 18,803 (6.5%) | 6,389 (7.9%) | 1,889 (13.2%) |
| Vomiting | 9,493 (3.3%) | 5,203 (6.4%) | 1,875 (13.1%) |
| Acute Respiratory Distress Syndrome | 1,596 (0.6%) | 1,963 (2.4%) | 1,466 (10.3%) |
Note: OSC: Ongoing COVID-19 Symptomatic COVID-19 (4-12 weeks); PCS: Post-COVID-19 Syndrome (>12 weeks)
Table 3: Key patient symptoms at time of acute COVID-19 disease, stratified by duration of disease.
After 12 weeks, patients with PCS (N=14,301) suffered mostly from continued shortness of breath and malaise (24% and 16%, respectively), as shown in Table 4. Pneumonia, respiratory failure and chest pain also affected 10%, 9% and 9% of patients, respectively. A complete list of all the CSSs at time of PCS is shown in Supplemental Table 6.
| COVID-19 Sign or Symptom | N (%) |
|---|---|
| Shortness of breath | 3,415 (23.7) |
| Malaise | 2,286 (15.9) |
| Pneumonia | 1,483 (10.3) |
| Respiratory Failure | 1,363 (9.5) |
| Chest Pain | 1,263 (8.8) |
| Fatigue | 1,098 (7.6) |
| Confusion | 1,010 (7.0) |
| Cough | 937 (6.5) |
| Sore Throat | 878 (6.1) |
Note: 100%=14,301 patients with PCS.
Table 4: Key COVID-19 signs and symptoms observed in patients with PCS, after 12 weeks post-index. Only diagnoses affecting more than 5% of patients are listed herein.
In the overall COVID-19 population, severity at time of acute disease was the most significant risk factor for PCS (odds ratio for PCS in severe vs. mild population (OR): 3.113 (95% confidence interval (CI): 2.336-4.149), OR for PCS in moderate vs. mild population: 1.911 (95%CI: 1.838-1.988)). Females ( males) were also at increased odds of PCS (OR: 1.763 (95%CI: 1.667-1.864)). The odds for PCS also increased with age (independently of severity-in 10-year increment: OR for PCS: 1.746 (95%CI: 1.466-2.079). From all the CSSs, somnolence and thrombosis at acute disease had the greatest association with PCS (OR: 1.911 (95%CI: 1.838-1.988) and OR: 1.787 (95%CI: 1.717- 1.860), respectively). Patient comorbidities, defined as chronic diseases identified prior to COVID-19, had much weaker associations to PCS. The outputs of the logistic regression models are shown in Supplemental Tables 7A-7D. While PCS was rare in mild cases, somnolence, myalgia and neurological symptoms were associated with greater odds for PCS in this population (OR: 8.650 (95%CI: 4.662-16.050), OR: 7.937 (95%CI: 6.414-9.823) and OR: 5.908 (95%CI: 4.304-8.110), respectively). In the moderate COVID-19 population, thrombosis, somnolence and acute respiratory failure were the top 3 CSSs associated with PCS (OR: 4.128 (95%CI: 3.472-4.908), OR: 3.041 (95%CI: 1.965-4.706), and OR: 2.687 (95%CI: 2.289-3.155), respectively). Thrombosis, hepatic failure and somnolence were the top 3 CSSs associated with PCS in the severe/ critical population (OR: 2.178 (95%CI: 1.336-3.551), OR: 2.171 (95%CI: 1.644-2.867) and OR: 2.136 (95%CI: 1.883-2.423), respectively).
Discussion
This study was designed to analyze disease duration and risk for PCS in the general US population. For this purpose, a nationwide commercial database (with patients up to age 65) and a Medicare supplemental database (with patients mostly 65 and above) were analyzed. A characteristic of COVID-19 is its widely heterogenous clinical manifestation, with disease severity ranging from asymptomatic to deadly. To better characterize disease duration, and the risks associated with an extended duration (PCS), we analyzed patients se parately based on the severity of their acute episode. Our analysis suggests that duration of disease averaged approximately 19 days for mild cases, and up to 61 days for severe cases. The rates of PCS differed significantly by group, with less than 1% of mild cases vs. 4% moderate and 20% severe cases developing PCS. The severity of the acute episode was therefore the main predictor for PCS. Other factors independently associated with PCS included female sex ( male), increased age, as well as some clinical diagnosis at time of index disease, specifically somnolence in mild cases and thrombosis in moderate and severe cases.
To evaluate the severity of the patients, clinical signs and symptoms, as captured in the diagnoses fields of claims databases, were analyzed. The algorithms used to define mild, moderate and severe, were based on the Janssen ENSEMBLE Phase 3 clinical trial, as shown in Supplemental Table 2A. The definitions for mild and severe/critical cases in the Janssen trial are similar to those of the National Institute of Health (NIH) (See Supplemental Table 2B). Both the mild and moderate Janssen trial definitions also include detailed descriptions of diagnoses and capture all the signs and symptoms typically described in the literature as associated with COVID-19. This definition of disease severity allows for a very unambiguous, and exhaustive, categorization of patients as mild/moderate and severe/critical. As expected, our mild, moderate and severe/critical patient populations exhibited known characteristics of patients defined with other severity definitions, such as increasing comorbidities and age with increasing COVID-19 severity [22].
A major assumption in our analysis was the maximum 35-day gap allowed between CSSs. We used this gap based on review of histograms and feasibility analyses not presented here. This maximum gap in care was important, because CSSs are very generic (e.g., headache, myalgia, gastrointestinal symptoms) and patients will return with these generic signs and symptoms, whether or not related to their initial COVID-19. Our analysis is therefore possibly conservative, as patients returning for care due to PCS after a >35-day break from healthcare would not be characterized in our research as having PCS.
Our study has some key limitations: The study included data from late 2020 to early 2021, describing outcomes with a COVID-19 variant that may not be dominant anymore. However, our focus on disease severity and clinical presentation, and analysis of risk factors based thereof, may provide a framework for understanding PCS in patients with COVID-19, regardless of variant. An additional limitation is our use of claims databases for this analysis. All signs and symptoms may not be exhaustively captured in this dataset, as might be the case in a prospective clinical trial. However, conducting such a prospective trial with large-enough sample size to evaluate duration of disease across different severity of COVID-19, would be challenging. As discussed above, the assumption related to the calculation of disease duration, allowing for a maximum 35-day gap between visits also represents a limitation. Finally, individuals included in this study were identified from US-based Commercial and Medicare Supplemental databases. It is unclear whether the findings of this study would apply to other geographies or populations, but the cohorts selected for this analysis had medical coverage for the entire duration of the study, and hence had access-from a financial standpoint-to needed healthcare. It is therefore very likely that these patients would have used healthcare for signs and symptoms post-COVID and thus would have been identifiable as having long COVID in our analysis.
Conclusion
In conclusion, we evaluated the duration of COVID-19 in more than 300K patients, of which 34,317 were severely/critically ill. Our analysis showed that duration of COVID-19 increased from an average of 19 days in mild cases to 61 days in severe/critical cases. Post-COVID-19 syndrome affected less than 1% of mild cases vs. 4% moderate and 20% severe cases. The severity of the acute COVID-19 episode was the main predictor for PCS. Other factors included female sex ( male), increased age, somnolence and thrombosis at time of acute disease.
Acknowledgments
The authors wish to acknowledge Dr. Lilit Hovhannisyan, for editorial support.
References
- Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, et al. (2021) Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 384:2187-2201.
[Crossref] [Google Scholar] [PubMed]
- Van Kessel SAM, Olde Hartman TC, Lucassen P, van Jaarsveld CHM (2022) Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam Pract 39:159-167.
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the long-term effects of COVID-19 (NG188). United Kingdom2021.
[PubMed]
- Office for National Statistics. The prevalence of long COVID symptoms and COVID-19 complications.
- Nehme M, Braillard O, Alcoba G, Aebischer Perone S, Courvoisier D, et al. (2021) COVID-19 symptoms: Longitudinal evolution and persistence in outpatient settings. Ann Intern Med 174:723-725.
[Crossref] [Google Scholar] [PubMed]
- Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, et al. (2020) Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems Network-United States, March-June 2020. Morb Mortal Wkly Rep 69:993-998.
[Crossref] [Google Scholar] [PubMed]
- Post COVID-19 condition (Long COVID).World Health Organization.2022.
- Tosato M, Carfi A, Martis I, Pais C, Ciciarello F, et al. (2021) Prevalence and persistence of COVID-19 symptoms in older adults: A single-center study. J Am Med Dir Assoc 22:1840-1844.
[Crossref] [Google Scholar] [PubMed]
- Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, et al. (2021) Post discharge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 93:1013-1022.
[Crossref] [Google Scholar] [PubMed]
- Huang C, Huang L, Wang Y, Li X, Ren L, et al. (2021) 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397:220-232.
[Crossref] [Google Scholar] [PubMed]
- Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, et al. (2021) Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 76:399-401.
[Crossref] [Google Scholar] [PubMed]
- Lee AM, Wong JG, McAlonan GM, Cheung V, Cheung C, et al. (2007) Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry 52:233-240.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, et al. (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26:1017-1032.
[Crossref] [Google Scholar] [PubMed]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, et al. (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584:430-436.
[Crossref] [Google Scholar] [PubMed]
- Menendez ME, Neuhaus V, van Dijk CN, Ring D (2014) The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 472:2878-2886.
[Crossref] [Google Scholar] [PubMed]
- Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36:8-27.
[Crossref] [Google Scholar] [PubMed]
- Sharma N, Schwendimann R, Endrich O, Ausserhofer D, Simon M (2021) Comparing Charlson and Elixhauser comorbidity indices with different weightings to predict in-hospital mortality: An analysis of national inpatient data. BMC Health Serv Res 21:13.
[Crossref] [Google Scholar] [PubMed]
- Tsai KY, Hsieh KY, Ou SY, Chou FH, Chou YM (2020) Comparison of Elixhauser and Charlson methods for discriminative performance in mortality risk in patients with schizophrenic disorders. Int J Environ Res Public Health.
[Crossref] [Google Scholar] [PubMed]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. (2020) Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med 383:2603-2615.
[Google Scholar] [PubMed]
- A Randomized, double-blind, placebo-controlled phase 3 study to assess the efficacy and safety of Ad26.COV2.S for the prevention of SARS-CoV-2-mediated COVID-19 in adults aged 18 years and older-Ensemble. 2020.
- Reinsdorf DS, Richburg CA, Czerniecki JM, Aubin PM (2019) Development of a robotic unloader brace for investigation of conservative treatment of medial knee osteoarthritis. Int Conf Rehabil Robot 2019:931-937.
[Crossref] [Google Scholar] [PubMed]
- Fathi M, Vakili K, Sayehmiri F, Mohamadkhani A, Hajiesmaeili M, et al. (2021) The prognostic value of comorbidity for the severity of COVID-19: A systematic review and meta-analysis study. PloS one 16:0246190.
[Crossref] [Google Scholar] [PubMed]
Citation: Patterson BJ, Ruppenkamp JW, Richards F, Debnath R, El Khoury AC, et al. (2023) Duration of COVID-19 and Predictors for Long COVID- A Retrospective US Healthcare Database Analysis. J Infect Dis Ther S1:001. DOI: 10.4173/2332-0877.23.S1.001
Copyright: © 2023 Patterson BJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2184
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1867
- PDF downloads: 317


 ) Mild; (
) Mild; ( ) Moderate; (
) Moderate; ( ) Severe
) Severe