Dual mechanism of action of PTC299 and other DHODH inhibitors in suppressing SARS-CoV-2 replication and cellular cytokine storms
Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 /
Abstract
COVID-19, the pandemic arising from the third coronavirus outbreak in the past 20 years, will not be the last. Identifying therapies for COVID-19, and possibly future outbreaks, is of great importance. COVID-19 is characterized by an initial phase of viral replication followed by an excessive pro-inflammatory response (cytokine storm). PTC299 is an orally available compound that is a potent inhibitor of dihydroorotate dehydrogenase (DHODH), the rate-limiting enzyme in the de novo pyrimidine biosynthesis pathway. Recent in vitro findings indicate that PTC299 acts via a dual mechanism to inhibit viral replication and the cytokine storm, both of which are dependent on intracellular pyrimidine levels. Consistent with PTC299 targeting a host enzyme, the drug demonstrates broad antiviral activity, and is likely to be impervious to viral resistance. These characteristics may be critical when SARS-CoV-2 becomes endemic or mutates sufficiently to be resistant to current vaccines, as well as during future coronavirus outbreaks.
Keywords
PTC299; SARS-CoV-2; COVID-19; Antiviral; Cytokine; Dihydroorotate Dehydrogenase; DHODH; Coronavirus; Cytokine storm.
Introduction
The pandemic widely known as coronavirus disease of 2019 (COVID-19) is the third coronavirus outbreak of zoonotic origin in the past 20 years, having been preceded by coronavirus-caused respiratory syndromes generally referred to as SARS [1] and MERS [2]. COVID-19 is characterized by high rates of infection and lethality, and has significantly strained economic, healthcare, and political systems. The disease was recognized as a global pandemic by the World Health Organization on 11 March 2020 and has since resulted in more than two million deaths worldwide [3,4]. Although several vaccines that prevent COVID-19 have already been developed [5], the need for effective therapeutics is paramount, particularly as the vaccines are not currently available in many countries, substantive logistical challenges remain for manufacturing and global distribution, and certain high-risk populations are unable to be vaccinated. Moreover, it is unclear what the future medical landscape may look like should SARS-CoV-2 become endemic or mutate to a structure poorly recognized by immune systems primed with vaccines targeted against the current versions of the virus’ spike protein.
Literature Review
COVID-19 is considered to have two critical elements: (1) uncontrolled SARS-CoV-2 replication in the early stage of the disease and (2) an over reactive inflammatory response, referred to as a cytokine storm, in the later stage of the disease [6]. Virus-induced cytokine storms are not unique to SARS-CoV-2, as both SARS and MERS also manifest this excessive immune response, with replication of the three respective disease-causing viruses stimulating increased levels of a subset of pro-inflammatory cytokines, including Interleukin (IL)-6, IL-17, and Vascular Endothelial Growth Factor (VEGF) [7].
The uncontrolled inflammation can result in hyper permeability of the vasculature, multi-organ failure, Acute Respiratory Distress Syndrome (ARDS) and death [7]. ARDS is a leading cause of mortality in COVID-19 and elevated levels of interleukin IL-6 and IL-17 are reported to be associated with severe pulmonary complications and death.
Multiple therapies are under development for treating COVID-19, but only a few of these potentially address both viral replication and the excessive pro-inflammatory immune response. A therapeutic that can inhibit SARS-CoV-2 replication while suppressing the cytokine storm would seem likely to be highly beneficial for both the early and late stages of the disease. Experience with COVID-19 and other viral infections indicates that treating the excessive inflammatory immune events downstream of the infection can be vital to patient recovery [8,9] with the provision that drugs utilized to modify the cytokine storm do not compromise viral clearance [9].
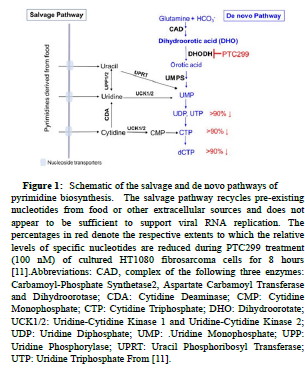
Targeting the cellular de novo pyrimidine biosynthesis pathway through inhibition of Dihydroorotate Dehydrogenase (DHOHD) may facilitate a dual mechanism COVID-19 therapeutic that is distinct from the use of separate antiviral or anti-inflammatory drugs. Pyrimidines are crucial building blocks for the biosynthesis of RNA and DNA and cellular pyrimidine levels control both viral replication and production of pro-inflammatory cytokines. DHODH is the rate limiting enzyme in this pathway and is expressed in all tissues examined, including the lung, heart, and liver. The enzyme is located on the inner membrane of mitochondria and catalyzes the Dehydrogenation of Dihydroorotate (DHO) to orotic acid, ultimately resulting in the production of Uridine and Cytidine Triphosphates (UTP and CTP) (Figure 1). In support of the notion of targeting DHODH for the treatment of viral infections, several high-throughput screens for broad-spectrum antivirals identified compounds that target the de novo pyrimidine biosynthesis pathway, in particular DHODH [9]. In addition, a recent multi-omics study of drug targets for viral infections prioritized DHODH inhibition as one of the top three mechanisms to consider for COVID-19 therapy [10]. It might be thought that DHODH inhibition would have adverse consequences for human patients, but pyrimidines can also be supplied by a salvage pathway, which recycles pre-existing nucleotides from extracellular sources such as food (Figure 1). This pathway does not appear to play a major role in the replication of SARS-CoV-2 and by itself would likely be inadequate to support the extensive viral replication requirements of SARS-CoV-2.
Figure 1:Schematic of the salvage and de novo pathways of pyrimidine biosynthesis. The salvage pathway recycles pre-existing nucleotides from food or other extracellular sources and does not appear to be sufficient to support viral RNA replication. The percentages in red denote the respective extents to which the relative levels of specific nucleotides are reduced during PTC299 treatment (100 nM) of cultured HT1080 fibrosarcoma cells for 8 hours [11].Abbreviations: CAD, complex of the following three enzymes: Carbamoyl-Phosphate Synthetase2, Aspartate Carbamoyl Transferase and Dihydroorotase; CDA: Cytidine Deaminase; CMP: Cytidine Monophosphate; CTP: Cytidine Triphosphate; DHO: Dihydroorotate; UCK1/2: Uridine-Cytidine Kinase 1 and Uridine-Cytidine Kinase 2; UDP: Uridine Diphosphate; UMP: .Uridine Monophosphate; UPP: Uridine Phosphorylase; UPRT: Uracil Phosphoribosyl Transferase; UTP: Uridine Triphosphate From [11].
PTC299 is an orally bioavailable molecule that directly binds DHODH and inhibits the activity of the enzyme [11]. Treatment of cultured cells with PTC299 results in reduction of DHODH activity, leading to increased levels of DHO, the substrate for the enzyme [11]. Similar findings were observed in PTC299-treated cancer patients, where administration of PTC299 caused increased levels of DHO in the blood, indicating successful inhibition of DHODH in these patients [11]. The ability of PTC299 to inhibit DHODH in vivo is further supported by the observation that PTC299 also normalized VEGF levels in cancer patients who characteristically have high levels of this stress-regulated cytokine [11]. Importantly, the clinical characteristics of PTC299 are well known, as it has been extensively studied in clinical trials of more than 300 healthy volunteers and cancer patients and shown to have a favorable Pharmaco Kinetic (PK) profile and to be well tolerated [12] (PTC Therapeutics, data on file).
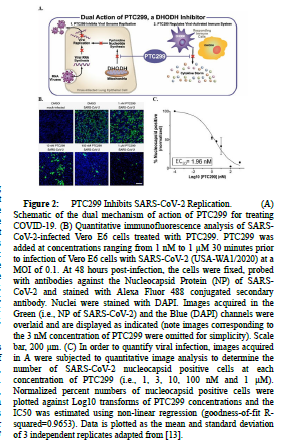
Recently, Luban and colleagues have demonstrated that, in vitro, PTC299 blocks SARS-CoV-2 replication and attenuates the production of a subset of inflammatory cytokines implicated in COVID-19 disease severity and prognosis (Figure 2A) [13]. PTC299 was found to inhibit SARS-CoV-2 replication in vitro with an EC50 ranging from 2.0 to 31.6 nM (Figures 2B and 2C) and with an antiviral The components of the inflammatory response inhibited by PTC299 in cell culture include MCP-1, IL-6, TNFα, VEGF, and IL-17, and the production of IgG [14]. Reduction of IgG levels by PTC299 may be clinically relevant as an elevated antibody titer has been shown to be associated with increased COVID-19 disease severity [15], and the inhibition of IL-6, IL-17, and VEGF by PTC299 may be of particular importance for treating COVID-19. IL-6 appears to play a key role in COVID-19 by promoting excessive cytokine production resulting from viral infection and pulmonary complications. Consistent with this, preliminary results from two small studies suggest inhibition of the IL-6 pathways by tocilizumab and siltuximab resulted in a treatment benefit for COVID-19 patients [16,17]. Similarly, IL-17 has been associated with disease severity and lung disease in COVID-19 and increased VEGF levels promote vascular permeability and leakage, contributing to the pathophysiology of hypotension and pulmonary dysfunction [18]. In short, the dual mechanism of action and the favorable PK and safety profiles of PTC299 support its investigation for use as a therapeutic for COVID-19.
Figure 2:PTC299 Inhibits SARS-CoV-2 Replication.(A)Schematic of the dual mechanism of action of PTC299 for treating COVID-19. (B) Quantitative immunofluorescence analysis of SARSCoV- 2-infected Vero E6 cells treated with PTC299. PTC299 was added at concentrations ranging from 1 nM to 1 μM 30 minutes prior to infection of Vero E6 cells with SARS-CoV-2 (USA-WA1/2020) at a MOI of 0.1. At 48 hours post-infection, the cells were fixed, probed with antibodies against the Nucleocapsid Protein (NP) of SARSCoV- 2 and stained with Alexa Fluor 488 conjugated secondary antibody. Nuclei were stained with DAPI. Images acquired in the Green (i.e., NP of SARS-CoV-2) and the Blue (DAPI) channels were overlaid and are displayed as indicated (note images corresponding to the 3 nM concentration of PTC299 were omitted for simplicity). Scale bar, 200 μm. (C) In order to quantify viral infection, images acquired in A were subjected to quantitative image analysis to determine the number of SARS-CoV-2 nucleocapsid positive cells at each concentration of PTC299 (i.e., 1, 3, 10, 100 nM and 1 μM). Normalized percent numbers of nucleocapsid positive cells were plotted against Log10 transforms of PTC299 concentrations and the IC50 was estimated using non-linear regression (goodness-of-fit Rsquared= 0.9653). Data is plotted as the mean and standard deviation of 3 independent replicates adapted from [13].
In addition to PTC299, a number of other DHODH inhibitors (brequinar, IMU-838, S312, S416, leflunomide and its active metabolite teriflunomide) have shown activity against SARS-CoV-2 and other RNA viruses. Leflunomide and teriflunomide are respectively FDA approved treatments for rheumatoid arthritis and relapsing forms of multiple sclerosis, whereas IMU-383 is a proposed treatment for multiple sclerosis. Leflunomide, teriflunomide, S312 and S416 have also been found to inhibit components of the cytokine storm [19] consistent with a dual mechanism of action for these DHODH inhibitors as well. Due to differences in experimental design it is difficult to compare findings across the group of DHODH inhibitors with respect to anti-SARS-CoV-2 activity but the available data suggest that PTC299 is highly potent compared with the other molecules whose EC50 at a Multiplicity of Infection (MOI) of 0.05 range from 0.017 to 41.5 μmol/L and whose selectivity indices range from 21 to 1050.
Currently, the efficacy and safety of PTC299 are being investigated in the randomized, placebo controlled, phase 2/3 study PTC299- VIR-015-COV19 in hospitalized adult patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04439071; also referred to as FITE19). Two other DHODH inhibitors, brequinar and IMU-838, are also in clinical trials of adult patients with COVID-19. Continued evidence of DHODH inhibitor efficacy in the clinic would not only be a reassuring development of a therapeutic approach to COVID-19, but also support the concept that a COVID-19 progression is mediated in part by cytokine storm [20].
Discussion and Conclusion
An important advantage of anti-viral drugs that target host cellular enzymes, e.g., DHODH, is that they act independently of virusencoded proteins, thus making them potentially impervious to the development of viral resistance. This attribute may be important as variants of SARs-CoV-2 with increased infectivity, e.g., B.1.1.7 and 50LV2. B.1.1.7, continue to arise B.1.17 is rapidly spreading around the globe due to increased rate of infection and 50LV2 is not only highly infectious, but also carries a mutation in the spike protein that reduces antibody recognition. Hence, concern has been raised that current vaccines, and those under development, may not be as efficacious against certain new variants as with the original SARSCoV- 2 strains. The ease of intercontinental travel and increased manmade changes to the environment will undoubtedly result in closer physical association of humans with zoonotic carriers of pathogens, including coronaviruses. Viral spillover events are thus likely to become more frequent potential health threats to the human population and identifying therapies that may treat such outbreaks is of great importance. The findings to date indicate that PTC299 acts by a dual mechanism of action to block SARS-CoV-2 replication and suppress the cytokine storm triggered by viral infection. This dual mechanism of action makes PTC299 a strong candidate as therapy for COVID-19, and potentially other future viral outbreaks.
Disclosures
E.G., M.W., J.D.G., E.M.W., K.O’K, and S.W.P are employed by PTC Therapeutics Inc. and have received salary compensation for time and effort and hold financial interest in the company. A.J. is a consultant and director for PTC Therapeutics Inc., receives compensation for those roles, and holds a financial interest in the company.
Funding Sources
This work was funded by PTC Therapeutics Inc. A.J. was supported by NIH grant R35GM122468.
References
- Hui DSC, Zumla A (2019) Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect Dis Clin North Am 33: 869-889.
- Groot RJ, Baker SC,Baric RS, Brown CS, Drosten C, et al. (2013) Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol 87: 7790-2.
- Cucinotta D, Vanelli M (2020) WHO Declares COVID-19 a Pandemic. Acta Biomed 91: 157-160.
- Dong H, Du H, Gardner L (2020) An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect Dis 20: 533-534.
- The Lancet (2020) COVID-19 vaccines: No time for complacency. Lancet 396: 1607.
- Berber B, Doluca O (2021) A Comprehensive Drug Repurposing Study for COVID19 Treatment: Novel Putative Dihydroorotate Dehydrogenase Inhibitors Show Association to Serotonin-Dopamine Receptors. Brief Bioinform
- Zhang YY,Li BR,Ning BT (2020) The Comparative Immunological Characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 Coronavirus Infections. Front Immunol 11: 2033.
- Quartuccio L,Semerano L,Benucci M,Boissier MC,Vita SD (2020) Urgent Avenues in the Treatment of COVID-19: Targeting Downstream Inflammation to Prevent Catastrophic Syndrome. Joint Bone Spine 87: 191-193.
- Sterne JAC, Murthy S, Diaz JV, Slutsky AS,Villar J, et al. (2020) Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. Jama 324: 1330-1341.
- Zheng J, Zhang Y, Liu Y, Baird D, Liu X, et al. (2020) Multi-Omics Study Revealing Tissue-Dependent Putative Mechanisms of SARS-CoV-2 Drug Targets on Viral Infections and Complex Diseases. MedRxiv
- Cao L,Weetall M,Trotta C, Cintron K, Ma J, et al. (2019) Targeting of Hematologic Malignancies with PTC299 A Novel Potent Inhibitor of Dihydroorotate Dehydrogenase with Favorable Pharmaceutical Properties. Mol Cancer Ther 18: 3-16.
- Weetall M, Davis T, Elfring G, Northcutt V ,Cao L, et al. (2016) Phase 1 Study of Safety, Tolerability, and Pharmacokinetics of PTC299, an Inhibitor of Stress-Regulated Protein Translation. Clin Pharmacol Drug Dev 5: 296-305.
- Luban J,Sattler RA,Mühlberger E,Graci JD and Cao L, et al. (2021) The DHODH Inhibitor PTC299 Arrests SARS-CoV-2 Replication and Suppresses Induction of Inflammatory Cytokines. Virus Res 292: 198246.
- Lafita-Navarro MC, Venkateswaran N, Kilgore JA, Kanji S and Han J, et al. (2020) Inhibition of the de novo Pyrimidine Biosynthesis Pathway Limits Ribosomal RNA Transcription Causing Nucleolar Stress in Glioblastoma Cells. PLoS Genet 16: e1009117.
- Zhao J, Yuan Q, Wang H, Liu W and Liao X, et al. (2020) Antibody Responses to SARS-CoV-2 in Patients of Novel Coronavirus Disease 2019. Clin Infect Dis.
- Xu X,Han M,Li T ,Sun W and Wang D, et al. (2020) Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv.
- Gritti G, Raimondi F, Ripamonti D, Riva I and Landi F, et al. (2020) Use of Siltuximab in Patients with COVID-19 Pneumonia Requiring Ventilatory Support. MedRxiv.
- Pacha O, Sallman MA,Evans SE (2020) COVID-19: A Case For Inhibiting IL-17? Nat Rev Immunol 20: 345-346.
- Xiong R, Zhang L ,Li S, Sun Y and Ding M, et al. (2020) Novel And Potent Inhibitors Targeting DHODH, A Rate-Limiting Enzyme in de novo Pyrimidine Biosynthesis, are Broad-Specturm Antiviral Against RNA Viruses Including Newly Emerged Coronavirus SARS-CoV-2. Protein & Cell 11: 723-739.
- CDC. Interim: Implications of the Emerging SARS-CoV-2 Variant VOC 202012/01. 2021
Citation: Peltz SW, Goodwin E, Weetall M, Graci JD, Welch EM,et al. (2021) Dual Mechanism of Action of PTC299 and other DHOHD Inhibitors in Suppressing SARS-CoV-2 Replication and Cellular Cytokine Storms. J Cytokine Biol 6:2:100037.
Copyright: © 2021 Peltz SW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1200
- [From(publication date): 0-2021 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 516
- PDF downloads: 684