Dual Phase 3D Angiography with 64 MDCT: The Evaluation of Vascular Anatomy before Laparoscopic Gastrectomy
Received: 21-Jan-2019 / Accepted Date: 08-Feb-2019 / Published Date: 18-Feb-2019 DOI: 10.4172/2161-069X.1000592
Abstract
Objective: We evaluated the efficiency of 3-dimensional Computed Tomographic Angiography (3D CTA) performed with 64-channel Multidetector Computed Tomography (MDCT) to assess vascular anatomy before laparoscopic gastrectomy.
Materials and Methods: 20 patients with early gastric cancer scheduled for laparoscopic gastrectomy were evaluated. Dual phase IV contrast CTA was performed before laparoscopic gastrectomy. Arterial and venous phase images were obtained after rapid infusion of contrast agent with an interval of 15 seconds serially during single breath hold of 31 seconds.
Results: In all patients, 3D CTA showed Left Gastric Artery (LGA), Left Hepatic Artery (LHA), Right Gastric Artery (RGA) and Left Coronary Vein (LCV) with celiac trunk. Celiac trunk branching pattern was classified according to Michel’s method and in 18 cases, Michel’s type 1, in 2 cases Michel’s type 2 were identified. RGA was originated from Gastro Duodenal Artery (GDA) in 16 cases, from Superior Mesenteric Artery (SMA) in 2 cases and from Proper Hepatic Artery (PHA) in 2 cases. LCV was draining into Superior Mesenteric Vein (SMV) in 18 cases and into Portal Vein (PV) in 2 cases. In 12 of the cases LCV was coursing dorsal to PHA, Common Hepatic Artery (CHA) and Splenic Artery (SA), in 6 cases LCV was coursing ventral to these vessels and was joined to SMV. In 2 cases whose LCV was draining into PV, it was coursing ventral to CHA.
Conclusion: Dual phase CTA is successful to define perigastric vascular structures and reduces the risk of vascular injuries when performed before laparoscopic gastrectomy.
Keywords: Gastric cancer; Laparoscopic gastrectomy;: Computedtomographic angiography; Vascular anatomy and variations
Introduction
Laparoscopic gastrectomy for the treatment of early gastric cancer attracts more attention day by day and its use has been increasing due to expectation of increased quality of life of the patients. However, this technique has also some disadvantages. Before all else, an entire view of the operation area is difficult to obtain during the procedure. Moreover, the lesion, organ and its vascular structures may not be directly manipulated by the surgeon.
This causes difficulty in the ligation of vessels and the procedures take longer time. In addition, during dissection of lymph nodes and ligation of vessels some structures may be injured. This results in unexpected large hemorrhages in the operation area causing more restriction of the view. Because of these, before laparoscopic gastrectomy a simultaneous establishment of arterial and venous structures and their courses by Computed Tomography Angiography (CTA) is important. In this way, more controlled ligation of arteries and dissection of lymph nodes are possible [1].
Our main aim in this study is to evaluate gastric vascular anatomy, to establish their variations by Multidetector Computed Tomography (MDCT) in patients with early gastric cancer who were scheduled for laparoscopic gastrectomy.
Material and Methods
Study design
33 patients diagnosed with stage 1a and stage 1b gastric cancer according to TNM Classification [2] that is, early stage gastric cancer, were included in the study. With consideration of patients’ preferences their associated comorbities were evaluated and 25 of 33 patients were scheduled for laparoscopic gastrectomy. Due to sensitivity to contrast agent and claustrophobia, 5 patients were excluded from the study. Vascular anatomy of 20 patients was evaluated by preoperative CTA.
CTA were performed 4-15 days before the operation (average 9 days). Ethics committee approval was obtained from the local ethics committee and written informed consent from all patients were taken. In our study; 18 of 20 patients were male and 2 were female with age range was 38-68 (mean age 53 ± 7.3 years).
CT Protocol
The CT images were obtained using 64 channel MDCT scanners (Philips Medical Systems, Brilliance 64, and the Netherlands). Before the procedure 5 gr effervescent granule (Enhos; Kansuk Pharmaceutical Industries. Istanbul, Turkey) was administered with little water to distend the stomach in order to obtain better images of the vessels around the stomach. Imaging was performed by using these parameters; slice thickness; 1 mm, reconstruction intervals; 50.5 mm, tube voltage; 120 kVp and tube current; 300 mA. An 18 G IV catheter was inserted into the right superficial cubical vein.
After nonionic contrast agent (300 mg/ml, iohexol; Omnipaque 300, Daiichi Sankyo) was infused rapidly at 5 ml/min, arterial and venous phase images were obtained. The examination was performed using bolus-tracking method. The amount of intravenous contrast agent was calculated as, 100 ml contrast agent for the patients weighing less than 40 kg, 2.5 ml per one kg for the patients weighing between 40-60 kg and 150 ml for the patients weighing more than 60 kilograms (the mean amount of the contrast agent was 133 ml). Arterial phase images were obtained using computer assisted bolus-tracking method that sets a Region of Interest (ROI) at the center of aorta at the level of the branching point of celiac trunk.
When the attenuation value measured by Region of Interest (ROI) was 50 HU greater than the value measured before the administration of the contrast agent, the arterial phase CTA was started. 40 seconds after that (the start of injection) venous phase imaging was performed. All the examination was performed at just one breath hold.
Image analysis
The slice data obtained from arterial and venous phases were transferred to the workstation where they were converted into Maximum Intensity Projection (MIP), Multi Planar Reconstruction (MPR) and Volume Rendering Technique (VRT). All the images having 1 mm of slice thickness from both of the phases were examined at axial, coronal and sagittal plans.
3D CTA images were evaluated by two different radiology experts and findings were discussed with the surgeons. The perigastric arteries and veins assessed before the operation were evaluated at the time of laparoscopic gastrectomy and the correlation between findings was given attention.
Results
After the arteries around the stomach were viewed at arterial phase (3D CT Arteriography) and veins at venous phase (3D CT Venography), reconstructed images were obtained by using MIP, MPR and VRT.
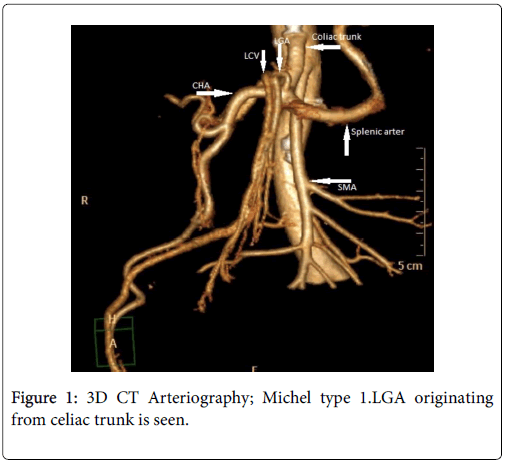
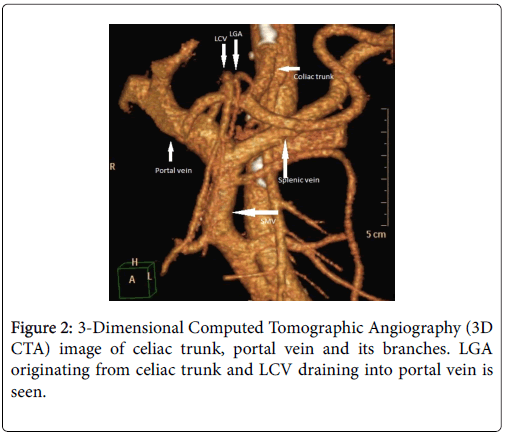
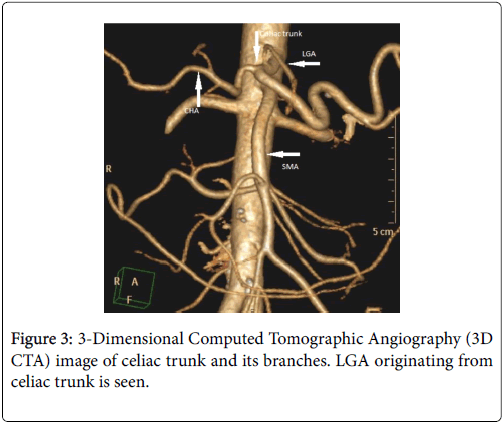
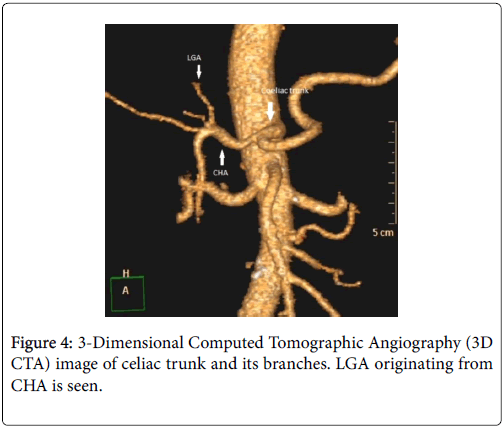
The celiac trunk branching pattern was classified according to Michel’s classification at 3D CT Arteriography [3,4] (Table 1 ). In 18 patients Michel’s type 1 (Figures 1-3) was seen and in the other 2 patients Michel’s type 2 (Figure 4) was present.
| Michels classification at 3D CT arteriography | |
|---|---|
| Hepatosplenogastric trunk (Type 1) | LGA, CHA and SA originate from celiac trunk |
| Hepatosplenic trunk (Type 2) | CHA and SA originate from celiac trunk, LGA originates from aorta, SA or hepatic artery |
| Hepatosplenomesenteric trunk (Type 3) | LGA originates from celiac trunk, CHA and SA originate from SMA |
| Hepatogastric trunk (Type 4) | LGA and CHA originate from celiac trunk, SA originates from SMA |
| Splenogastric trunk (Type 5) | LGA and SA originate from celiac trunk, CHA originates from SMA |
| Celiacomesenteric trunk (Type 6) | LGA, CHA and SA originates from one trunk |
Table 1: Michel’s classification at 3D CT Arteriography.
In all the 20 patients the course of Right Gastric Artery (RGA) could be followed and it was consistent with intraoperative findings. In 16 of the 20 patients it was seen that RGA was originating from GDA, in 2 patients it was originating from AHP and in the other 2 patients from SMA.
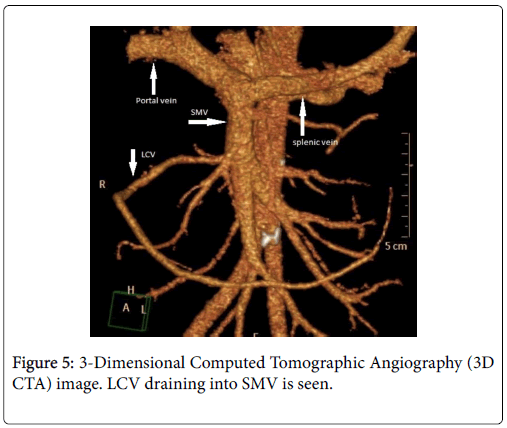
The course of the Left Gastric (coronary) Vein (LGV, LCV), established at 3D CT Venography was also 100% consistent with the intraoperative findings. While in 18 of the 20 patients the LCV was draining into SMV (Figure 5), in the other 2 patients it was draining into portal vein (Figure 2). While in 12 of the cases LCV was coursing at the dorsal edge of AHP, CHA and SA, in the other 6 cases it was coursing at the ventral part of these vessels and then draining into SMV. The course of LCV in 2 cases was draining into the portal vein at the ventral part of AHC.
All these findings were consistent with the findings gained from the operation. The sensitivity and positive predictive value of 3D CTA to establish these vessels was calculated as 100%.
In all the patients the laparascopic gastrectomy performed with the help of CTA findings was completed successfully and open gastrectomy was not needed. In addition, in local lymph node dissections metastatic foci were not encountered.
Discussion
Laparoscopic gastrectomy is attracting more attention day by day. It can be used for both treatment and staging of gastric cancer. It has many advantages when it is compared with open gastrectomy. The most important ones are; minimal surgical incision, low risk of intraoperative hemorrhage, less hospitalization time, the faster return of intestinal movements to normal, better respiratory functions and fast immune response [5,6].
Starting from the laparoscopic cholesistectomy performed by Dubois and his colleagues in 1989, the laparoscopic technique has improved fastly. Laparoscopic gastrectomy has been firstly described and performed in Japan in 1994. In the last studies it was reported that in early gastric cancer laparoscopic gastrectomy is more advantageous than open surgery [7,8].
Laparoscopic gastrectomy is indicated when no metastatic lymph nodes can be established by US, CT or endosonography. This technique includes wide resection, intragastric mucosal resection and removal of local lymph nodes. In our study, the lymph nodes around LGA, CHA and celiac trunk were evaluated and the lymph nodes having short diameter over 1 cm were accepted as metastatic. By taking Japan Gastric Cancer notification into account, lymph node dissection was performed for the nodes located at perigastric area, anteriosuperior of LGA, CHA and the lymph nodes around celiac trunk [9,10]. However, patients in whom mucosal resection was possible, that means that their tumors are limited to mucosa or submucosa, and without any ulceration and which are smaller than 2 cm in diameter were excluded from this group. The candidates for distal gastrectomy are generally gastric cancer cases whose tumors are located at antrum or corpus and limited to mucosa and submucosa.
The most important disadvantage of laparoscopic gastrectomy is limited vision of the operation area. It takes generally a long time to find the origins of arteries and veins due to the narrow manipulation area. Therefore, preoperative knowledge of vascular anatomy is extremely important. Digital Subtraction Angiography (DSA) is known as the gold standard method. However, it is a time consuming, expensive and invasive procedure having many risks. On the other hand, in MDCT at just one breath hold, images having high contrast resolution can be obtained. By using reconstructions the establishment of anatomic variations is facilitated.
The most important restriction in our study is the limited number of cases. In the literature the frequency of celiac trunk trifurcation, that is type 1 pattern at Michel Classification, is reported as 72-90% [11-14]. This rate was established as 98% in our study and although it was similar to the results found in the study of Matsuki and his colleagues [14], compared to other studies in literature this higher frequency was found firstly related with the limited number of cases. The frequency of type 2 pattern (Hepatosplenic trunk) was 2% and LGA was originating directly from aorta. This rate is similar with that of literature [11]. In addition, in 4 cases variations at the origin of RGA were established. In 2 cases RGA was originating from PHA and in the other 2 cases it was originating from SMA instead of GDA. Different manipulations are described in the operation depending on the origin of the RGA [15,16]. Since during the operation before dissection of lymph nodes LCV was ligated, the course of LCV was particularly described. Also, the trunk of Henle which connects the stomach and colon draining veins is significant in surgeries of stomach [17]. It can have different variations and the most common of these is gastro-pancreato-colic trunk. The presence of this trunk introduces an additional risk for bleeding [18]. If the ligation of these veins is not performed, venous injuries can be seen resulting in massive hemorrhages [16,19-21].
Conclusion
As a result, in laparoscopic gastrectomy assessment of origins and courses of perigastric arteries and veins is challenging and due to variations, the procedure takes more time. In addition, during ligation of arteries and dissection of lymph nodes, venous structures can be injured. Being aware of these variations before the operations is very helpful in retention of possible complications. Therefore, before performing the laparoscopic gastrectomy, radiological evaluation of arterial and venous structures of the operation area is extremely important.
Conflicts of Interest
Conflict of interest disclosed was none.
References
- Yamashita K, Sakuramoto S, Mieno H, Shibata T, Nemoto M, et al. (2014) Preoperative dual-phase 3D CT angiography assessment of the right hepatic artery before gastrectomy. Surg Today 44: 1912-1919.
- Kwee RM, Kwee TC (2007) Imaging in local staging of gastric cancer: A systematic review. J Clin Oncol 25: 2107–2116.
- Michels NA (1995) Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. JB Lippincott Company, Philadelphia 172.
- Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G (2004) Anatomic variations of the hepatic arteries. Surg Radiol Anat 26: 239-244.
- Azagra JS, Goergen M, De Simone P, Ibanez-Aguirre J (1999) Minimally invasive surgery for gastric cancer. Surg Endosc 13: 351-357.
- Yano H, Monden T, Kinuta M, Nakano Y, Tono T, et al.( 2001) The usefulness of laparoscopy-assisted distal gastrectomy in comparison with that of open distal gastrectomy for early gastric cancer. Gastric Cancer 4:93-97.
- Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, et al.(2011) Meta-analysis of laparoscopy-assisted and open distal gastrectomy for gastric cancer. J Surg Res 171:479-85.
- Orsenigo E, Di Palo S, Tamburini A, Staudacher C (2011) Laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: A monoinstitutional Western center experience. Surg Endosc 25: 140-145.
- Japanese Research Society for Gastric Cancer (1995) Japanese classification of gastric carcinoma, 1st English ed. Tokyo, Japan: Kanehara Co.
- Pavlidis T, Pavlidis E, Sakantamıs A (2012) The role of laparoscopic surgery in gastric cancer. J Minim Access Surg 8: 35-38.
- Ugurel M, Battal B, Bozlar U, M S Nural, M Tasar, et al.(2010) Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol 83: 661-667.
- Matoba M, Tonami H, Kuginuki M, Yokota H, Takashima S, et al.(2003) Comparison of high-resolution contrastenhanced 3D MRA with digital subtraction angiography in the evaluation of hepatic arterial anatomy. Clin Radiol 58: 463-468.
- Vandamme JP, Bonte J (1985) The branches of the coeliac trunk. Acta Anat(Basel) 122: 110-114.
- Li XH, Sun CH, Feng ST, Yan CG, He YL,et al.(2012) Assessment of 64-slice spiral computed tomography angiography with image fusion for perigastric arteries anatomy Zhonghua Wei Chang Wai Ke Za Zhi 15: 594-598.
- Matsuki M, Tanikake M, Kani H, Tatsugami F, Kanazawa S, et al.(2006) Dual phase 3D CT Angiography During a Single Breath-Hold Using 16MDCT: Assessment of Vascular Anatomy Before Laparoscopic Gastrectomy. AJR Am J Roentgenol 186: 1079-1085.
- Matsuki M, Kani H, Tatsugami F, Yoshikawa S, Narabayashi I, et al. (2004) Preoperative assessment of vascular anatomy around the stomach by 3D imaging using MDCT before laparoscopy-assisted gastrectomy. AJR Am J Roentgenol 183: 145–151.
- Gao Y, Lu Y (2018) Variations of Gastrocolic Trunk of Henle and Its Significance in Gastrocolic Surgery. Gastroenterol Res Pract. 6:3573680.
- Stefura T, Kacprzyk A, Droś J, Pędziwiatr M, Major P, et al.(2018) The venous trunk of henle (gastrocolic trunk): A systematic review and meta-analysis of its prevalence, dimensions, and tributary variations. Clin Anat 31: 1109-1121.
- Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, et al.(1999) Completely laparoscopic extraperigastric lymph node dissection for gastric malignancies located in the middle or lower third of the stomach. Gastric Cancer 2: 186-190.
- Li X, Chu J, Sun C, Pui MH, Huang S, et al. (2013) Sixty-four-slice computed tomography angiography of perigastric veins with image fusion. J Comput Assist Tomogr 37: 165-170.
- Takiguchi S, Sekimoto M, Fujiwara Y, Yasuda T, Yano M, et al. (2004) Laparoscopic lymph node dissection for gastric cancer with intraoperative navigation using three-dimensional angio computed tomography images reconstructed as laparoscopic view. Surg Endosc 18: 106-110.
Citation: Atça AO, Erok B, Yilmaz E, Oktar SF, Eyuboglu E, et al. (2019) Dual Phase 3D Angiography with 64 MDCT: The Evaluation of Vascular Anatomy before Laparoscopic Gastrectomy. J Gastrointest Dig Syst 9: 592. DOI: 10.4172/2161-069X.1000592
Copyright: © 2019 Atça AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5006
- [From(publication date): 0-2019 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 4057
- PDF downloads: 949