Dual Roles of IL-15 in Cancer Biology
Received: 01-Dec-2015 / Accepted Date: 30-Jan-2016 / Published Date: 05-Feb-2016 DOI: 10.4172/2576-3881.1000103
Abstract
IL-15 is an immune-enhancing cytokine belonging to the IL-2 family, which supports survival, proliferation and functional activities of NK, NK-T, T and B cells. Moreover, IL-15 may support the growth and survival of different lymphoid malignancies, suggesting that targeting of the IL-15/IL-15R system or its downstream signaling cascade may result in therapeutic effects, in these tumors. On the other hand, given its immune-enhancing activities IL-15 has been considered a good candidate for cancer immunotherapy. Indeed, IL-15 or IL-15 super agonists have shown anti-tumor activity in several animal tumor models either alone or combined with other immune-enhancing molecules. Therefore, clinical trials of IL-15 or IL-15 super-agonists are ongoing in different cancers. Here we will summarize the biological features of the IL-15/IL-15R system and discuss its duality in tumor biology and the potential applications of IL-15 agonists and antagonists in cancer.
Keywords: IL-15; IL-15Ra; Cancer immunotherapy; Lymphoid malignancy
6188Introduction
IL-15 belongs to a family of cytokines that bind to receptor (R) complexes sharing the common γ chain (γc or CD132), which is essential for signaling through the associated tyrosine kinase JAK3. Besides IL-15, this family also comprises IL-2, IL-4, IL-7, IL-9, and IL-21, which regulate the development and functions of lymphoid cells [1,2]. IL-4, IL-7, IL-9 and IL-21 receptor complexes consist of a cytokine-specific α chain and the γc, while IL-2 and IL-15, besides having specific α chains, also share the usage of a promiscuous IL-2/ IL-15Rβ (CD122) chain [3,4]. Therefore, both IL-2 and IL-15 signal through JAK1/3 and STAT3/5 pathways and mediate the proliferation and differentiation of NK, NK-T and activated T and B cells, in vitro. The IL-2/IL-15Rβ/γc is constitutively expressed on resting NK cells and on T cell subsets, which can directly respond to IL-2 or IL-15 and acquire potent cytolytic activity against cancer cells [5]. This effect is related to the induction of granzyme B and perforin expression [6]. In addition, IL-15 cooperates with IL-12 to induce secretion of different cytokines such as IFN-γ, TNF-α, and MIP-1α in NK cells [7].
In spite of a functional overlap in vitro, IL-2 and IL-15 have specific functions in vivo, as demonstrated by the study of KO models. In particular, IL-15 or IL-15Rα are essential for the development and survival of NK, NK-T and specific T cell subsets [8,9]. Differently, IL-2 and IL-2Rα have a specific role in immune regulation and their deficiency results in lympho proliferation and autoimmunity [10,11]. These phenotypes may reflect immune-regulatory activities of IL-2, including the induction of the activation-induced cell death of T cells and the expansion and fitness of CD4+CD25 high regulatory T cells. The specific functions of the two cytokines in vivo are related, at least in part, to their differential expression and regulation [12]. Indeed, IL-2 is specifically produced by activated T lymphocytes during the immune response, while IL-15 is expressed in different cell types, including monocytes, macrophages, DCs, stromal and some epithelial cells, in response to different signals [3,12]. Moreover, the IL-2Rα and IL-15Rα are also differentially regulated, are present in different cell types, and have different functional activities and affinities for their ligands [12,13].
Biology of the IL-15/IL-15R system
The human IL15 gene consists of six coding exons and maps to chromosome 4q31. The study of an IL-15 reporter transgenic mouse showed that IL15 promoter activity is largely limited to myeloid lineages while lymphoid cells express very low IL15 promoter activity. Hematopoietic stem cells show high levels of IL-15 expression, which is down-regulated during T cell differentiation in a stepwise and Notchdependent fashion [14]. Different transcription factors, including NFkB, IRF-E, Myb, and INF2 mediate transcriptional activation of the IL-15 gene [15-17]. In addition to transcriptional control, the IL-15 expression is also regulated at the level of mRNA splicing and translation and protein intracellular trafficking [12,17]. Alternative splicing of the IL-15 transcript results in the generation of two mRNA isoforms encoding for IL-15 proteins bearing either a short (SSP) or a long signal peptide (LSP) [18]. The different hydrophobicity of the two signal peptides dictates differential intracellular trafficking, as the LSPIL- 15 enters the ER and can be exported outside the cell, while the SSP-IL-15 localizes to the cytoplasm and nucleus [19,20]. Finally, IL-15 mRNA translation is limited by the presence of multiple AUG codons in the 3’UTR, upstream the initiation codon [21]. These mechanisms greatly limit IL-15 secretion in cells expressing IL-15 mRNA, and IL-15 release or surface exposure occurs only in activated monocytes, macrophages, DCs and stromal cells.
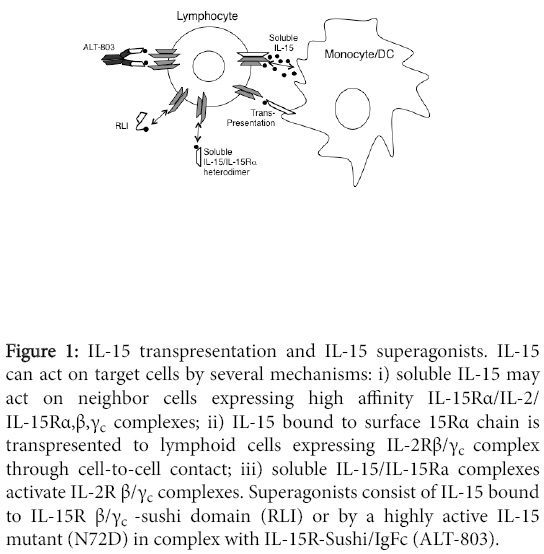
The human Il15RA consists of seven exons, and alternative mRNA splicing may result in eight molecular IL-15Rα isoforms with different extra- or intracellular domains [13]. Full-length isoforms consist of an extracellular portion containing the Sushi (i.e. IL-15-binding) domain, a trans-membrane domain, and an intracellular tail. Different from IL-2Rα, the isolated IL-15α chain is a high-affinity receptor with a KD <10-11M. The high affinity is an important property of the IL-15Rα, which can bind IL-15 at the surface of myeloid cells, in the absence of the IL-2/IL-15Rβ/γc dimers. In this way, activated DCs or macrophages can trans-present surface IL-15Rα/IL-15 complexes to IL-2/IL-15Rβ/γc + lymphocytes, through a “juxtacrine” mechanism involving cell-to-cell contacts [22-24] (Figure 1).
Figure 1: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Several pieces of evidence support the concept that IL-15 transpresentation is the most important mechanism of action of IL-15. The IL-2/IL-15Rβ/γc dimer present on T or NK cells has a low/intermediate affinity for free IL-15, and its activation requires high IL-15 concentrations, which are not found in vivo . The study of KO models revealed an essential role of IL-15Rα in IL-15 biology. Both IL-15- and IL-15Rα-deficient mice displayed a defective development of NK, NKT and intestinal intraepithelial CD8+ T cells [8,9,25]. Other studies showed that IL-15 mediates the commitment of hematopoietic progenitors of the bone marrow, lymphoid organs and cord blood to the NK cell lineage [26-29], and IL-15 trans-presentation is essential for NK cell development in vivo [30]. T or NK cell responses require the presence of IL-15Rα on interacting activated myeloid cells coexpressing IL-15, whereas expression of IL-15Rα on T or NK cells is not necessary [25,30]. Trans-presentation of IL-15 also mediates NK cell survival, as NK cells from wild-type mice showed reduced survival when implanted into Il15ra-/- mice [9]. Indeed, IL-15 is an important survival factor for NK and T cells in peripheral tissues through Erk-1/2 and PI-3 kinase-mediated inhibition of the apoptosis-inducing molecule Bim, and up-regulation of the anti-apoptotic molecule Mcl-1 [31]. In addition, reduced CD8+CD44 high memory T cells were reported in Il15ra-/- mice, indicating a role for IL-15Rα in the development of memory T cells. Again the presence of IL-15Rα on CD8+ T cells is dispensable for T cell memory differentiation, indicating a prominent role for trans-presentation [32]. Membranebound IL-15 is an important mediator of the cross-talk between DCs and NK cells in secondary lymphoid organs [33]. DCs produce IL-15 in response to CD40 signalling and trans-present it to NK cells mediating their activation and proliferation [34]. On the other hand, IFN-γ produced by NK cells enhances surface IL-15 expression on the DCs [35]. Also, TLR agonists trigger DCs to produce IFN-type I, which induce IL-15 trans-presentation. Induction of IL-15 expression on DCs is relevant for adaptive immunity against pathogens [36], as it supports the proliferation of memory CD45RO+ CD4+ and CD8+ T cells and naive CD45RO-CD8+ T cells.
The IL-15Rα/IL-15 complex may activate IL-2Rβ/γc+ T or NK cells not only as membrane-bound form but also as soluble complex (Figure 1). An IL-15RαΔ3 soluble isoform generates functional complexes with IL-15, which exert potent biological activity on lymphoid cells [37]. Type I IFNs, viral infection, and CD40 stimulation induce the release of soluble IL-15Rα/IL-15 complexes, which may enhance immune responses, in vivo [38]. The demonstration that IL-15/IL-15Ra-Sushi domain complexes strongly stimulate lymphoid cells expressing IL-2/ IL-15Rβ/γc provided the basis for the generation of new IL-15 superagonists [39].
IL-15Rα may also act as a signaling molecule in myeloid cells, as delivery of an IL15 gene in Rag2-/-γc-/- mice increased the number of myeloid cells in the spleen and IL-15 induced RANTES production through activation of JNK and NF-?B, in these cells [40].
Besides IL-15Rα-bound IL-15, alternative membrane forms of GPIlinked or trans-membrane IL-15 have been described on human monocytes [41]. The trans-membrane IL-15 may deliver signals by cell-to-cell contacts to lymphoid cells and could also mediate a reverse signal to monocytes through the Rho-GTPase Rac3 and the MAPK pathway, resulting in increased adhesion and IL-8 secretion [42].
Pro-tumor effects of IL-15
IL-15 stimulates growth and survival of normal T, B and NK cells, and may have similar effects on their neoplastic cellular counterparts. Indeed, several pieces of evidence indicate that IL-15 supports proliferation and survival of different types of neoplastic lymphoid cells, including those from Large Granular Lymphocyte Leukemia (LGLL) [43-45], B-Chronic Lymphocytic Leukemia (B-CLL) [46-48], Follicular Lymphoma (FL) [49] Hodgkin’s Lymphoma (HL) [50] Cutaneous T cell Lymphoma (CTC) [51], Multiple Myeloma (MM) [52], Enteropathy-Associated T cell Lymphoma (EATL) [53,54], and Adult T cell Leukemia (ATL) [55].
An early study showed that IL-15 mediates the proliferation of T- or NK-type LGLL cells, which express IL-15Rα and IL-2/IL-15Rβ/γc suggesting that it may act as a growth factor in these lymphoproliferative disorders [44]. Also, the study of IL-15-transgenic mice showed that chronic hyper-expression of IL-15 in vivo may result in the development of LGLL, sharing similarities with the human disease [43]. Further studies confirmed that chronically high levels of IL-15 alone are sufficient to drive the neoplastic transformation of normal LGL in the mouse, through induction of JAK1/3 and STAT3/5 signalling [45]. The role of STAT3 in LGLL genesis was also indicated by the high frequency (30-40%) of STAT3 mutations, involving the SH2 domain in human T and NK-LGLL [56]. Chronic activation of the STAT3 pathway mediates high Myc expression resulting in: i) overexpression of Aurora kinase A and B, which mediate amplification of centrosomes and chromosome instability; ii) down regulation of mir-29b, which results in enhanced expression of DNA methyl transferases and epigenetic silencing of oncosuppressor genes [45]. Antibodies blocking the IL-2Rβ/γccomplex such as the Mikβ1 antibody inhibit the effects of IL-15 on LGL proliferation, in vitro [44]. However, clinical studies of murine or humanized Mikβ1antibody showed no significant clinical benefit in LGLL patients [57], possibly suggesting the involvement of other factors and/or the loss of IL-15- dependency during progression.
An initial report indicated that IL-15 supports B-CLL proliferation in vitro through the IL-2R [47]. Further studies showed that IL-15 triggers STAT3/5 and ERK1/2 activation, mediating proliferation and survival of B-CLL cells in vitro and that stimulation via CD40L increased sensitivity to IL-15 effects [46]. B-CLL cells express TLR9, which upon ligand engagement drives their apoptosis. However, a recent report showed that IL-15 inhibits TLR-9-induced apoptosis and that TLR9 and IL-15 rather co-stimulate B-CLL clonal expansion. BCLL cells with chromosomal anomalies showed stronger proliferative responses, which correlated with reduced patient survival. In addition, the presence of IL-15-producing cells and apoptotic cells near B-CLL pseudofollicles in the spleen, suggest that DNA and IL-15 may costimulate B-CLL growth in secondary lymphoid organs [48]. Also, FL cells proliferate in response to IL-15 trans-presented by macrophages and CD40L signaling further increases this response [49]. Collectively, these studies indicate that IL-15 may cooperate with other stimuli such as TLR and CD40L to support neoplastic B cell growth in the microenvironment of lymphoid organs.
The IL-15/IL-15R system mediates mitogenic and anti-apoptotic signals in Hodgkin’s and Reed Stenberg cells through the phosphorylation of ERK1/2 and STAT5, and enhanced the expression of inflammatory factors including IL-1α, IL-6, IL-9, IL-12β, and CCL3 [50].
In ATL, an autocrine IL-15 loop supports the proliferation of neoplastic cells. In this disease the human HTLV-1 transactivating protein TAX drives IL-15 and IL-15Rα over-expression, thus generating an autocrine IL-15 loop that may play a role in disease development and progression [12,16,17].
IL-15 has also been involved in CTC, where the skin shows overexpression of IL-15, which could mediate paracrine effects on CTC cells. Since IL-15 can act as a chemoattractant for T cells ( ), it is conceivable that it may be involved in the tropism of CTC cells for the skin. At advanced stages also CTC cells acquire the ability to produce autocrine IL-15, which can render cells less dependent on the support provided by the skin environment [51]. Similarly, autocrine IL-15 expression by MM cells supports their proliferation and survival, protecting them from spontaneous or activation-induced apoptosis and rendering them independent from the microenvironment [52].
IL-15, which is highly expressed in the gut of Celiac Disease (CD) patients, may also play a role in some complications of this disease, including Refractory CD and EATL. Indeed, in type II Refractory CD there is an accumulation of abnormal intraepithelial lymphocytes with a CD3- and CD8-negative phenotype and clonal rearrangements of the TCR, which are considered a low-grade intraepithelial lymphoma [53]. Instead, EATL is a rare but aggressive T cell lymphoma with inflammatory features. It is likely that chronic antigenic stimulation may act in concert with IL-15 to support the expansion of intraepithelial lymphocytes, a first step in the development of oligoclonal and monoclonal expansions and subsequent lymphoma development [53].
Collectively these data support the concept that IL-15 may play an important role as a paracrine growth factor in some lymphoid malignancies, where agents blocking the IL-15/IL-15Rα system or its downstream JAK/STAT3/5 pathway may provide potential therapeutic tools.
These agents include antibodies blocking the IL-15R complex, such as the Mikb1 antibody. This antibody showed no effects in LGLL [57], but two studies in advanced CD (NCT01893775) and HTLV-1- Associated Myelopathy/Tropical Spastic Paraparesis (NCT00076843) are recruiting patients. However, in some situations IL-15 may not be the only cytokine driving lymphoma pathogenesis. In this case, the use of small molecule inhibitors targeting common downstream signalling pathways of tumor supportive cytokines, such as the JAK inhibitors ruxolitinib or tofacitinib, may represent more powerful strategies. Indeed, CP-690,550 (tofacitinib) treatment prolonged the survival of mice bearing a CD8+ JAK3-mutant T-ALL [58] or a CD8+ T cell IL-15-transgenic leukemia [59]. In addition, a clinical study of ruxolitinib in ATL is currently recruiting patients (NCT01712659) and a very recent pilot study on nine patients with rheumatoid arthritisassociated LGLL suggested clinical benefit [60].
Anti-tumor activities of IL-15
IL-2 has been a milestone in cancer immunotherapy, but it has a remarkable toxicity and is active only in a limited proportion of melanoma or renal cancer patients. The immune-enhancing activities and data from pre-clinical studies of IL-15 suggested that it could represent an alternative candidate [12,17,61]. Indeed, several studies in pre-clinical models of cancer supported this hypothesis. An early study showed that recombinant simian IL-15 has low toxicity in mice, and inhibits the growth of lung metastases in a syngeneic sarcoma model [62]. Also, mammary adenocarcinoma cells, genetically modified to secrete human IL-15, showed reduced growth rates when implanted in syngeneic mice. Vaccination with irradiated IL-15-secreting tumor cells inhibited the growth of lung metastases of parental adenocarcinoma. These effects were mediated by the induction of T and NK-cell responses and by IFN-γ [63]. Another strategy is based on the expression of IL-15 induced by an oncolytic virus, harboring the IL15 gene, which mediates T cell responses and survival of mice bearing colon carcinoma implants [64].
The IL-15 anti-tumor activity can be increased by co-administration of other immune-enhancing molecules or immune checkpoint inhibitors. For example, combined IL-12 and IL-15 gene transfer in human small cell lung cancer cells resulted in complete loss of their tumorigenic potential upon implant in SCID or nude mice, while each cytokine alone had limited activity [65]. This cooperative effect was mediated by the activation of cytotoxic macrophages. The combined use of IL-12 gene-modified tumor cells and IL-15 administration showed synergistic effects also in a melanoma model through stimulation of CTLs and IFN-γ [66]. Differently, the combined use of IL-12 and IL-15 gene transfer in breast adenocarcinoma cells induced anti-tumor immunity through the induction of CD8+ T cells and TNF- α, in syngeneic IFN-γ-deficient mice [67]. These data indicate that IL-15 combined with IL-12 mediates the activation of different antitumor mechanisms, in pre-clinical models.
IL-15 anti-tumor activity can also be enhanced by combination with agonistic anti-CD40 mAbs, which enhance the expression of IL-15Rα on DC and their ability to trans-present IL-15. Treatment of syngeneic mice bearing colon cancer with IL-15 combined with anti-CD40 mAb resulted in increased mice survival, compared to each treatment alone [68].
Other studies combined IL-15 with immune checkpoint blockers. Indeed, IL-15 has immune stimulatory effects, but it also induces the expression of the PD-1 inhibitory receptor on CTLs, suggesting that IL-15 anti-tumor activity may be enhanced by simultaneous checkpoint blockade. The co-treatment of colon carcinoma-bearing mice with IL-15, anti-CTLA-4 and anti-PD-L1 mAbs produced much stronger therapeutic effects than each treatment alone [69]. This combined treatment showed enhanced efficacy also in a transgenic mouse prostate cancer model [70].
A different approach to enhance IL-15 activity is based on the generation of super-agonists consisting of IL-15 bound to part of the extracellular domain of IL-15Rα, to mimic IL-15 trans-presentation in a soluble form. A fusion protein, termed RLI, was constructed by binding IL-15 to the Sushi domain of IL-15Rα via an amino acid linker [39]. RLI was a more potent stimulator of NK and T cells than IL-15, on a molar basis and showed prolonged half-life and stronger antitumor activity than IL-15 or IL-2 in metastatic B16F10 melanoma models [71]. Moreover, RLI reduced tumor growth and metastasis of human colon carcinoma cells in an orthotopic nude mouse model.
A further enhancement of IL-15 anti-tumor properties was achieved by the generation of fusion proteins consisting of RLI linked to antibodies targeting tumor-associated antigens, such as the ganglioside GD2 [72], the CD20 B-cell lymphoma antigen [73], or the Fibroblast Activation Protein (FAP) of the tumor stroma [74]. These reagents showed that antibody-targeted delivery of an IL-15-transpresenting moiety at the tumor site is suitable to enhance IL-15 activity for tumor therapy.
Finally, a complex of a mutant IL-15 superagonist and a dimeric Sushi domain/Fc fusion protein termed ALT-803 showed much more potent biologic activity on NK and T cells than IL-15, in vivo [75]. A single dose of ALT-803 prolonged survival of syngeneic mice bearing 5T33P and MOPC-315P myeloma while IL-15 was ineffective [75]. ALT-803 promoted rapid expansion of CD8+CD44high memory T cells in vivo , resulting in CTL- and IFN-γ-dependent immunity to rechallenge with the same tumor cells. Similarly, ALT-803 in combination with stereotactic surgery or anti-PD-1 antibody induced potent antitumor immune responses resulting in prolonged survival and complete remissions in a syngeneic glioblastoma model. These effects required both CD4+ and CD8+ T cells and IFN-γ production [76]. Moreover, ALT-803 augmented ADCC activity and IFN-γ secretion by human NK cells targeted by anti-CD20 mAbs against Bcell lymphoma cells. The combination of ALT-803 and anti-CD20 mAb significantly reduced mouse B cell lymphoma growth and increased survival [77].
Another report showed that IL-15 may directly act on a peculiar population of human CD105+ renal cancer stem cells (CSCs) in vitro . These are cell populations resistant to conventional therapy, capable of self-renewal and driving tumorigenesis and relapses. IL-15 mediated epithelial differentiation of CD105+ renal CSCs, which lose their stem cell characteristics, and acquire epithelial markers and the capability to self-produce IL-15 [78].
All these studies exploited the anti-tumor activity of IL-15 in various tumor models. Differently a recent report addressed the role of endogenous IL-15 in inflammation-induced colon cancer [79]. Il15-/- but not Il15rα-/- mice showed higher tumor incidence and increased colon weight than wild-type mice. Gene expression analysis showed up-regulation of pro-inflammatory cytokines involved in progression, such as IL-1β, IL-22, IL-23, Cxcl5, and Spp1 in tissues from Il15-/- mice [79]. Altogether, these findings suggest that IL-15 signaling via low-affinity IL-2/IL-15Rβ/γc suppresses colon carcinogenesis through induction of antitumor immune-surveillance and modulation of the tumor-associated inflammation.
A TAX-LUC mouse model of ATL allows to study lymphomagenesis by transgenic expression of HTLV-1 Tax, which drives the development of luciferase expressing lymphomas. As IL-15 is an autocrine factor in HTLV-1 adult T cell leukemia [16], the role of IL-15 in lymphomagenesis was studied in IL-15 TAX-LUC mice. Unexpectedly, the study of this model showed increased lymphomagenesis and mortality, indicating that IL-15 is not strictly required for the development of Tax-mediated lymphomas, whereas IL-15 seems involved in anti-lymphoma immune surveillance. Lymphomas developing in the absence of IL-15 showed a significant increase in IL-1α and IL-1α-regulated cytokine expression, suggestive of a lymphoma-promoting role of these cytokines, in the absence of IL-15 [80].
In view of the anti-tumor effects of IL-15 in preclinical models, recombinant human IL-15 was further developed at clinical grade and tested for toxicity in rhesus macaques using different schedules [81,82]. IL-15 was biologically active particularly in the i.v. settings, as it increased circulating NK cells and central and effector memory CD8+ T cells. An initial phase I clinical trial of IL-15 was performed in refractory metastatic renal cancer and/or melanoma, which are sensitive to IL-2-based immunotherapy. In principle, the use of IL-15 may better support effector memory T cell survival and functions than IL-2 and avoid the induction of activation-induced cell death and the stimulatory activity on Treg cells functions and fitness, which are typical of IL-2 [83]. In this clinical study IL-15 showed toxicity, including grade 3 fever, hypotension, thrombocytopenia, and increased transaminase levels, at 3 or 1 μg/kg/day for 12 days dose levels. The maximal tolerated dose was established at 0.3 μg/kg/day. After an initial rapid efflux of NK and memory CD8 T cells from the blood within minutes of IL-15 administration, influx and hyperproliferation resulted in 10-fold expansions of NK cells. Serum levels of multiple inflammatory cytokines, including IFN-g and IL-6 increased up to 50- fold, and may be involved in some toxic effects. No objective responses were observed and disease stabilization was recorded as best response [84]. A phase I/II study of i.v. IL-15 administration following a nonmyeloablative lymphocyte depleting chemotherapy and autologous tumor-infiltrating lymphocytes transfer in metastatic melanoma was recently terminated due to autoimmune toxicity (NCT01369888). Other trials of IL-15 using different schedules are ongoing, e.g. a phase I/II study of s.c. rIL-15 in adults with advanced cancers (NCT01727076). In addition, other trials will address the use of IL-15 super agonists in cancer patients. A clinical study of s.c. hetIL-15, are combinant heterodimer of IL15/sIL-15Rα, in adults with metastatic cancers (NCT02452268) is recruiting patients. Two other studies of the IL-15 super agonist ALT-803 have been initiated in patients with advanced solid tumors [NCT01946789] and in relapses of hematologic malignancy after allogeneic stem-cell transplantation [NCT01885897]. It is hoped that ongoing studies will unravel the potential and the best formulation and schedules of IL-15-based immunotherapies in order to achieve clinical efficacy in cancer patients.
Conclusion
IL-15 is a pleiotropic cytokine, which is essential for NK cell development and promotes proliferation, differentiation and functions of T, B and NK cells. These effects are not limited to normal lymphoid cells, as IL-15 can promote the growth of several types of malignant lymphoid cells, in vitro . Also, IL-15-transgenic mice develop spontaneous T or NK-type LGL leukaemias, supporting an in vivo role of IL-15 in leukaemia genesis. Therefore, the use of IL-15 for the treatment of lymphoid tumors should be avoided and, instead, the use of antibodies blocking the IL-15/IL-15R system or inhibitors targeting its downstream JAK/STAT signalling pathway is currently investigated. In this context, preliminary findings suggest that the JAK inhibitor tofacitinib has clinical activity in rheumatoid arthritis-associated LGLL.
On the other hand, the ability of IL-15 to stimulate both NK and T cell responses, and its well-documented anti-tumor activity in preclinical models, support the development of clinical studies of IL-15 in cancer. In addition, IL-15 showed acceptable toxicity profiles in mouse and primate models. Therefore, clinical studies of IL-15 or IL-15 superagonists, consisting of IL-15 linked to IL-15Rα portions, have been initiated. Also, studies combining IL-15 with adoptive transfer of T or NK cells are ongoing in cancer patients. However, a study combining IL-15 and TILs was recently terminated due to autoimmune toxicity, suggesting that IL-15’s immune enhancing activity is powerful and may result in exaggerated reactions. The ongoing and future clinical studies will elucidate the potential of IL-15 or of its superagonists in immunotherapy and provide indications on the best treatment schedules. Finally, preclinical models have shown that the combination of IL-15 with other immune enhancing cytokines, CD40-agonists, or immune checkpoint blockers may result in cooperative anti-tumor effects, supporting the development of combinational therapies in clinical settings.
Acknowledgment
Financial support: AIRC (Associazione Italiana per la Ricerca sul Cancro,), Italian Ministry of Health (5 x 1000 Funds 2012) and Compagnia di San Paolo.
References
- Liao W, Lin JX, Leonard WJ (2011) IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. See comment in PubMed Commons below CurrOpinImmunol 23: 598-604.
- Meazza R, Azzarone B, Orengo AM, Ferrini S (2011) Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. See comment in PubMed Commons below J Biomed Biotechnol 2011: 861920.
- GrabsteinKH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, et al. (1994) Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. See comment in PubMed Commons below Science 264: 965-968.
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, et al. (1994) Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. See comment in PubMed Commons below J Exp Med 180: 1395-1403.
- Gamero AM, Ussery D, Reintgen DS, Puleo CA, Djeu JY (1995) Interleukin 15 induction of lymphokine-activated killer cell function against autologous tumor cells in melanoma patient lymphocytes by a CD18-dependent, perforin-related mechanism. See comment in PubMed Commons below Cancer Res 55: 4988-4994.
- Fehniger TA , Cai SF, Cao X, Bredemeyer AJ, Presti RM, et al. (2007) Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. See comment in PubMed Commons below Immunity 26: 798-811.
- Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, et al. (1999) Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. See comment in PubMed Commons below J Immunol 162: 4511-4520.
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, et al. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. See comment in PubMed Commons below J Exp Med 191: 771-780.
- Koka R, Burkett PR, Chien M, Chai S, Chan F, et al. (2003) Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. See comment in PubMed Commons below J Exp Med 197: 977-984.
- Sadlack B, Löhler J, Schorle H, Klebb G, Haber H, et al. (1995) Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. See comment in PubMed Commons below Eur J Immunol 25: 3053-3059.
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, et al. (1995) Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. See comment in PubMed Commons below Immunity 3: 521-530.
- Waldmann TA (2015) The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. See comment in PubMed Commons below Cancer Immunol Res 3: 219-227.
- Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, et al. (1995) Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. See comment in PubMed Commons below J BiolChem 270: 29862-29869.
- Colpitts SL, Stonier SW, Stoklasek TA, Root SH, Aguila HL, et al. (2013) Transcriptional regulation of IL-15 expression during hematopoiesis. See comment in PubMed Commons below J Immunol 191: 3017-3024.
- Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, et al. (1998) Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. See comment in PubMed Commons below Nature 391: 700-703.
- Azimi N, Brown K, Bamford RN, Tagaya Y, Siebenlist U, et al. (1998) Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site. See comment in PubMed Commons below ProcNatlAcadSci U S A 95: 2452-2457.
- Mishra A, Sullivan L, Caligiuri MA (2014) Molecular pathways: interleukin-15 signaling in health and in cancer. See comment in PubMed Commons below Clin Cancer Res 20: 2044-2050.
- Meazza R, Verdiani S, Biassoni R, Coppolecchia M, Gaggero A, et al. (1996) Identification of a novel interleukin-15 (IL-15) transcript isoform generated by alternative splicing in human small cell lung cancer cell lines. See comment in PubMed Commons below Oncogene 12: 2187-2192.
- Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, et al. (1997) Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. See comment in PubMed Commons below ProcNatlAcadSci U S A 94: 14444-14449.
- Gaggero A, Azzarone B, Andrei C, Mishal Z, Meazza R, et al. (1999) Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. See comment in PubMed Commons below Eur J Immunol 29: 1265-1274.
- Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA (1996) Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. See comment in PubMed Commons below ProcNatlAcadSci U S A 93: 2897-2902.
- Dubois S, Mariner J, Waldmann TA, Tagaya Y (2002) IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. See comment in PubMed Commons below Immunity 17: 537-547.
- Stonier SW, Schluns KS (2010) Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. See comment in PubMed Commons below ImmunolLett 127: 85-92.
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A (2008) IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. See comment in PubMed Commons below J Exp Med 205: 1213-1225.
- Lodolce JP1, Burkett PR, Boone DL, Chien M, Ma A (2001) T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. See comment in PubMed Commons below J Exp Med 194: 1187-1194.
- Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, et al. (2006) Evidence for discrete stages of human natural killer cell differentiation in vivo. See comment in PubMed Commons below J Exp Med 203: 1033-1043.
- Grzywacz B, Kataria N, Kataria N, Blazar BR, Miller JS, et al. (2011) Natural killer-cell differentiation by myeloid progenitors. See comment in PubMed Commons below Blood 117: 3548-3558.
- Perez SA, Sotiropoulou PA, Gkika DG, Mahaira LG, Niarchos DK, et al. (2003) A novel myeloid-like NK cell progenitor in human umbilical cord blood. See comment in PubMed Commons below Blood 101: 3444-3450.
- Vosshenrich CA1, Ranson T, Samson SI, Corcuff E, Colucci F, et al. (2005) Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. See comment in PubMed Commons below J Immunol 174: 1213-1221.
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, et al. (2009) IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. See comment in PubMed Commons below J Exp Med 206: 25-34.
- Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, et al. (2007) Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. See comment in PubMed Commons below Nat Immunol 8: 856-863.
- Burkett PR, Koka R, Chien M, Chai S, Chan F, et al. (2003) IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. See comment in PubMed Commons below ProcNatlAcadSci U S A 100: 4724-4729.
- Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, et al. (2004) Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. See comment in PubMed Commons below ProcNatlAcadSci U S A 101: 16606-16611.
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A (2007) Dendritic cells prime natural killer cells by trans-presenting interleukin 15. See comment in PubMed Commons below Immunity 26: 503-517.
- Morandi B, Mortara L, Carrega P, Cantoni C, Costa G, et al. (2009) NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs. See comment in PubMed Commons below IntImmunol 21: 599-606.
- Perera PY, Lichy JH, Waldmann TA, Perera LP (2012) The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. See comment in PubMed Commons below Microbes Infect 14: 247-261.
- Giron-Michel J, Giuliani M, Fogli M, Brouty-Boyé D, Ferrini S, et al. (2005) Membrane-bound and soluble IL-15/IL-15Ralpha complexes display differential signaling and functions on human hematopoietic progenitors. See comment in PubMed Commons below Blood 106: 2302-2310.
- Anthony SM, Howard ME, Hailemichael Y, Overwijk WW, Schluns KS (2015) Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways. See comment in PubMed Commons below PLoS One 10: e0120274.
- Mortier E, Quéméner A, Vusio P, Lorenzen I, Boublik Y, et al. (2006) Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. See comment in PubMed Commons below J BiolChem 281: 1612-1619.
- Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, et al. (2012) IL-15 can signal via IL-15Rα, JNK, and NF-κB to drive RANTES production by myeloid cells. See comment in PubMed Commons below J Immunol 188: 4149-4157.
- Musso T1, Calosso L, Zucca M, Millesimo M, Ravarino D, et al. (1999) Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. See comment in PubMed Commons below Blood 93: 3531-3539.
- Neely GG, Epelman S, Ma LL, Colarusso P, Howlett CJ, et al. (2004) Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling. See comment in PubMed Commons below J Immunol 172: 4225-4234.
- Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, et al. (2001) Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. See comment in PubMed Commons below J Exp Med 193: 219-231.
- Zambello R, Facco M, Trentin L, Sancetta R, Tassinari C, et al. (1997) Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. See comment in PubMed Commons below Blood 89: 201-211.
- Mishra A1, Liu S, Sams GH, Curphey DP, Santhanam R, et al. (2012) Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. See comment in PubMed Commons below Cancer Cell 22: 645-655.
- Trentin L, Cerutti A, Zambello R, Sancretta R, Tassinari C, et al. (1996) Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. See comment in PubMed Commons below Blood 87: 3327-3335.
- de Totero D, Meazza R, Capaia M, Fabbi M, Azzarone B, et al. (2008) The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. See comment in PubMed Commons below Blood 111: 517-524.
- Mongini PK, Gupta R, Boyle E, Nieto J, Lee H, et al. (2015) TLR-9 and IL-15 Synergy Promotes the In Vitro Clonal Expansion of Chronic Lymphocytic Leukemia B Cells. See comment in PubMed Commons below J Immunol 195: 901-923.
- Epron G, Ame-Thomas P, Le Priol J, Pangault C, Dulong J, et al. (2012) Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. See comment in PubMed Commons below Leukemia 26: 139-148.
- Ullrich K, Blumenthal-Barby F, Lamprecht B, Köchert K, Lenze D, et al. (2015) The IL-15 cytokine system provides growth and survival signals in Hodgkin lymphoma and enhances the inflammatory phenotype of HRS cells. See comment in PubMed Commons below Leukemia 29: 1213-1218.
- Leroy S, Dubois S, Tenaud I, Chebassier N, Godard A, et al. (2001) Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). See comment in PubMed Commons below Br J Dermatol 144: 1016-1023.
- Tinhofer I, Marschitz I, Henn T, Egle A, Greil R (2000) Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. See comment in PubMed Commons below Blood 95: 610-618.
- Malamut G, El Machhour R, Montcuquet N, Martin-Lannerée S, Dusanter-Fourt I, et al. (2010) IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. See comment in PubMed Commons below J Clin Invest 120: 2131-2143.
- Yokoyama S, Perera PY, Waldmann TA, Hiroi T, Perera LP (2013) Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J ClinImmunol 33: 586-94.
- Mariner JM, Lantz V, Waldmann TA, Azimi N (2001) Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. See comment in PubMed Commons below J Immunol 166: 2602-2609.
- Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, et al. (2012) Somatic STAT3 mutations in large granular lymphocytic leukemia. See comment in PubMed Commons below N Engl J Med 366: 1905-1913.
- Waldmann TA, Conlon KC, Stewart DM, Worthy TA, Janik JE, et al. (2013) Phase 1 trial of IL-15 trans presentation blockade using humanized Mikβ1 mAb in patients with T-cell large granular lymphocytic leukemia. See comment in PubMed Commons below Blood 121: 476-484.
- Degryse S, de Bock CE, Cox L, Demeyer S, Gielen O, et al. (2014) JAK3 mutants transform hematopoietic cells through JAK1 activation, causing T-cell acute lymphoblastic leukemia in a mouse model. Blood 124: 3092-3100.
- Ju W, Zhang M, Jiang JK, Thomas CJ, Oh U, et al. (2011) CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. See comment in PubMed Commons below Blood 117: 1938-1946.
- Bilori B, Thota S, Clemente MJ, Patel B, Jerez A, et al. (2015) Tofacitinib as a novel salvage therapy for refractory T-cell large granular lymphocytic leukemia. See comment in PubMed Commons below Leukemia 29: 2427-2429.
- Croce M, Orengo AM, Azzarone B, Ferrini S (2012) Immunotherapeutic applications of IL-15. See comment in PubMed Commons below Immunotherapy 4: 957-969.
- Munger W1, DeJoy SQ, Jeyaseelan R Sr, Torley LW, Grabstein KH, et al. (1995) Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. See comment in PubMed Commons below Cell Immunol 165: 289-293.
- Meazza R, Lollini PL, Nanni P, De Giovanni C, Gaggero A, et al. (2000) Gene transfer of a secretable form of IL-15 in murine adenocarcinoma cells: effects on tumorigenicity, metastatic potential and immune response. See comment in PubMed Commons below Int J Cancer 87: 574-581.
- Stephenson KB, Barra NG, Davies E, Ashkar AA, Lichty BD (2012) Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther 19: 238-246.
- Di Carlo E, Comes A, Basso S, De Ambrosis A, Meazza R, et al. (2000) The combined action of IL-15 and IL-12 gene transfer can induce tumor cell rejection without T and NK cell involvement. See comment in PubMed Commons below J Immunol 165: 3111-3118.
- Lasek W, Basak G, Switaj T, Jakubowska AB, Wysocki PJ, et al. (2004) Complete tumour regressions induced by vaccination with IL-12 gene-transduced tumour cells in combination with IL-15 in a melanoma model in mice. Cancer ImmunolImmunother 53: 363-372.
- Comes A, Di Carlo E, Musiani P, Rosso O, Meazza R, et al. (2002) IFN-gamma-independent synergistic effects of IL-12 and IL-15 induce anti-tumor immune responses in syngeneic mice. See comment in PubMed Commons below Eur J Immunol 32: 1914-1923.
- Zhang M, Yao Z, Dubois S, Ju W, Müller JR, et al. (2009) Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. See comment in PubMed Commons below ProcNatlAcadSci U S A 106: 7513-7518.
- Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA (2010) Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 16: 6019-6028.
- Yu P, Steel JC, Zhang M, Morris JC, Waitz R, et al. (2012) Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. See comment in PubMed Commons below ProcNatlAcadSci U S A 109: 6187-6192.
- Bessard A, Solé V, Bouchaud G, Quéméner A, Jacques Y (2009) High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. See comment in PubMed Commons below Mol Cancer Ther 8: 2736-2745.
- Vincent M, Bessard A, Cochonneau D, Teppaz G, Solé V, et al. (2013) Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. See comment in PubMed Commons below Int J Cancer 133: 757-765.
- Vincent M, Teppaz G, Lajoie L, Solé V, Bessard A, et al. (2014) Highly potent anti-CD20-RLI immunocytokine targeting established human B lymphoma in SCID mouse. See comment in PubMed Commons below MAbs 6: 1026-1037.
- Kermer V, Baum V, Hornig N, Kontermann RE, Müller D (2012) An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. See comment in PubMed Commons below Mol Cancer Ther 11: 1279-1288.
- Xu W, Jones M, Liu B, Zhu X, Johnson CB, et al. (2013) Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor αSu/Fc fusion complex in syngeneic murine models of multiple myeloma. See comment in PubMed Commons below Cancer Res 73: 3075-3086.
- Mathios D, Park CK, Marcus WD, Alter S, Rhode PR, et al. (2016) Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer 138: 187-194.
- Rosario M, Liu B2, Kong L, Collins LI, Schneider SE, et al. (2016) The IL-15-Based ALT-803 Complex Enhances FcγRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clin Cancer Res 22: 596-608.
- Azzi S, Bruno S, Giron-Michel J, Clay D, Devocelle A, et al. (2011) Differentiation therapy: targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst 103: 1884-1898.
- Bahri R, Pateras IS, D'Orlando O3, Goyeneche-Patino DA3, Campbell M4, et al. (2015) IL-15 suppresses colitis-associated colon carcinogenesis by inducing antitumor immunity. Oncoimmunology 4: e1002721.
- Rauch DA, Harding JC, Ratner L (2014) IL-15 deficient tax mice reveal a role for IL-1α in tumor immunity. PLoS One 9: e85028.
- Berger C, Berger M, Hackman RC, Gough M, Elliott C, et al. (2009) Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood 114: 2417-2426.
- Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, et al. (2011) Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood 117: 4787-4795.
- Malek TR, Bayer AL (2004) Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 4: 665-674.
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, et al. (2015) Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J ClinOncol 33: 74-82.
Citation: Fabbi and Ferrini (2016) Dual Roles of IL-15 in Cancer Biology. J Cytokine Biol 1:103. DOI: 10.4172/2576-3881.1000103
Copyright: © 2016 Fabbi M et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14026
- [From(publication date): 5-2016 - Apr 20, 2025]
- Breakdown by view type
- HTML page views: 12893
- PDF downloads: 1133