Research Article Open Access
Drug Abuse in People Living with HIV in the Era of Highly Active Antiretroviral Therapy: A Systematic Review and Meta-Analysis
Chidozie U Nduka1*, Olalekan A Uthman2,3, Peter K Kimani1 and Saverio Stranges1,41Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, CV4 7AL, UK
2Warwick-Center for Applied Health Research and Delivery (WCAHRD), Warwick Medical School, University of Warwick, Coventry, CV4 7AL, UK
3Center for Applied Health Research and Delivery (CAHRD), Liverpool School of Tropical Medicine, Liverpool, UK
4Epidemiology and Public Health Research Unit, Department of Population Health, Luxembourg Institute of Health, L-1445 Strassen, Luxembourg, UK
- Corresponding Author:
- Chidozie U Nduka
Division of Health Sciences, Warwick Medical School
University of Warwick, Coventry, CV4 7AL, United Kingdom
Tel: +44(0)7472730268
E-mail: C.U.Nduka@warwick.ac.uk
Received date: November 01, 2015 Accepted date: December 23, 2015; Published date: December 31, 2015
Citation: Nduka CU, Uthman OA, Kimani PK, Stranges S (2015) Drug Abuse in People Living with HIV in the Era of Highly Active Antiretroviral Therapy: A Systematic Review and Meta-Analysis. J Addict Res Ther 6:255. doi:0.4172/2155-6105.1000255
Copyright: © 2015 Nduka CU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: Little is known about the epidemiology of drug abuse in HIV-infected populations. Therefore, we aimed to estimate the prevalence of drug abuse among people living with HIV. We also sought to examine factors potentially associated with drug abuse in this high-risk population subgroup.
Methods: We searched EMBASE and PubMed databases from 1997 to September 2015 for studies that reported crude prevalence estimates of drug abuse in people living with HIV. Using random-effects meta-analysis, we pooled prevalence estimates of all forms of drug abuse, including alcohol, crack/cocaine, methamphetamine, heroin, over-the-counter, tobacco/nicotine, and prescription drugs. We defined drug abuse strictly in terms of its accompanying self-damaging effects. Random-effects meta-regression analysis was performed on all study-level characteristics to identify factors that may be associated with drug abuse in HIV-infected persons.
Results: Seventy two studies, comprising 153,711 HIV-infected participants, met our inclusion criteria. Majority (87%) of the study population was resident in the United States (US). Overall, the prevalence of drug abuse was 33.6% (95% confidence interval [CI] 28.2 to 39.3, I2=99.7%, 31 studies, 28,238 participants), with prescription drugs identified as the most abused (42.7%, 95% CI 25.7 to 60.6, I2=99.7%, 14 studies, 1775 participants). While HIV infection duration (coefficient 0.03, 95% CI 0.0003 to 0.05, P=0.49, explained variance [R2]=51.3%) and ethnicity (Hispanic/Latino) (coefficient 0.006, 95% CI 0.001 to 0.01, P=0.012, R2=23.2%) may be determinants of drug abuse in people living with HIV, exposure to antiretroviral treatment was a strong deterrent (coefficient -0.004, 95% CI -0.01 to -0.0001, P=0.048, R2=10.1%).
Conclusion: One in three HIV-infected persons may be affected by drug abuse, with HIV infection duration and ethnicity (Hispanic) identified as predictors of this disorder. However, most of the available evidence comes from US studies. More studies originating from low- and middle-income countries are needed to obtain more precise estimates.
Keywords
Drug abuse; HIV; Prevalence; Highly active antiretroviral therapy
Introduction
Drug abuse, as opposed to mere substance use, entails the illicit and self-damaging use of recreational or prescription drugs [1,2]. Although drug abuse is a well-established risk factor for HIV infection [1-3], the potential for reverse causation in this association may also be high, with evidence suggesting substantially higher rates of alcohol and other forms of substance abuse among people living with HIV, compared with the general population [1]. Of note, people living with HIV may use recreational drugs as coping mechanisms for relieving psychologically stressful events often associated with chronic diseases [4,5]. Given that HIV infection is an immunosuppressive disease, often requiring pharmacological treatment, the effects of drug abuse may also be more severe among HIV-infected patients, compared with the general population. For instance, studies show that HIV-infected drug abusers are likely to have inadequate antiretroviral adherence levels, which may lead to treatment failure and progression to more advanced clinical stages of HIV infection, marked by a high occurrence of opportunistic infections, non-AIDS-defining illnesses and HIV-related deaths [6,7].
However, without a comprehensive assessment of drug abuse prevalence in people living HIV, it would be impossible to accurately estimate or predict its burden in this high-risk group. While prevalence estimates of HIV infection in drug abusers have been reported to vary widely between 0.01% and 72.1% depending on geographical location [8], the epidemiology of drug abuse among people living with HIV remains largely unknown. Previous systematic reviews have either provided global estimates of HIV prevalence in substance abusers [8] or estimated the prevalence of drug use in the general population [9]. Given these gaps in the available literature, we aimed to provide estimates of drug abuse prevalence in people living with HIV. We also sought to identify factors that may be associated with drug abuse in HIV-infected populations.
Methods
Eligibility criteria
We included studies that reported prevalence estimates of drug abuse among HIV-infected patients. Of note, studies without clear characterization of drug use as abuse, in which case, drug abuse was not defined in terms of its accompanying self-damaging effects, were not eligible for inclusion [1,2] (see Box 1 for eligibility criteria).
| Inclusion | Exclusion | |
|---|---|---|
| Population | HIV-infected | HIV-seronegative |
| Adolescents and Adults | Children | |
| Outcome | Any drug abuse including: | Drug or substance use not associated with any self-damaging effect. |
| Alcohol | ||
| Crack cocaine | ||
| Methamphetamine | ||
| Heroin | ||
| Inhalants e.g. amyl nitrite | ||
| Prescription drugs | ||
| *Over-the-counter medications | ||
| Tobacco/Nicotine | ||
| Study types | Any study design including: | Expert reviews |
| Cross-sectional | Policy reports | |
| Case-control | ||
| Cohort | ||
| Randomized trials | ||
| Population-based studies | ||
| Hospital-based studies | ||
| Full-texts | ||
| Conference abstracts |
Box 1: Eligibility Criteria,*: Over-the-counter medications e.g., cough and cold medicines, non-steroidal anti-inflammatory drugs.
Search strategy and study selection
We sought for eligible studies from PubMed (1997 to 10 September 2015) and EMBASE (1997 to 11 September 2015) databases using the following medical subject heading (MeSH) terms and keywords: *drug abuse/, *illicit drug/, *cocaine/, heroin.mp./, *diamorphine/, *methamphetamine/, *amyl nitirite/, *alcohol abuse/, *prevalence/, HIV.mp./ (Appendices 1 and 2). We also scanned bibliographies of relevant articles identified by the electronic search. The electronic search was limited to studies published after 1996, the year marking the onset of the highly active antiretroviral therapy (HAART) era [10], so as to assess the influence of antiretroviral treatment on drug use behaviour. All articles obtained from the search were screened by their titles and abstracts initially, and by the full texts subsequently. CUN and OAU independently evaluated the eligibility of studies yielded by the search, and SS resolved any disparities.
Data extraction
CUN and OAU independently extracted data from each included study using a piloted data extraction form and any disparities were resolved by consensus with SS. Data extracted included: citation, study design, sample size, country, geographical region, country income group, mean age, sex distribution, proportion with a history of incarceration, proportion with same sex partners, duration of HIV-infection, HAART status, housing, educational status, and occupational grade. Crude prevalence of drug abuse in any form was the primary outcome; however, we also extracted data on prevalence estimates with regard to specific drugs of abuse, such as alcohol, crack/cocaine, methamphetamine, heroin, marijuana, over-the-counter, and prescription drugs.
Assessment of risk of bias
Using a domain-based checklist adapted from the Newcastle-Ottawa scale, we investigated potential sources of bias in each included study: selection of participants, sample size justification, outcome assessment and statistical test [11] (Appendix 3).
Statistical analysis
First, we stabilized the raw proportions of participants with drug abuse from each study using the Freeman-Turkey variant of the arcsine square root transformed proportion [12] (Appendix 4). For each drug reported, we performed random-effects meta-analyses to obtain overall prevalence estimates of drug abuse. Heterogeneity across the included studies was assessed by inspecting forest plots using the I2 statistic, for which a value greater than 75% indicated considerable heterogeneity [13]. Subgroup analysis was also performed using the random-effects model to assess for any differential impact of country income group, geographical region and study design on drug abuse prevalence. We performed leave-one-out sensitivity analysis by omitting the included studies one at a time in order to determine whether any of the individual studies had an undue influence on the overall prevalence of drug abuse. To examine for predictors of drug abuse in people living with HIV, we performed univariate random-effects meta-regression analyses on a number of study-level variables: age, sex, ethnicity, educational status, occupational status, housing, sexual orientation, history of incarceration, duration of HIV infection and HAART status. We also examined for evidence of secular trend in drug abuse prevalence by performing meta-regression analysis on the year of publication. Publication bias was assessed by funnel plot inspection and using Egger’s regression test for funnel plot asymmetry. Where publication bias was present, we ascertained its effect on the overall results using the ‘trim and fill’ analysis of Duval and Tweedie [14]. Prevalence estimates were reported with 95% confidence intervals (CI) and P<0.05 was considered statistically significant for meta-regression analysis. All analyses were conducted using Stata version 14 for Windows (Stata Corp, College Station, Texas).
Results
Search strategy and study selection
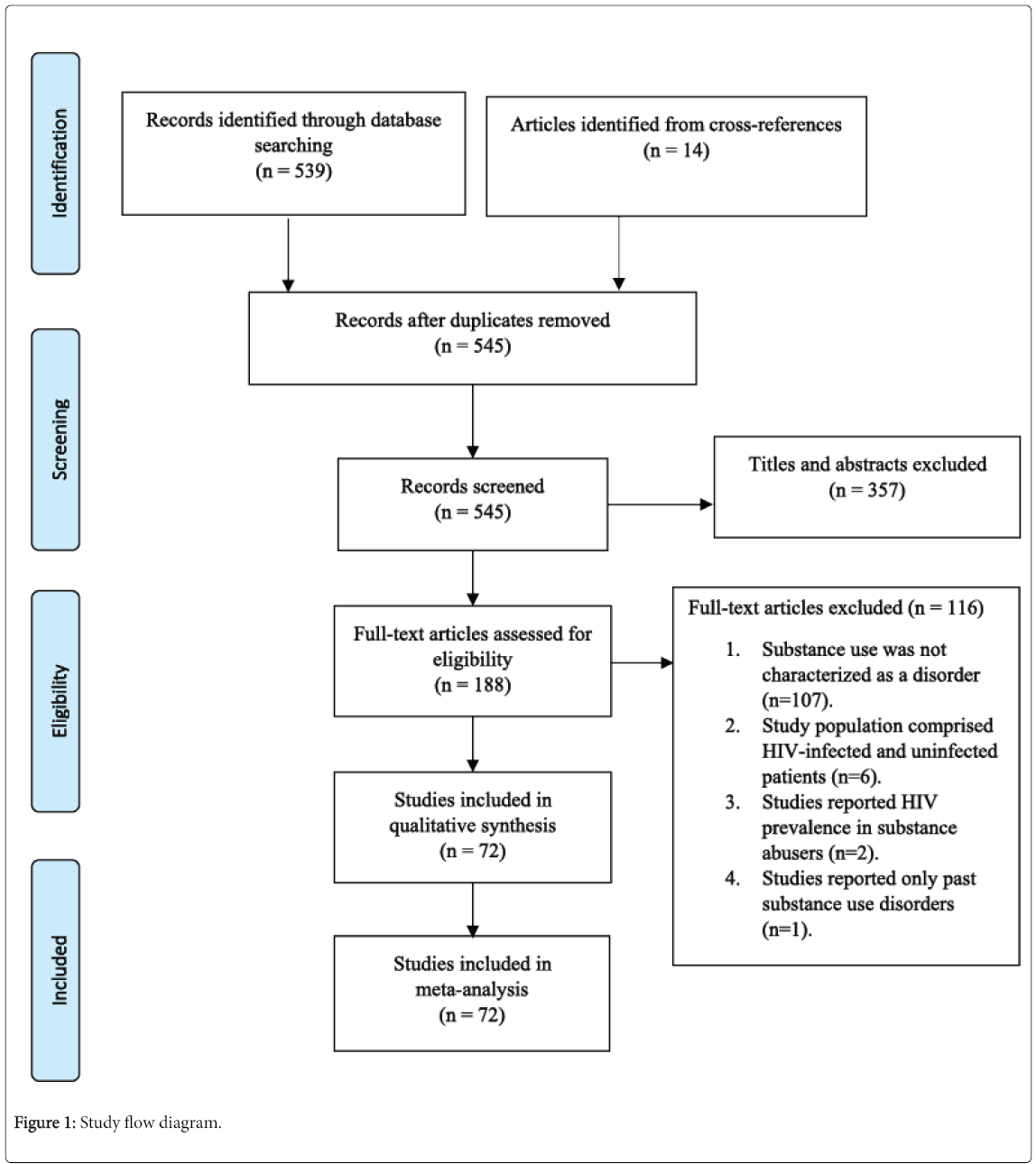
From 553 records yielded by the literature research, 357 articles were excluded by abstracts and 8 duplicate records were withdrawn, leaving 188 articles assessed to determine eligibility for inclusion. We excluded an additional 116 articles after reviewing the full texts, leaving 72 studies [15-86] eligible for inclusion in the systematic review and meta-analyses. The details of the study selection process are illustrated in Figure 1.
Characteristics of the included studies
We obtained data from 153,711 HIV-infected participants in studies conducted across 21 countries, with males accounting for more than 80% of the total study population. Table 1 summarizes the characteristics of the study participants in all 72 included studies. Most of the studies (n=60 studies; 149,074 participants) were conducted in high-income countries, including a substantial majority originating from the United States of America (n=44 studies; 133,865 participants). The total mean age was 43.0 ± 9.1 years, and participants from high-income countries (mean age 43.4 ± 8.7 years) were much older than those from low- and middle-income countries (mean age 38.2 ± 13.0 years). There were more participants of African descent (47.9%), compared with Caucasians (39.9%) and Hispanics (19.6%). The average duration of HIV infection was 8.1 ± 5.3 years, with 64% of patients exposed to HAART. More than one in three (34.7%) had a history of incarceration, and sexual orientation was homosexual in 37%. About half (46.9%) of the included participants had received less than 12 years of formal education, 60% were unemployed at the time, and the proportion of study participants who were homeless was 30%.
| Author | Year | Study design | Country | Income group | Region | Number analyzed | M (%) | Age (years) | HIV duration (years) | ART (%) | Incarcerated (%) | Homosexual (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ajuoga | 2009 | Cross-sectional | USA | High | America | 215 | 69 | 45 ± 8.3 | ||||
| Altice | 2011 | Cohort | USA | High | America | 295 | 68.1 | 45.2 ± 8.2 | 59.7 | 19 | ||
| Belloso | 2010 | Cohort | Multiple | LMIC | America | 520 | 5.5 ± 4.4 | 80 | ||||
| Berg | 2009 | RCT | USA | High | America | 77 | 53 | 47 ± 6.9 | 13 ± 4 | 100 | ||
| Bertolaccini | 2008 | Cohort | Italy | High | Europe | 26 | 85 | 39 ± 6.1 | 85 | 23 | ||
| Buchacz | 2008 | Cohort | USA | High | America | 7155 | 80.5 | 58.5 | ||||
| Castellares | 2008 | Cross-sectional | Spain | High | Europe | 2168 | 76.2 | 42 ± 6 | 34 | |||
| Chander | 2006 | Cohort | USA | High | America | 1957 | 63.7 | 73.2 | 23.6 | |||
| Chao | 2012 | Cohort | USA | High | America | 12872 | 90 | 40.1 ± 9.8 | 2.8 ± 4.1 | 21.1 | 61.6 | |
| Fuster | 2014 | Cohort | USA | High | America | 400 | 74.7 | 43 ± 7.4 | 62 | |||
| Fuster | 2013 | Cross-sectional | USA | High | America | 308 | 73.1 | 42.8 ± 7.3 | 60.4 | |||
| Goar | 2011 | Cross-sectional | Nigeria | LMIC | Africa | 160 | 35.6 | |||||
| Goh | 2007 | Cross-sectional | Singapore | High | W/Pacific | 96 | 89.6 | 40.2 ± 7.3 | 22 | |||
| Green | 2010 | Cohort | USA | High | America | 3160 | 97.5 | 49.4 ± 8.8 | ||||
| Grzeszczuk | 2015 | Cross-sectional | Poland | LMIC | Europe | 457 | 76.6 | 38 ± 6.1 | 10 ± 3.3 | 23.4 | 8.8 | |
| Hayashi | 2015 | Cross-sectional | Thailand | LMIC | W/Pacific | 128 | 80.4 | 54.7 | 93.3 | |||
| Helleberg | 2013 | Cohort | Denmark | High | Europe | 2921 | 77.5 | 43.3 ± 6.6 | 7 ± 2.6 | 77.4 | 52.9 | |
| Hsu | 2013 | Cohort | USA | High | America | 30533 | 95.5 | 50.3 ± 11 | ||||
| Ibrahim | 2014 | Cross-sectional | Nigeria | LMIC | Africa | 250 | 47.6 | |||||
| Ikeda | 2013 | Cross-sectional | Brazil | LMIC | America | 1240 | 50.6 | 39.1 ± 10 | 65.7 | |||
| Josephs | 2010 | Cross-sectional | USA | High | America | 951 | 68 | 69 | 34 | |||
| Justice | 2010 | Cohort | USA | High | America | 9784 | 97.9 | 45 | ||||
| Kabali | 2011 | Cohort | USA | High | America | 462 | 77 | |||||
| Kagimu | 2012 | Case-control | Uganda | LMIC | Africa | 106 | 18 | |||||
| Kellerman | 2003 | Cohort | USA | High | America | 16248 | 70.8 | 47.1 | 38.4 | |||
| Kellinghaus | 2008 | Cross-sectional | Germany | High | Europe | 51 | 74.5 | 37 ± 2.5 | 72.6 | |||
| Kim | 2006 | Cohort | USA | High | America | 349 | 89.3 | 40.7 ± 7.2 | 31.8 | 17.8 | ||
| Korthuis | 2015 | Cohort | USA | High | America | 3038 | 97.5 | 49 ± 8.8 | 83.9 | |||
| Korthuis | 2012 | Cross-sectional | USA | High | America | 3410 | 97.4 | 49.1 ± 8.8 | 83.2 | |||
| Krupitsky | 2005 | Cross-sectional | Russia | High | Europe | 201 | 62 | 26.6 ± 8.2 | ||||
| Lim | 2014 | Cross-sectional | USA | High | America | 2111 | 97.7 | 48.5 ± 2.5 | 82.6 | |||
| Luo | 2013 | Cross-sectional | China | LMIC | W/Pacific | 551 | 66.2 | 67.5 | 9.3 | |||
| Mayer | 2010 | Cross-sectional | USA | High | America | 398 | 100 | 41.5 ± 8.4 | 8.6 ± 6.7 | 66.1 | 100 | |
| Mayer | 2012 | Cohort | USA | High | America | 557 | 78.6 | 42 ± 2.8 | 4.9 ± 1.5 | 78 | 65.5 | |
| McGinnis | 2013 | Cohort | USA | High | America | 444 | 50 ± 8.4 | |||||
| Merlin | 2012 | Cohort | USA | High | America | 1521 | 42.3 | 43.7 | 54.9 | |||
| Merlin | 2013 | Cross-sectional | USA | High | America | 1903 | 77.4 | 43.6 | 52.7 | |||
| Metsch | 2009 | Cross-sectional | USA | High | America | 1038 | 62 | 42 | 31.4 | |||
| Miaskowski | 2011 | Cross-sectional | USA | High | America | 296 | 70.7 | 48.2 ± 7.3 | 74.4 | |||
| Mijch | 2006 | Cohort | Australia | High | W/Pacific | 2981 | 93.5 | 4.3 | ||||
| Moore | 2012 | Cross-sectional | USA | High | America | 117 | 87.5 | 43.7 ± 7.8 | ||||
| Nahvi | 2012 | RCT | USA | High | America | 77 | 53 | 47 ± 7 | 100 | |||
| Nakimuli-Mpungu | 2011 | Cross-sectional | Uganda | LMIC | Africa | 500 | 30.2 | |||||
| Newville | 2015 | Cross-sectional | USA | High | America | 295 | 67.8 | 47.5 ± 9.7 | 11.5 | 100 | ||
| Nicolleti | 2007 | Cross-sectional | Brazil | LMIC | America | 385 | 70 | 37.5 ± 8.6 | 70 | |||
| Nunes | 2006 | Cross-sectional | USA | High | America | 401 | 73 | 59 | 23 | |||
| Obel | 2011 | Cohort | Denmark | High | Europe | 2267 | 73.9 | 40.8 ± 3.6 | 5.3 | 38.5 | ||
| Omland | 2010 | Cohort | Denmark | High | Europe | 392 | 59.5 | 39 ± 2.6 | 6.5 ± 3 | 37.5 | ||
| Pace | 2012 | Cross-sectional | Russia | High | Europe | 682 | 60 | 30 ± 5.2 | 17.2 | 38.1 | 2.1 | |
| Pakkala | 2012 | Cohort | USA | High | America | 80 | 80 | 52 | 70 | 19 | ||
| Pintado | 2001 | Cross-sectional | Spain | High | Europe | 80 | 80 | 7.5 | ||||
| Robinson-Papp | 2012 | Cohort | USA | High | America | 636 | 77 | 41 | ||||
| Ruan | 2007 | Cohort | China | LMIC | W/Pacific | 229 | 82.1 | |||||
| Ryan | 2004 | Cross-sectional | USA | High | America | 107 | 81.3 | 43.5 ± 7.2 | ||||
| Salmon-Ceron | 2009 | Cohort | France | High | Europe | 898 | 75 | 46 | 11.8 | 88 | 26 | |
| Sambamoorthi | 2000 | Cross-sectional | USA | High | America | 5559 | 54.2 | 58.8 | ||||
| Scribner | 2000 | Case-control | USA | High | America | 75 | 89.3 | 39.5 | 61 | |||
| Siemieniuk | 2012 | Cross-sectional | Canada | High | America | 687 | 45 ± 10.2 | 10.5 | ||||
| Silverberg | 2013 | Cohort | USA | High | America | 4137 | 87.6 | 47.2 ± 8.8 | 10 ± 5.9 | 81.1 | 61.6 | |
| Sullivan | 2006 | RCT | USA | High | America | 16 | 94 | 47.2 ± 8.5 | ||||
| Surrat | 2013 | Cross-sectional | USA | High | America | 503 | 59.5 | 46.1 ± 7.8 | 13.3 ± 7.3 | |||
| Tabarsi | 2012 | Cross-sectional | Iran | LMIC | E/Mediterranean | 111 | 96.4 | 38 ± 9 | 26.1 | 88.1 | 0.9 | |
| Tetrault | 2012 | Cohort | USA | High | America | 114 | 97 | 49 | 52 | |||
| Towner | 2012 | Cohort | USA | High | America | 20775 | 91 | 41 | 3.5 | 33 | 59 | |
| Tsui | 2012 | Cohort | USA | High | America | 397 | 74.8 | 42.5 ± 7.5 | ||||
| Tyurina | 2013 | Cohort | Russia | High | Europe | 700 | 59.3 | 30.1 ± 5.2 | 17.3 | |||
| Vagenas | 2014 | RCT | USA | High | America | 85 | 78.8 | 46 | 81.2 | |||
| Vallecillo | 2013 | Cross-sectional | Spain | High | Europe | 91 | 63.7 | 44.5 ± 8 | 74.7 | |||
| Van der Werf | 2006 | Cross-sectional | Ukraine | High | Europe | 968 | 73.6 | 12.1 | ||||
| Vergara-Rodriguez | 2011 | Cohort | USA | High | America | 303 | 60 | |||||
| Vidrine | 2006 | RCT | USA | High | America | 95 | 77.9 | 42.9 ± 8.1 | 37.9 | |||
| Whetten | 2012 | Cross-sectional | USA | High | America | 611 | 68.7 | 40.1 ± 25 | 44.5 | |||
Table 1: Characteristics of eligible studies, ART: antiretroviral therapy; E/Mediterranean: eastern mediterranean; LMIC: low- and middle-income country; M: males; W/Pacific
Methodological quality in the included studies
eTable 1 summarizes the quality assessment of the included studies. Sampling bias was low in only 13 studies [18,20–23,32,56,57,61,69,70,78,85]; sample size was justified and satisfactory in 15 studies [17,18,20–23,28,32,34,35,38,39,70,78]; respondents to questionnaires were no less than 70% of the study population and comparable in baseline characteristics to non-respondents in 18 studies [18,20–22,27,31,33–35,38,39,46,50,51,53,57,63,85]; and drug abuse was assessed using a validated instrument in 39 studies [15–17,23–26,28,33,34,41–45,49–51,53–58,61,63,66,68,70,73–75,79–82,84–86]. Nonetheless, statistical tests were described and appropriate in all 72 studies.
Overall prevalence of drug abuse in people living with HIV
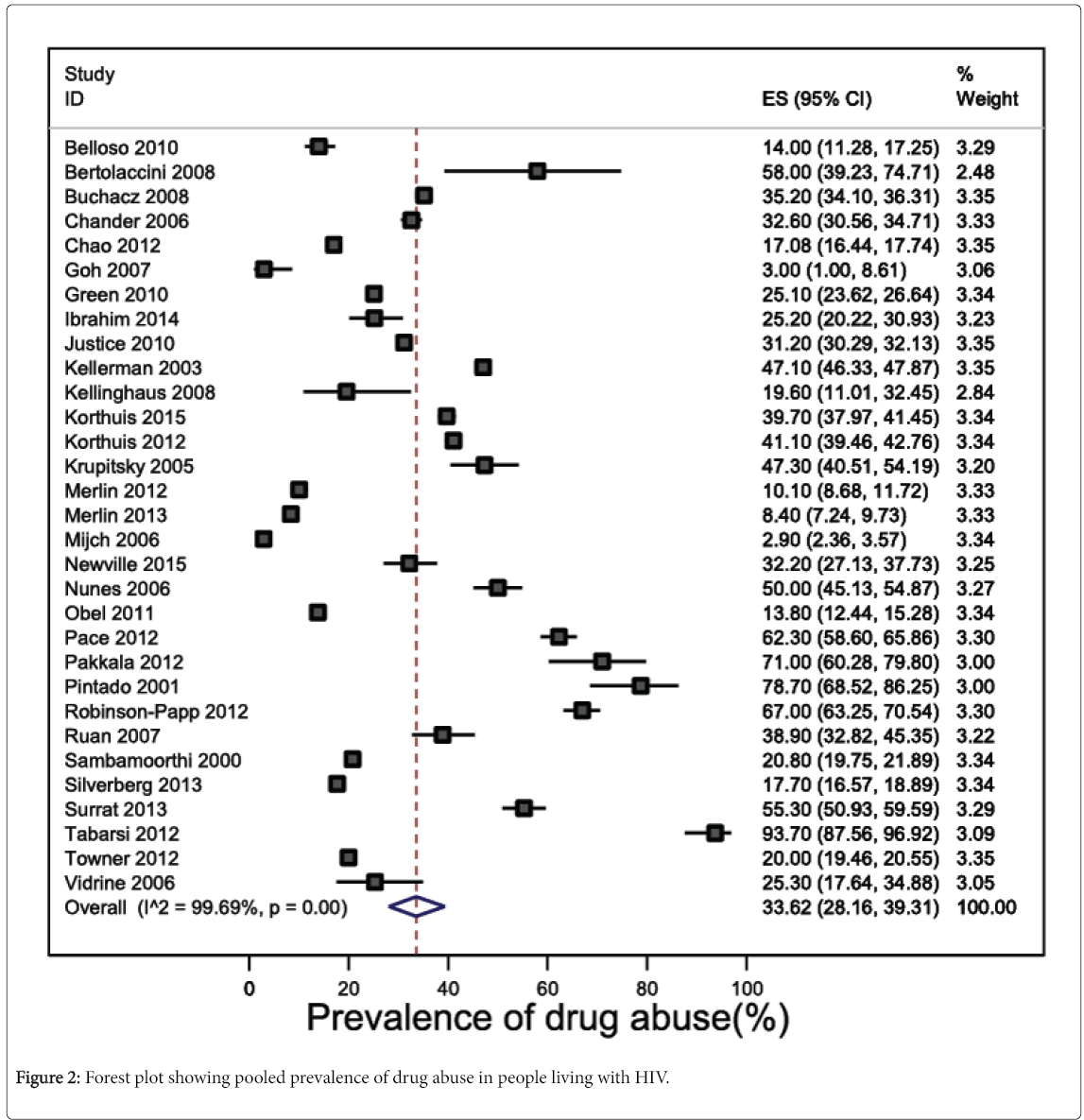
Prevalence estimates of drug abuse in any form were reported in 31 studies (n=101,023 participants), and widely varied between 2.9% [54] and 93.7% [76]. The pooled prevalence of drug abuse was 33.6% (95% CI 28.2 to 39.3, 28,238 participants) (Figure 2). Heterogeneity across the 31 studies was considerable and statistically significant (I2 statistic=99.7%, P<0.001). Funnel plot asymmetry was absent, suggesting no evidence of publication bias (P=0.46 for Egger’s regression test for funnel plot asymmetry) (eFigure 1). Leave-one-out sensitivity analysis showed than no study included in the meta-analysis had an undue influence on the pooled prevalence of drug abuse as to change the confidence interval significantly (eFigure 2).
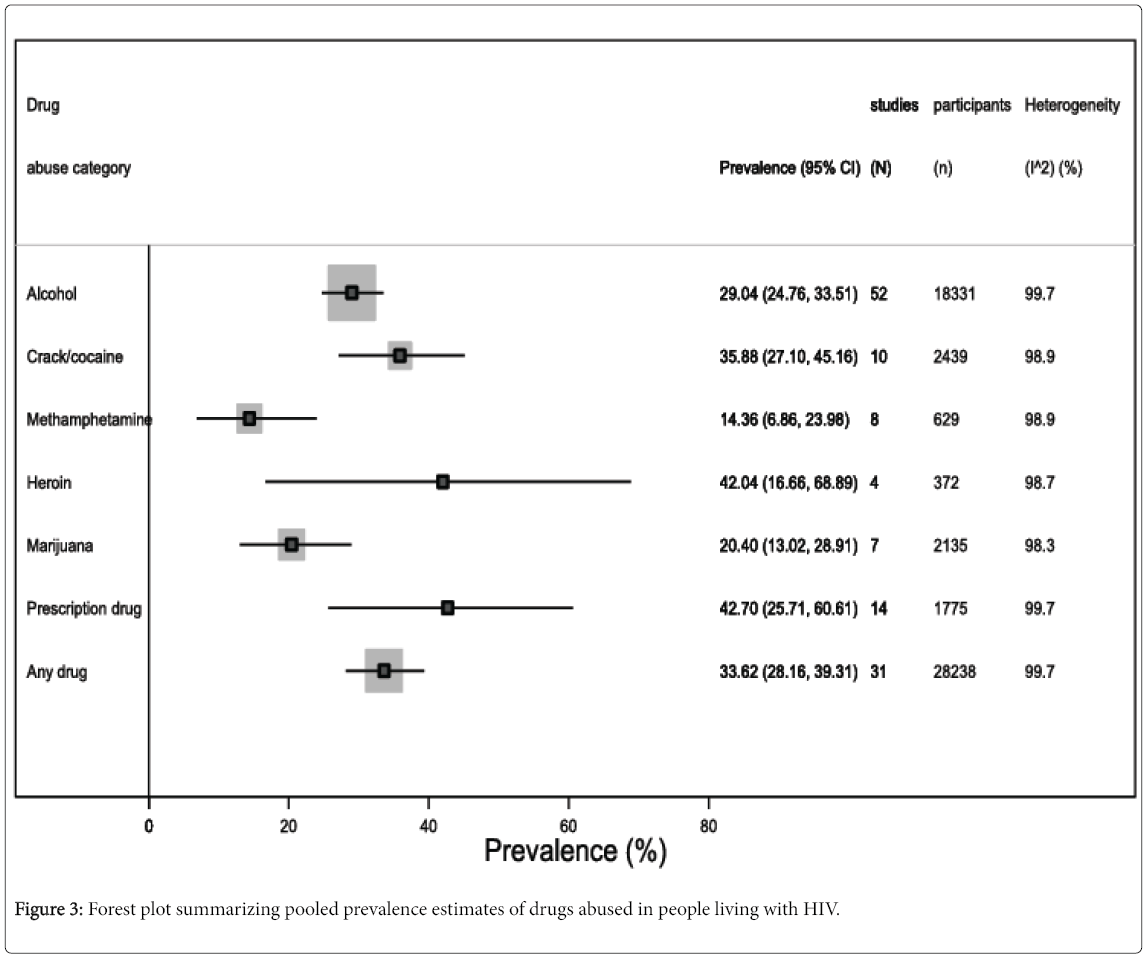
Figure 3 summarizes the prevalence estimates regarding specific drugs of abuse. For instance, alcohol abuse was found in 29% of the included study population (95% CI 24.8 to 33.5, I2=99.6%, 52 studies, 18,331 participants) (eFigure 3); prevalence of crack/cocaine abuse was 35.9% (95% CI 27.1 to 45.2, I2=98.8%, 10 studies, 2439 participants) (eFigure 4); methamphetamine abuse prevalence was 14.4% (95% CI 6.9 to 24.0, I2=98.9%, 8 studies, 629 participants) (eFigure 5); 42% (95% CI 16.7 to 68.9, I2=98.7%, 4 studies, 372 participants) abused Heroin (eFigure 6); 20.4% (95% CI 13.0 to 28.9, I2=98.3%, 7 studies, 2135 participants) abused marijuana (eFigure 7); and 42.7% (95% CI 25.7 to 60.6, I2=99.7%, 14 studies, 1775 participants) abused prescription drugs (eFigure 8).
Subgroup estimates of drug abuse prevalence in people living with HIV
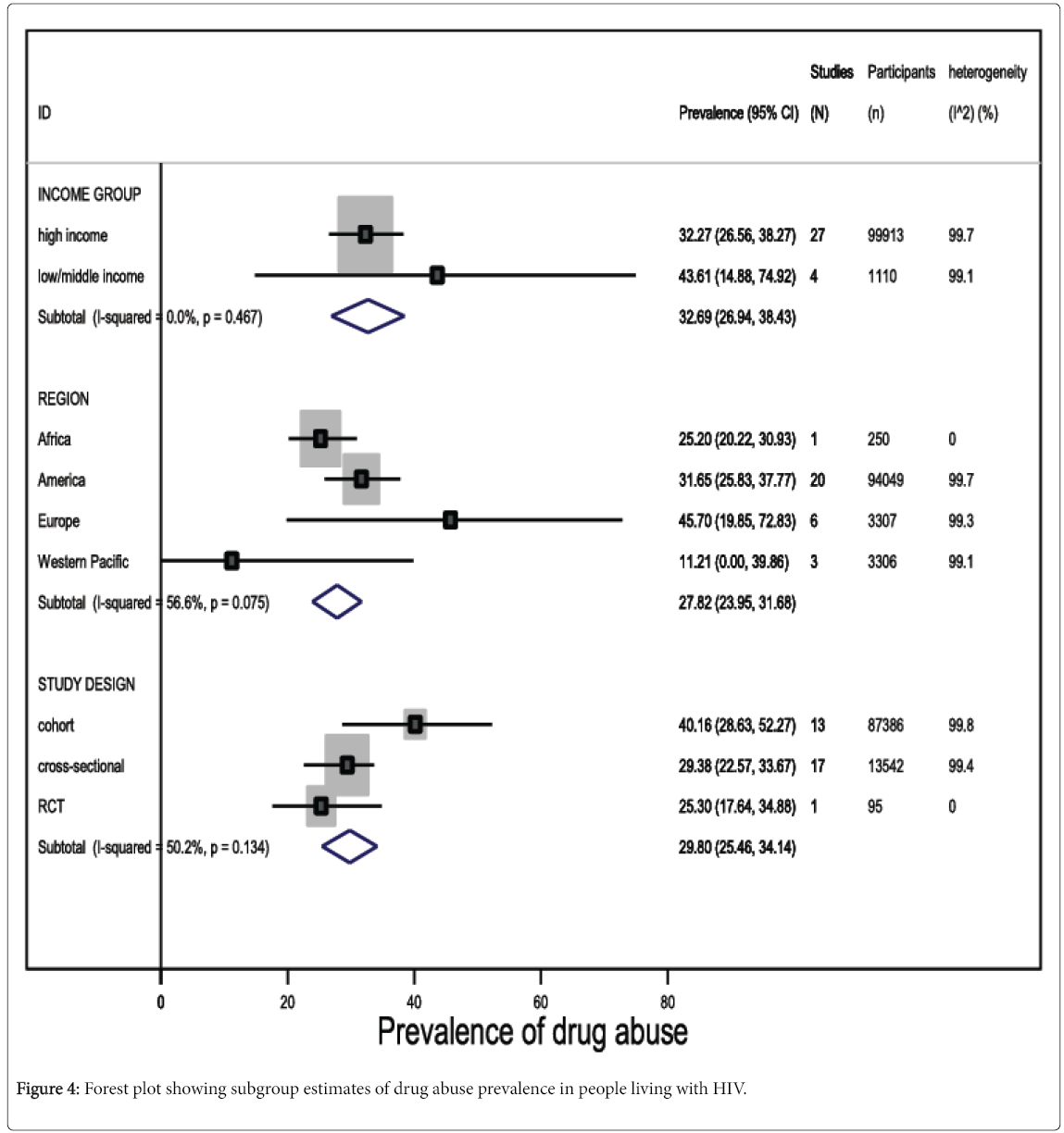
Among studies reporting any form of drug abuse in people living with HIV in high income countries, the pooled prevalence was 32.3% (95% CI 26.6 to 38.3, I2=99.7%, 27 studies, 99,913 participants). Although drug abuse prevalence was higher among people living with HIV in low- and middle-income countries (pooled prevalence 43.6%, 95% CI 14.9 to 74.9, I2=99.1%, 4 studies, 1110 participants), the difference was not statistically significant (Figure 4; eTable 2).
In general, studies conducted in European countries reported higher prevalence estimates of drug abuse, compared with other regions (pooled prevalence 45.7%, 95% CI 19.9 to 72.8, I2=99.3%, 6 studies, 3307 participants) (Figure 4). Although, we observed a 93.7% prevalence of drug abuse among HIV-infected patients resident in the Middle-East, this estimate was based on evidence from one study only, which comprised a highly selected population. Nonetheless, the difference in drug abuse prevalence in people living with HIV was not statistically significant across geographical regions (eTable 2).
Prevalence estimates of drug abuse in HIV-infected subjects were higher (P=0.05) in cross-sectional studies (pooled prevalence 40.2%, 95% CI 28.6 to 52.3, I2=99.8%, 13 studies, 13,542 participants), compared with cohort studies (pooled prevalence 29.4%, 95% CI 22.6 to 33.7, I2=99.4%, 17 studies, 87,386 participants), but the difference was also not statistically significant at P<0.05 (Figure 4).
Furthermore, we observed that most of the included studies did not consider alcohol abuse to be a form of drug abuse. Therefore, it was additionally important to perform a subgroup analysis of the prevalence of alcohol abuse. Prevalence of alcohol abuse was higher among people living with HIV in high income countries (pooled prevalence 30.6%, 95% CI 26.0 to 35.5, I2=99.6%, 42 studies, 17,515 participants), compared with those in low- and middle-income countries (pooled prevalence 24.8%, 95% CI 9.3 to 44.7, I2=99.4%. 10 studies, 2923 participants). However, there was no statistically significant difference between both subgroup estimates. By region, alcohol abuse prevalence was highest among people living with HIV in the Western Pacific (pooled prevalence 74.4%, 95% CI 71.0 to 77.6, I2=99.4%, 2 studies, 501 participants); whereas, HIV-infected subjects in the Africa region had the lowest prevalence of alcohol abuse (pooled prevalence 20.2%, 95% CI 1.0 to 53.9, I2=96.7%, 3 studies, 106 participants).
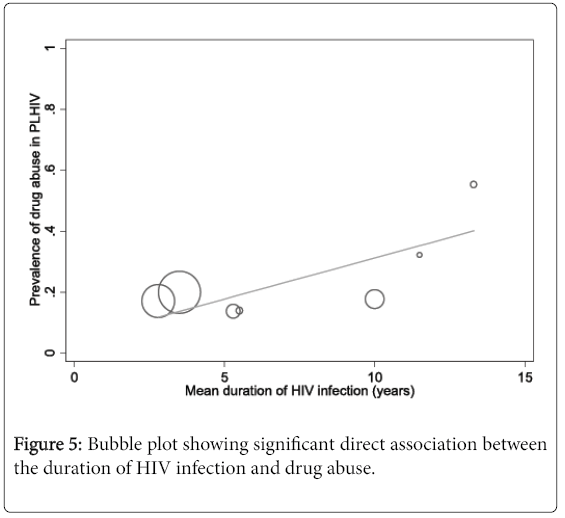
Factors associated with drug abuse in people living with HIV
The results of meta-regression analyses showed a significant direct association between the duration of HIV infection and drug abuse of any form in people living with HIV (coefficient 0.03, 95% CI 0.0003 to 0.05, P=0.048) (Figure 5). In fact, variations in the mean duration of HIV infection between the included studies accounted for more than half of the heterogeneity in the pooled prevalence of drug abuse (explained variance R2=51.3%) (eTable 2). We also found a significant association between ethnicity (Hispanic/Latino) and alcohol abuse in people living with HIV (coefficient 0.006, 95% CI 0.001 to 0.01, P=0.012, R2=23.2%) (eTable 3). In contrast, HIV-infected persons exposed to HAART (coefficient -0.004, 95% CI -0.01 to -0.0001, P=0.048) and those with same sex partners were significantly less likely to abuse drugs (coefficient -0.007, 95% CI -0.01 to -0.001, P=0.019) (eTable 3). Meta-regression analysis performed on publication year revealed no evidence of a secular trend in the prevalence of drug abuse in people living with HIV (coefficient -0.002, 95% CI -0.02 to 0.19, P=0.847) (eTable 2).
Discussion
We present the first comprehensive systematic review examining the epidemiology of drug abuse in HIV-infected populations. Overall, our findings suggest that drug abuse in people living with HIV is a global public health problem, with one in three HIV-infected persons affected, and no evidence suggesting substantial regional, or socio-economic variation in the occurrence of this disorder. Although, in absolute terms, alcohol abuse may be more common among people living with HIV, compared with other drugs of abuse, we observe that prescription drug abuse, notably opioids, may be most prevalent, with approximately one in two HIV-infected persons affected. Interestingly, our analyses suggest that the likelihood for drug abuse among people living with HIV increases substantially as the duration of HIV infection increases. As is the case with other chronic diseases, we speculate that the increased life-expectancy of people living with HIV since the onset of the HAART era may be accompanied by increased levels of psychosocial stress, for which substance use may be a coping mechanism [5,87,88]. Our analyses also suggest that there might be an ethnic predilection for alcohol abuse among people living with HIV, specifically those of Hispanic origins. However, taking into account the occurrence of drug abuse of all forms, there was no evidence of ethnic variation.
Our findings may have important clinical and public health implications for HIV-infected populations worldwide. For instance, beyond the baseline assessment of recreational drug use among HIV-infected persons newly enrolled into care, our results support the inclusion of comprehensive routine screening and on-going assessment for substance users as part of care and treatment guidelines for HIV-infected patients [89]. Drug abusers may constitute a higher-risk subgroup within HIV-infected populations, emphasizing the importance of early identification in the clinic setting, especially as the duration of infection progresses.
In addition to differences in the duration of HIV infection, other factors accounting for some of the observed heterogeneity in the prevalence estimates of drug abuse include varying proportions of participants exposed to ART, and those with same sex partners. We observed that exposure to ART may deter drug abuse among HIV-infected patients, and this may be linked to an increased likelihood to quit the illicit use of drugs following the initiation of ART [89]. Similarly, while previous studies identify homosexuality to be associated with drug abuse in the general population [90,91], we find that the reverse may be the case among people living with HIV: our results suggest that homosexuality may also deter drug abuse among people living with HIV. However, while we hypothesize those HIV-infected persons with same sex partners may quit the illicit use of recreational substances and other drugs of abuse due to the underlying HIV infection; it is equally plausible that this proposed scenario may also apply to HIV-infected persons with heterosexual partners [89].
Strengths and limitations
Our findings must be interpreted with caution given that the population samples across the included studies were mostly limited to people living with HIV in the Americas, predominantly within the United States. In fact, less than 5% of the total population sample was resident in low-and middle-income countries - where the burden of HIV infection is most severe - potentially reducing generalizability of our findings across different geographic and socio-economic settings. In order to obtain a more comprehensive estimate of the prevalence of drug abuse among people living with HIV, studies originating from low- and middle-income countries in the Africa, Middle-East and South-East Asia regions need to be adequately represented. Secondly, the methodological quality across the included studies was moderate at best, with only 18% (n=13) of the studies assessed as having a low risk of sampling bias. Nonetheless, using meta-regression analysis, we affirm that sampling bias had no significant impact (P=0.121) on the pooled prevalence of drug abuse [13]. Furthermore, alcohol abuse was not considered in estimating the prevalence of drug abuse in some of the included studies. In fact, 16 of the 31 studies reporting prevalence estimates of drug abuse assessed alcohol abuse separately [17,22,23,28,36,39,42–44,58,60,63,66,73,76,85], which may potentially suggest that the pooled prevalence of drug abuse in our study population of HIV-infected patients is likely to be underestimated. The dearth of studies reporting abuse of nicotine and over-the-counter medications (such as non-steroidal anti-inflammatory drugs and cold and cough medicines) precluded estimates of the prevalence of these potential drugs of abuse among people living with HIV. Although study-level analyses such as meta-regression allow multiple factors to be examined simultaneously, regression analyses using individual participant data (IPD) would be considered to be more robust in examining factors that may potentially influence drug abuse in persons living with HIV [92]. However, we did not have access to data recorded for each patient in each study.
Nonetheless, the strengths of our study should also be highlighted. For instance, we present the most comprehensive evidence and first pooled analyses investigating the prevalence, patterns and predictors of drug abuse including prevalence estimates of specific recreational drugs and controlled substances -among people living with HIV. Secondly, by identifying HIV infection duration as a potential predictor of drug abuse, we contribute a novel finding which strategically fills an important gap in the literature on a potentially neglected issue affecting people living with HIV worldwide [8,9]. Furthermore, the absence of small-study effects, and any undue influence on the overall prevalence of drug abuse by any of the included studies are also important strengths of our study.
Conclusion
This meta-analysis of over 150,000 subjects is the first of its kind to provide contemporary and up-to-date estimates that reflect the potential burden of drug abuse among people living with HIV worldwide. On average, one in three HIV-infected persons are affected by this disorder, which occurs more commonly as the duration of HIV infection increases. Although our findings may be limited by a high risk of sampling bias and considerable differences in the prevalence estimates of drug abuse between studies included in the meta-analysis, all analyses were performed using the random-effects model and meta-regression revealed that sampling bias had no significant impact on the pooled prevalence of drug abuse. However, future studies could employ IPD meta-analysis to investigate potential predictors of drug abuse in persons living with HIV. More prevalence studies originating from low- and middle-income countries are also needed to obtain more precise estimates across different geographic regions and socio-economic settings, as well as to accurately predict future trends of the global prevalence of drug abuse in people living with HIV.
Authorship
CUN conceived of the study, and participated in designing the study, data extraction, analysis and interpretation, and drafted the manuscript. OAU participated in designing the study, data extraction, analysis and interpretation. PKK participated in data analysis and interpretation. SS participated in designing the study and interpretation of the data. All authors critically revised the manuscript and approved submission of final draft.
References
- National Institute on Drug Abuse (2015) Drug and alcohol use - a significant risk factor for HIV.
- Centre for Disease Control and Prevention (2015) HIV and substance use in the United States.
- AIDS (2014) Substance abuse/use.
- Pence BW, Thielman NM, Whetten K, Ostermann J, Kumar V, et al. (2008) Coping strategies and patterns of alcohol and drug use among HIV-infected patients in the United States Southeast.AIDS Patient Care STDS 22: 869-877.
- Leiphart JM (2014) Coping with stress
- Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, et al. (2007) The impact of illicit drug use and substance abuse treatment on adherence to HAART. AIDS Care 19: 1134–1140.
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, et al. (2013) Mortality among people who inject drugs: a systematic review and meta-analysis.Bull World Health Organ 91: 102-123.
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, et al. (2008) Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 372: 1733–1745.
- Aceijas C, Stimson GV, Hickman M, Rhodes T (2004) Global overview of injecting drug use and HIV infection among injecting drug users. AIDS 18: 2295–2303.
- Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, et al. (2007) Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS 21: 2093–2100.
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, et al. (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses.
- Miller JJ (1978) Inverse of the Freeman-Tukey Double Arcsine Transformation. The American Statistician 32: 138.
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558.
- Duval S, Tweedie R (2000) A non-parametric “Trim and Fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 95: 89–98.
- Ajuoga E, Sansgiry SS, Ngo C, Yeh RF (2008) Use/misuse of over-the-counter medications and associated adverse drug events among HIV-infected patients. Res Social Adm Pharm 4: 292–301.
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, et al. (2011) HIV Treatment Outcomes Among HIV-Infected, Opioid-Dependent Patients Receiving Buprenorphine/Naloxone Treatment within HIV Clinical Care Settings: Results From a Multisite Study. J Acquir Immune DeficSyndr. 56: S22–S32.
- Belloso WH, Orellana LC, Grinsztejn B, Madero JS, La Rosa A, et al. (2010) Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Medicine 11: 554–564.
- Berg KM, Mouriz J, Li X, Duggan E, Goldberg U et al. (2009) Rationale, design, and sample characteristics of a randomized controlled trial of directly observed antiretroviral therapy delivered in methadone clinics. ContempClin Trials 30: 481–489.
- Bertolaccini L, Lybéris P, Soncini S, Di Perri G, Manno E (2008) Clinical characteristic lung cancer in HIV-infected patients. Cancer Therapy 6: 903–906.
- Buchacz K, Baker RK, Moorman AC, Richardson JT, Wood KC, et al. (2008) Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS 22:1345–1356.
- Castellares C, Barreiro P, Marti´n-Carbonero L, Labarga P, Vispo ME, et al. (2008) Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 15: 165–172.
- Chander G, Lau B, Moore RD (2006) Hazardous Alcohol Use: A Risk Factor for Non-Adherence and Lack of Suppression in HIV Infection. J Acquir Immune DeficSyndr 43: 411–417.
- Chao C, Leyden WA, Xu L, Horberg MA, klein D, et al. (2012) Exposure to Antiretroviral Therapy and Risk of Cancer in HIV-infected Persons. AIDS 13; 26: 2223–2231.
- Fuster D, Tsui JI, Cheng DM, Quinn EK, Bridden C, et al. (2013) Impact of lifetime alcohol use on liver fibrosis in a population of HIV-infected patients with and without hepatitis C coinfection. Alcohol ClinExp Res. 37: 1527–1535.
- Fuster D, Cheng DM, Quinn EK, Armah KA, Saitz R, et al. (2014) Inflammatory cytokines and mortality in a cohort of HIV-infected adults with alcohol problems. AIDS 28: 1059–1064.
- Goar SG, Audu MD, Agbir MT, Dochalson E (2011) Prevalence and socio-demographic correlates of alcohol use disorders among HIV patients. African Journal of Drug and Alcohol Studies 10.
- Goh B, Chan RKW, Sen P, Theng CTS, Tan H, et al. (2007) Spectrum of skin disorders in human immunodeficiency virus-infected patients in Singapore and the relationship to CD4 lymphocyte counts. Int J Dermatol 46: 695–699.
- Green TC, Kershaw T, Lin H, Heimer R, Goulet JL (2010) Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend 110: 208–220.
- Grzeszczuk A, Wandalowicz AD, Jaroszewicz J, Flisiak R (2015) Prevalence and risk factors of HCV/HIV co-infection and HCV genotype distribution in North-Eastern Poland. Hepat Mon 15: e27740.
- Hayashi K, Ti L, Avihingsanon A, Kaplan K, Suwannawong P, et al. (2015) Compulsory drug detention exposure is associated with not receiving antiretroviral treatment among people who inject drugs in Bangkok, Thailand: a cross-sectional study. Subst Abuse Treat Prev Policy 10:16.
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, et al. (2013) Mortality Attributable to Smoking Among HIV-1–Infected Individuals: A Nationwide, Population-Based Cohort Study. Clin Infect Dis 56: 727–734.
- Hsu JC, Li Y, Marcus GM, Hsue GY, Scherzer R, et al. (2013) Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am CollCardiol 61: 2288–2295.
- Ibrahim AW, Sale S (2014) Prevalence, Predictors and Patterns of Psychoactive substance use among HIV seropositive adults at Aminu Kano Teaching Hospital Kano, North Western Nigeria. International Journal of Medicine and Medical Sciences1: 81–90.
- Ikeda ML, Barcellos NT, Alencastro PR, Wolff FH, Brandão AB, et al. (2013) Association of blood pressure and hypertension with alcohol consumption in HIV-infected white and non-white patients.ScientificWorldJournal 2013: 169825.
- Josephs JS, Fleishman JA, Korthuis PT, Moore RD, Gebo KA, et al. (2010) Emergency department utilization among HIV-infected patients in a multisite multistate study. HIV Med 11: 74–84.
- Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, et al. (2010) Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med 11: 143–151.
- Kabali C, Cheng DM, Brooks D, Bridden C, Horsburgh R, et al. (2011) Recent cigarette smoking and HIV disease progression: no evidence of an association. AIDS Care 23: 947–956.
- Kagimu M, Guwatudde D, Rwabukwali C, Kaye S, Walakira Y, et al. (2012) Religiosity for HIV prevention in Uganda: a case study among Christian youth in Wakiso district. African Health Sciences 12: 17–25.
- Kellerman SE, Hanson DL, McNaghten AD, Fleming PL (2003) Prevalence of Chronic Hepatitis B and Incidence of Acute Hepatitis B Infection in Human Immunodeficiency Virus–Infected Subjects. J Infect Dis 188: 571–577.
- Kellinghaus C, Kovac ES, Moddel G, Boesebeck F (2008) Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure 17: 27–33.
- Kim TW, Kertesz SG, Horton NJ, Tibbetts N, Samet JH (2006) Episodic homelessness and health care utilization in a prospective cohort of HIV-infected persons with alcohol problems. BMC Health Services Res 6:19.
- Korthuis PT, McGinnis KA, Kraemer KL, Gordon AJ, Skanderson M, et al. (2015) Quality of HIV Care and Mortality in HIV-Infected Patients. Clin Infect Dis.
- Korthuis PT, Fiellin DA, McGinnis KA, Skanderson M, Justice AC, et al. (2012) Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. Acquir Immune DeficSyndr 61: 171–178.
- Krupitsky EM, Horton NJ, Williams EC, Lioznova D, Kuznetsova M, et al. (2005) Alcohol use and HIV risk behaviors among HIV-infected hospitalized patients in St. Petersburg, Russia. Drug Alcohol Depend 79: 251–256.
- Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, et al. (2014) Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus–infected, and uninfected patients. Clin Infect Dis 58:1449–1458.
- Luo X, Duan S, Duan Q, Pu Y, Yang Y, et al. (2013) Alcohol use and subsequent sex among HIV-infected patients in an ethnic minority area of Yunnan Province, China.PLoS One 8: e61660.
- Mayer KH, O’Cleirigh C, Skeer M, Covahey C, Leidolf E, et al. (2010) Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: findings from a Boston community health centre. Sex Transm Infect 86: 66–70.
- Mayer KH, Bush T, Henry K, Overton ET, Hammer J, et al. (2012) Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis 39:1-7.
- McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, et al. (2013) Comparing alcohol screening measures among HIV-infected and -uninfected men. Alcohol ClinExp Res 37: 435-442.
- Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, et al. (2012) Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, antiretroviral therapy adherence, and virologic failure. J Acquir Immune DeficSyndr 61: 164-70.
- Merlin JS, Westfall AO, Chamot E, Overton ET, Willig JH, et al. (2013) Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med 14: 1985-1993.
- Metsch LR, Bell C, Pereyra M, Cardenas G, Sullivan T, et al. (2009) Hospitalized HIV-infected patients in the era of highly active antiretroviral therapy. Am J Public Health 99: 1045-1049.
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, et al. (2011) Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain 12: 1004–1016.
- Mijch A, Burgess P, Judd F, Grech P, Komiti A, et al. (2006) Increased health care utilization and increased antiretroviral use in HIV-infected individuals with mental health disorders. HIV Med 7: 205–212.
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, et al. (2012) Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care 24: 1504–1513.
- Nahvi S, Litwin AH, Heo M, Berg KM, Li X, et al. (2012) Directly observed antiretroviral therapy eliminates adverse effects of active drug use on adherence. Drug Alcohol Depend 120: 174–180.
- Nakimuli-Mpungu E, Musisi S, Katabira E, Nachega J, Bass J (2011) Prevalence and factors associated with depressive disorders in an HIV+ rural patient population in southern Uganda. J Affect Disord 135: 160–167.
- Newville H, Roley J, Sorensen JL (2015) Prescription medication misuse among HIV-infected individuals taking antiretroviral therapy. J Subst Abuse Treat 48: 56–61.
- Nicoletti C, Brandileone MC, Guerra ML, Levin AS (2007) Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. DiagnMicrobiol Infect Dis 57:259–265.
- Nunes D, Saitz R, Libman H, Cheng DM, Vidaver J, et al. (2006) Barriers to treatment of hepatitis C in HIV/HCV-coinfected adults with alcohol problems. Alcohol ClinExp Res 30:1520–1526.
- Obel N, Omland LH, Kronborg G, Larsen CS, Pedersen C, et al. (2011) Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: a population-based nationwide cohort study. PLoS One 6: e22698.
- Omland LH, Jepsen P, Weis N, Christensen PB, Laursen AL, et al. (2010) Mortality in HIV-infected injection drug users with active vs cleared hepatitis C virus-infection: a population-based cohort study. J Viral Hepat 17: 261–268.
- Pace CA, Lioznov D, Cheng DM, Wakeman SE, Raj A, et al. (2012) Sexually transmitted infections among HIV-infected heavy drinkers in St Petersburg, Russia. Int J STD AIDS 23: 853–858.
- Pakkala S, Chen Z, Rimland D, Owonikoko TK, Gunthel C, et al. (2012) Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy.Cancer 118: 164-172.
- Pintado V, Martín-Rabadán P, Rivera ML, Moreno S, Bouza E. Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients. A comparative study. Medicine (Baltimore) 80: 54–73.
- Robinson-Papp J, Gelman BB, Grant I, Singer E, Gensler G, et al. (2012) Substance abuse increases the risk of neuropathy in an HIV-infected cohort. Muscle Nerve 45: 471–476.
- Ruan Y, Qin G, Yin L, Chen K, Qian HZ, et al. (2007) Incidence of HIV, hepatitis C and hepatitis B viruses among injection drug users in southwestern China: a 3-year follow-up study. AIDS 21: S39–S46.
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P, et al. (2004) Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology 62: 957–962.
- Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, et al. (2009) Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol 50: 736–745.
- Sambamoorthi U, Walkup J, Olfson M, Crystal S (2000). Antidepressant treatment and health services utilization among HIV-infected medicaid patients diagnosed with depression. J Gen Intern Med 15: 311–320.
- Scribner AN, Troia-Cancio PV, Cox BA, Marcantonio D, Hamid F, et al. (2000) Osteonecrosis in HIV: a case-control study. J Acquir Immune DeficSyndr 25: 19–25.
- Siemieniuk RA, Miller P, Woodman K, Ko K, Krentz HB, et al. (2013) Prevalence, clinical associations, and impact of intimate partner violence among HIV-infected gay and bisexual men: a population-based study. HIV Med 14: 293–302.
- Silverberg MJ, Ray GT, Saunders K, Rutter CM, Campbell CI, et al. (2012) Prescription long-term opioid use in HIV-infected patients. Clin J Pain 28: 39–46.
- Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, et al. (2006) A trial of integrated buprenorphine/naloxone and HIV clinical care. Clin Infect Dis 43: S184–S190.
- Surratt HL, Kurtz SP, Cicero TJ, O'Grady C, Levi-Minzi MA (2013) Antiretroviral medication diversion among HIV-positive substance abusers in South Florida. Am J Public Health 103: 1026–1028.
- Tabarsi P, Chitsaz E, Moradi A, Baghaei P, Farnia P, Marjani M, et al. (2012) Treatment outcome, mortality and their predictors among HIV-associated tuberculosis patients. Int J STD AIDS 23: e1–e4.
- Tetrault JM, Tate JP, McGinnis KA, Goulet JL, Sullivan LE, et al. (2012) Hepatic safety and antiretroviral effectiveness in HIV-infected patients receiving naltrexone. Alcohol ClinExp Res 36: 318–324.
- Towner WJ, Xu L, Leyden WA, Horberg MA, Chao CR, et al. (2012) The effect of HIV infection, immunodeficiency, and antiretroviral therapy on the risk of hepatic dysfunction. J Acquir Immune DeficSyndr 60: 321–327.
- Tsui JI, Cheng DM, Libman H, Bridden C, Samet J (2012) Hepatitis C virus infection is associated with painful symptoms in HIV-infected adults. AIDS Care 24: 820–827.
- Tyurina A, Krupitsky E, Cheng DM, Coleman SM, Walley AY, et al. (2013) Is cannabis use associated with HIV drug and sex risk behaviors among Russian HIV-infected risky drinkers? Drug Alcohol Depend 132: 74–80.
- Vagenas P, Di Paola A, Herme M, Lincoln T, Skiest DJ, et al. (2014) An evaluation of hepatic enzyme elevations among HIV-infected released prisoners enrolled in two randomized placebo-controlled trials of extended release naltrexone. J Subst Abuse Treat 47: 35–40.
- Vallecillo G, Mojal S, Roquer A, Martinez D, Rossi P, et al. (2013) Risk of QTc prolongation in a cohort of opioid-dependent HIV-infected patients on methadone maintenance therapy. Clin Infect Dis 57: 1189–1194.
- van der Werf MJ, Yegorova OB, Chentsova N, Chechulin Y, Hasker E, et al. (2006) Tuberculosis-HIV co-infection in Kiev City, Ukraine. Emerg Infect Dis 12: 766–768.
- Vergara-Rodriguez P, Tozzi MJ, Botsko M, Nandi V, Altice F, et al. (2011) Hepatic safety and lack of antiretroviral interactions with buprenorphine/naloxone in HIV-infected opioid-dependent patients. J Acquir Immune DeficSyndr 56: S62–S67.
- Vidrine DJ, Arduino RC, Lazev AB, Gritz ER (2006) A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 20: 253–260.
- Whetten K, Reif S, Toth M, Jain E, Leserman J, et al. (2012) Relationship between trauma and high-risk behavior among HIV-positive men who do not have sex with men (MDSM).AIDS Care 24: 1453-1460.
- Dawes MA, Antelman SM, Vanyukov MM, Giancola P, Tarter RE, et al. (2000) Developmental sources of variation in liability to adolescent substance use disorders. Drug Alcohol Depend 61: 3-14.
- Sinha R, Fuse T, Aubin LR, O'Malley SS (2000) Psychological stress, drug-related cues, and cocaine craving. Psychopharmacology 152: 140-148.
- National Institute on Drug Abuse (2012) Drug abuse and HIV.
- McKirnan DJ, Peterson PL (1989) Alcohol and drug use among homosexual men and women: epidemiology and population characteristics. Addict Behav 14: 545-553.
- Centres for Disease Control and Prevention (2015) Gay and bisexual men’s health: substance abuse.
- Teramukai S, Matsuyama Y, Mizuno S, Sakamoto J (2004) Individual patient-level and study-level meta-analysis for investigating modifiers of treatment effect.Jpn J ClinOncol 34: 717-721.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 11652
- [From(publication date):
December-2015 - Dec 12, 2024] - Breakdown by view type
- HTML page views : 10872
- PDF downloads : 780