Drought Tolerance Mechanisms in Plants: Physiological Responses Associated with Water Deficit Stress in Solanum lycopersicum
Received: 11-Apr-2018 / Accepted Date: 10-May-2018 / Published Date: 17-May-2018 DOI: 10.4172/2329-8863.1000362
Abstract
Drought is among the top largest causes of agricultural productivity losses globally. Tomato (Solanum lycopersicum L.) is a commercially important crop considerably hampered by drought. It is considered a drought sensitive crop with a yield response factor (Ky) 1.05. Although advancements in molecular research and plant breeding have led to release of drought tolerant cultivars in most developed countries, breeding efforts have focused on yield as the core selection index particularly in Sub-Saharan Africa (SSA) with less regard for drought tolerance. Several studies, however, have documented various physiological, morphological and biochemical adaptive drought tolerance and avoidance strategies in tomatoes and other crop species. It is argued that selection efficiency for drought tolerance breeding programs would be improved if physiological traits linked to drought tolerance are considered. This review presents an overview of previous research efforts in understanding physiological responses to drought, in crop species with particular attention to Solanum lycopersicum (Tomato). It further highlights research gaps, identifying unexplored domains and suggesting recommendations for future investigation.
Keywords: Drought; Physiological traits; Chlorophyll fluorescence; Photosynthesis; Reactive oxygen species
Introduction
It is well documented that tomato (Solanum lycopersicum L. ) plays a critical role in meeting domestic nutritional food requirements, generation of income, foreign exchange earnings and creation of employment [1] in Africa and globally. However, notwithstanding its contribution to poverty alleviation, the tomato industry is faced with a myriad of constraints along its value chain. These include pest and disease infestation [1], physiological disorders and drought. Drought remains a major constraint in tomato farming [2]. Tomatoes are very sensitive to water stress [3]. It is estimated that reduction in watering by 15% and 30% would reduce gross revenue by 15% and 22%, respectively [4]. While production in rainy season may appear attractive, low yields have been reported [5]. This is attributed to many leaf diseases that affect the plant in rainy season, such as Phytophthora infestans , Cladosporium fulvum , Stemphylium solani , Xanthomonas campestris , and viruses [5]. For example, in a fresh market tomato trial during a rainy season in Malawi, the highest yielding variety produced a yield of 36 t/ha, while during the dry season, the highest yielding variety was at 85.9 t/ha [6]. It is reasonable to infer that dry seasons are convenient for tomato production. However, limited moisture levels during dry seasons are equally a major impediment to yield, particularly when irrigation water supply is limited. Recently, many parts of the tropics, particularly Southern Africa experienced the El Nino related drought effects in 2015/2016 growing season, which reduced cereal yield by 30% and subjected 2.8 Million (16.4% of population) Malawians to food insecurity [7]. These effects continue to affect agriculture, hence the need for drought resistant crop cultivars.
During water deficit stress, many physiological and biochemical pathways are perturbed [8]. An understanding of a myriad of mechanisms by which plants respond to water deficit has been named as a challenge to enhancing crop drought tolerance [9,10]. Quantification of physiological responses of plants under water stress is a viable, reliable and accurate approach in studying water stress tolerance [11-13]. It is suggested that selection efficiency in breeding for water stress tolerance could be enhanced if particular physiological and/or morphological attributes related to yield under a stress environment could be identified and employed as selection criteria for complementing traditional plant breeding [14]. However, under drought conditions, yield has invariably remained the core selection index in many crops. In developing a breeding program to improve drought resistance of a crop plant, it is necessary to gain knowledge concerning both the genetics and physiology of tolerance mechanisms [ 15]. Therefore, physiological traits in various plant species and varieties, with strong correlation with response of plants to drought are crucial in understanding and exploring water stress tolerance mechanisms [16]. To achieve effective drought tolerance, crop improvement and plant breeding programs demand the pyramiding of many dissimilar traits suitable for different growing environments [17]. Invariably, tolerance to water stress and tissue water deficits often involves maintenance of turgor under low tissue water potential, through osmotic adjustment (OA) [18-20] or as a result of the presence of rigid cell walls or decreased cell size [21].
This review therefore aims at uncovering critical physiological traits associated with water stress in tomatoes and other plant species. It further highlights research gaps for future exploration. Understanding physiology of plants under water stress will provide for a comprehensive and integrated selection basis in water stress tolerance breeding programs.
Physiological Traits Associated with Water Stress Tolerance in Plants
Chlorophyll fluorescence parameters
Chlorophyll fluorescence (CF) has been widely used in water stress studies in various plants including tomatoes [22], maize [23], potato [ 24], cotton and peanut [25]. It is defined as light that chlorophyll molecules re-emit upon return from excited to non-excited states [26]. It is used as an indicator of photosynthetic organisms’ ability and efficiency in photosynthetic energy conversion. It is therefore closely related to and reflective of plant photosynthesis and the physiological state of plant. As a consequence, chlorophyll fluorescence has on numerous instances been utilized as a powerful, non-destructive and dependable tool for studying the photosynthetic behavior of plants under water stress [27-30]. When a plant leaf is illuminated, the leaf ’s chlorophyll absorbs the light which can serve three functions.
• It can be used for the light dependent processes of photosynthesis in the thylakoids (Photochemistry), excess energy can be dissipated.
• As heat (or)
• It can be re-emitted as light, herein referred to as chlorophyll fluorescence [31-33].
These processes compete with each other. As a consequence, an increased efficiency in chlorophyll fluorescence will result into a decrease in the other two; photochemistry (photosynthesis) and heat dissipation. Therefore, an accurate measure of chlorophyll fluorescence yield, can supply reliable information regarding changes in the efficiency of photochemistry and heat dissipation [31].
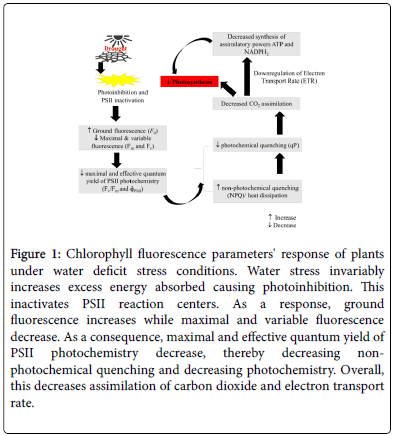
Fluorescence can therefore potentially not only inform tomato’s ability to withstand environmental stresses such as drought, but also signal stress induced damage on photosynthetic apparatus [31,34]. Water stress is invariably associated with an increase in minimal fluorescence parameter (Fo) and an increase in maximal fluorescence [ 35]. This leads to a decline in maximal quantum yield of PSII photochemistry (Fv/Fm) ascribed to inhibited activity of PSII photosystems reducing the effective quantum yield of PSII photochemistry (ɸPSII) [36]. For detailed understanding of chlorophyll fluorescence parameters, refer to Maxwell and Johnson, [34]. It is substantially corroborated that a decline in Fv/Fm and ɸPSII indicates photoinbitory damage of water stress to PSII photosystems. Due to generally low carbon assimilation under these conditions, plants adaptively reduce electron transport rate (ETR) [22], a phenomenon which Baker and Rosenqvist 2004 suggest to be an adaptive strategy to down regulate electron transport to maintain an equilibrium with production of assimilatory powers; Adenosine Triphosphate (ATP) and Nicotinamide Adenosine Dinucleotide Phosphate (NADP). These events in turn increase non-photochemical quenching (NPQ), which is double edged; may signal reduction in photochemistry or may indicate plant’s ability to dissipate excess energy through carotenoids in form of heat (Figure 1). In many cases, these processes are caused as a consequence of water stress induced oxidative stress, causing plants to deactivate antennae of PSII, eliciting the above processes. While pursuit for water stress tolerance continues, chlorophyll fluorescence serves as an important tool to screen for genotypes that can both maintain photochemistry, protect PSII reaction centers and dissipate excess energy while maintaining productivity under water stress.
Figure 1: Chlorophyll fluorescence parameters' response of plants under water deficit stress conditions. Water stress invariably increases excess energy absorbed causing photoinhibition. This inactivates PSII reaction centers. As a response, ground fluorescence increases while maximal and variable fluorescence decrease. As a consequence, maximal and effective quantum yield of PSII photochemistry decrease, thereby decreasing nonphotochemical quenching and decreasing photochemistry. Overall, this decreases assimilation of carbon dioxide and electron transport rate.
Photosynthesis, stomatal conductance, and transpiration rate
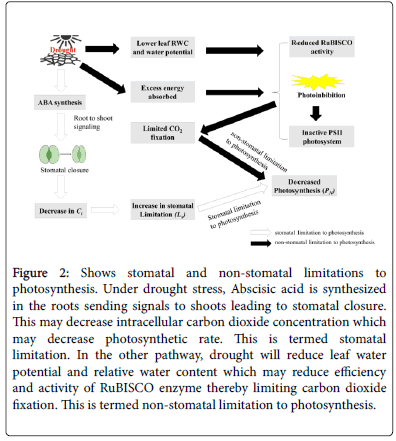
On the outermost surface of plant leaves is a waxy cuticle that prevents loss of water. Generally, however opening and closing of stomata on the leaf surface controls transpiration rate [37]. Water loss in plant leaves through transpiration is partitioned into two; stomatal transpiration and cuticular transpiration. Stomatal transpiration remains the primary pathway of water loss in plants accounting for 99% while only ~1% of water loss is through cuticles [38]. Therefore, monitoring stomatal conductance can provide a better insight into plants water loss through transpiration. Stomatal conductance is essentially an estimate of transpiration (water loss) rate and gas exchange (CO2 uptake) through the leaf stomata [39,40]. Decreased conductance lowers transpiration rate since most stoma are virtually closed. This is hardly a consequence when water is abundant, for a growth response to elevated CO2 since c i (internal concentration of CO2) is sufficient to support photosynthesis. In water limited conditions, a growth retardant hormone Abscisic Acid (ABA) is exuded by roots, which acts as root-shoot signals eliciting stomatal closure [41-43] and reducing stomatal conductance and transpiration. In a study by Yuan et al. all levels of water stress significantly reduced stomatal conductance (gs). The study also observed that mild water stress reduced intracellular carbon dioxide concentration (Ci), and net photosynthetic rate (PN) suggesting that under mild water stress (55 to 60% of field capacity), the reason for decline in photosynthesis was stomatal limitation (Ls). However, intensifying the stress to moderate and severe (45-50% FC and 35-40% FC), (Ci) increased, yet PN declined. This led the authors to conclude that under moderate to severe water stress, non-stomatal limitation is the primary cause for decline in photosynthesis [22]; inhibition of RuBISCO enzyme, photooxidation and photorespiration. Considering the crucial role of water in photosynthesis, it is apparent that water stress is highly likely to negatively impact photosynthesis through both stomatal and nonstomatal limitations. Consequently, photosynthesis inhibition (Figure 2) has been substantially reported as one of the prime physiological implications of drought [41,44]. In another study by Easlon and Richards [43] both tomatoes and its wild type Lycopersicon pennelli showed nearly complete stomatal closure when moisture was below 50% of field capacity. In another study by Tembe et al. considerable decrease in stomatal conductance was observed among water stress subjected plants in 20 tomato genotypes [45]. Stomatal conductance at different levels of moisture ranged from 207.7 mmol/m2s to 287.5 mmol/m2s at 100% of the field capacity, 115.5 mmol/m2s to 196.7 mmol/m2s at 80% of the field capacity, 104.0 mmol/m2s to 100.1 mmol/m2s at 60% of the field capacity and 74.0 mmol/m2s to 100.1 mmol/m2s at 40% of the field capacity [45]. This has been generally accepted as a strategy to aid conservation of moisture and maintain an adequate leaf water status, consequently reducing leaf internal CO2 concentration and photosynthesis [46]. Active stomatal regulation in tomatoes is generally indicative of drought avoidance strategy at species level. Tomato genotypes and its wild types such as Lycopercon pennelli , that are able to reduce stomatal conductance under water stress are promising gene pools for water tolerance cultivar development. Adaptive research may consider producing cultivars that hyper express ABA to elicit stomatal closure. However, a delicate balance between reduced stomatal conductance and photosynthetic rate is more prime. Reduction in water loss may not be justifiable if associated with a reduction in PN.
Figure 2: Shows stomatal and non-stomatal limitations to photosynthesis. Under drought stress, Abscisic acid is synthesized in the roots sending signals to shoots leading to stomatal closure. This may decrease intracellular carbon dioxide concentration which may decrease photosynthetic rate. This is termed stomatal limitation. In the other pathway, drought will reduce leaf water potential and relative water content which may reduce efficiency and activity of RuBISCO enzyme thereby limiting carbon dioxide fixation. This is termed non-stomatal limitation to photosynthesis.
Chlorophyll content
Photosynthetic pigments remain major drivers of plants photosynthetic capacity due to their crucial role in both absorption (chlorophyll) and dissipation (carotenoids) light energy. Drought has severally been reported to affect chlorophyll content in plant leaves, and the effects vary among species [22,47]. In some species, drought lowers chlorophyll content while in some no changes are observed. The intensity of the change depends on the rate of stress and duration [ 48,49]. In a study by Pirzad et al. introduced low water stress increased chlorophyll a, b and total in Matricaria chamomilla L while high water stress considerably reduced them [47]. In another study by Beeflink et al. increasing drought stress in onions increased chlorophyll [ 50]. Similar results were obtained in other separate [51,52]. It is thus evident that effects of water stress on chlorophyll is species specific. In young peach trees for example, drought stress has been reported to decrease chlorophyll [53,54]. These results attributed the reductions in chlorophyll concentrations to the increased electrolyte leakage due to leaf senescence [47]. Water deficit affects chlorophyll content by destroying the chlorophyll or simply preventing its synthesis [55]. Several other hypotheses have been suggested explaining reduced chlorophyll under water stress. Some authors suggest that the decline is an adaptive strategy by the plant to reduce chlorophyll content so as to minimize absorption of excess energy [56] while others assert that it is as a result of photo-oxidatory damage by excess light energy absorbed [ 57]. Besides, it has been investigated that light, even of low photon flux density (PFD) becomes excess under low water conditions [35]. In a study by Pirzad et al. it was concluded that water stress; both excess water and deficit significantly decreases leaf chlorophyll (chlorophyll a, b and total chlorophyll) concentrations [47].
Since effects of water deficit stress on chlorophyll content is species dependent, it also remains highly likely that different tomato landraces may exhibit altered responses to drought. Considering the higher diversity of tomato landraces particularly in the tropics, most of which are at risk of extinction, it is an urgent call for plant breeders to characterize and document tomato landraces with such important attributes as resistance to chlorophyll degradation under abiotic stress. These may be priorities of conservational efforts to serve as germplasm sources for new cultivar development.
Accumulation of reactive of oxygen species (ROS) and antioxidants
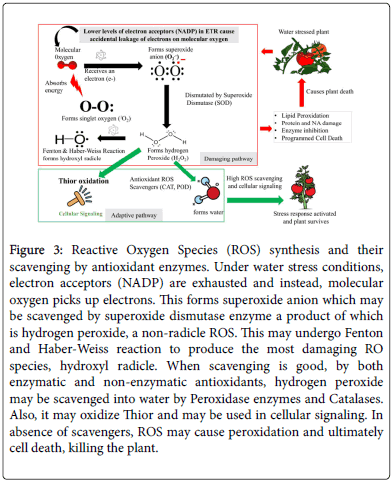
A common consequence of abiotic stress, drought inclusive is the accumulation of ROS. A number of RO species are reported, including radicles like superoxide anion (O2.-), singlet oxygen (-O2) and hydroxyl onion (.OH) while non-radical forms include hydrogen peroxide (H2O2). For a detailed review on ROS types, formation and antioxidants, refer to Sharma et al. [58]. These ROS species have been found to be cause peroxidation of lipids, oxidation of proteins, damage to nucleic acids, enzyme inhibition, eliciting programmed cell death (PCD) pathway and ultimately causing death [58]. However, notwithstanding their potentially destructive nature, recent studies report that moderate accumulations, balanced with sufficient scavenging ability may act as second messengers under abiotic stresses ( Figure 3). Therefore, ROS will only be damaging when their synthesis way supersedes their scavenging. A number of studies have demonstrated that water stress induces hyper accumulation of ROS [ 58-61]. A common approach to assess this is to quantify scavenging activity by monitoring antioxidant enzymes. These include superoxide dismutase (SOD), peroxidase (POD e.g., guaiacol peroxidase and ascorbate peroxidase) and catalase (CAT). Yuan et al. while working on water stress in tomato, they found a significant increase in SOD, CAT and POD [22]. This may implicitly indicate accumulation of ROS, which these antioxidants are scavenging. The study further found an increase in Malondialdehyde (MAD), which is indicative of peroxidation of lipid membranes. This made them conclude that water stress caused an oxidative stress induced membrane damage. In a heat stress study, it was demonstrated that ROS were the prime cause of lethality under heat stress in yeast [62]. Growing yeast anaerobically, prevented accumulation of ROS, and consequently prevented cell death. This led to a conclusion that ROS is what causes cell death under heat stress. While this conclusion has been only demonstrated in heat stress induced yeast, it is plausible that a similar phenomenon may occur in plants. If ROS may prove to be primary lethal agents under stress, finding ways to eliminate them may offer new strategies for achieving tolerance under drought stress. This may inform such breeding efforts as to overexpress antioxidants under drought stress. Moreover, moderate accumulation is suggested to aid in secondary signaling. These are wakeup calls for more research attention on ROS.
Figure 3: Reactive Oxygen Species (ROS) synthesis and their scavenging by antioxidant enzymes. Under water stress conditions, electron acceptors (NADP) are exhausted and instead, molecular oxygen picks up electrons. This forms superoxide anion which may be scavenged by superoxide dismutase enzyme a product of which is hydrogen peroxide, a non-radicle ROS. This may undergo Fenton and Haber-Weiss reaction to produce the most damaging RO species, hydroxyl radicle. When scavenging is good, by both enzymatic and non-enzymatic antioxidants, hydrogen peroxide may be scavenged into water by Peroxidase enzymes and Catalases. Also, it may oxidize Thior and may be used in cellular signaling. In absence of scavengers, ROS may cause peroxidation and ultimately cell death, killing the plant.
Concentration of potassium (K+) in leaf tissues
Potassium is the crucial essential macronutrient required by plants for optimum growth. Various studies have supported their critical role in plants biochemical and physiological processes key in growth and metabolism [63]. Substantial evidence supports the role of K in biotic and abiotic plant stress tolerance [64-66]. In many plants, potassium remains the primary inorganic cation to accumulate in leaf tissues subjected to water stress and is found in abundance as a solute in leaves [67,68].
Water maintains turgidity of cells which leads to enlargement and growth of cells. Potassium plays a central role as an osmolyte, aiding osmotic adjustment [64], a phenomenon in which solutes accumulate in plant cells in response to a decline in cellular water potential. This enables continued growth under low water potential. Osmotic adjustments have been observed in several species and substantiated by evidence to aid in stomatal conductance, photosynthesis, leaf water volume and growth [18,19]. Many studies have reported a positive correlation between osmotic adjustment and drought tolerance in several plant species [20]. Overall, enhanced osmotic adjustment aided by adequate K enables maintenance of higher turgor pressure, relative water content and lower osmotic potential, thereby enhancing plants ability to tolerate drought stress.
Additionally, maintenance of a higher Potassium concentration in plant tissues promotes root growth by increasing root elongation [69], consequently increasing root surface area that is exposed to soil as a result of increased root water uptake [65]. Other researchers have also confirmed the role of K in water retention in plant tissues under water stress [70]. Furthermore, K improves cell membrane integrity [63] which normally decline under drought stress [71]. Moreover, it is well documented that K+ regulates opening and closure of stomata. Therefore, in water deficit conditions, rapid closure of stomata and conservation of internal moisture are desirable adaptive traits [69]. It is suggested that under drought conditions, an adequate K+ concentration will enable stomata closure. However, contrasting views exist as to whether presence of K in leaf tissues under water stress would enable opening or closing of stomata [69,72,73]. Some studies suggest that under well-watered conditions, K would promote stomatal opening and transpiration, hence no effect was observed on stomata conductance and photosynthesis while the converse is hypothesized under water stress conditions [74,75].
Since maintenance of adequate K nutrition is critical in precluding drought stress damage by adjusting water balance, there is a need, therefore for more studies on such nutrient cum stomatal conductance relationships in selected cultivars of Tomato. Information on the influence of K content in leaf tissues on water stress adaptation, may further accurately guide farmers in selection of appropriate fertilization and soil health management options aimed at attaining cation balance ideal for water deficient conditions.
Electrolyte leakage (EL)
Electrolyte leakage is an upcoming technique widely being used to assess the effect of biotic and abiotic stresses on the physiology of plants. Two forms of electrolyte leakage are usually assessed; root electrolyte leakage (REL) and shoot electrolyte leakage SEL). They both elucidate the integrity of cell membranes to hold water and minerals. In plants, water is stored in two key pathways; the symplast and apoplast separated by a cell membrane. Water resident in the apoplast is nearly pure, while symplast water contains a variety of ions. Considering the semi-permeable nature of cell membranes, passage of water across the membrane only depends on the plants need for water while ions are restricted in entirety. As a consequence, under drought conditions, membranes are damaged by reactive oxygen species (ROS) released perturbing membrane integrity allowing ion leakage. Hence quantifying the amount of ions leaking into the solution across the membrane can indicate viability of the root and shoot system [76]. In this view, EL has been adopted in understanding water stress [77,78], heat and salt tolerance in various crop species [79]. In many crop species including tomatoes, EL is assessed using procedures described by Lutts et al. [80] and Mao et al. [81]. In a study by Jungklang et al. subjecting Curcuma alismatifolia plants to water deficit stress considerably reduced electrolyte leakage [78]. This is indicative of drought tolerance mechanism in the species through maintenance of membrane integrity and reduction of electrolyte leakage. The study further established that EL was significantly reduced when drought subjected plants are treated with a growth retardant Paclobutrazol (PBZ), a clear indication of its critical role in protecting membranes from damage in drought stress [78]. While electrolyte leakage has been widely used in many crop species and tree seedlings, to assess salt, heat, water and biotic stress tolerance, research efforts must be scaled up to authenticate its effectiveness in monitoring water deficit stress in tomatoes.
Leaf relative water content (RWC)
Leaf RWC has recently emerged to be a popular trait in assessing drought tolerance in crops and has slowly replaced leaf water potential [ 82]. It is reasonable to assert that leaf water content contributes to plant-level physiological drought tolerance [16]. Consequently, from mid-80’s, leaf Relative Water Content (RWC) is now regarded as a best criterion for plant water status. Leaf RWC is defined as the percentage of water present at the time of sampling, in relation to the amount of water in a saturated leaf [83]. Since RWC relates well with cell volume, it can accurately indicate the balance between absorbed water by plant and that lost though transpiration [82,84], hence it is an important indicator of water status in plants. Furthermore, the close correlation of RWC with a plants physiological activities and soil water status [ 85,86], qualifies it as a reliable trait, for assessing plants tolerance to drought stress.
While screening for water stress tolerance in long storage tomato genotypes, it was observed that leaf RWC significantly decreased as drought stress progressed [87] in all genotypes. In another water stress tolerance study in wheat by Schonfeld et al. it was shown that wheat cultivars having high RWC, are more tolerant to drought stress. The rate of RWC in plants with high water stress tolerance is high relative to susceptible plants/cultivars. It is therefore expected that plants having higher yields under drought stress should exhibit high RWC [ 82]. In another study by Soltys-Kalina et al. [8] subjecting Solanum tuberosum plants to drought for 3 weeks significantly decreased leaf water content of the 18 cultivars. The study further correlated RWC with relative yield decrease and found low but statistically significant correlation (R=-0.18) [8]. Pirzad et al. found no significant differences in leaf RWC in Matricaria chamomilla L . [47] signifying high water maintenance under both low and high-water conditions, which may be indicative of water deficit stress tolerance in the species. Jungklang et al. [78] in their study found a significant decline in leaf RWC in Curcuma alismatifolia leaves at 30 days after withholding water. However, under drought conditions, the study established that RWC was enhanced when plants were treated with a growth retardant PBZ. It is apparent, therefore, that leaf RWC examines water balance in plants. More studies to elucidate growth factors and traits that enhance maintenance of leaf RWC are of gross need in achieving drought tolerance.
Proline Accumulation
Some of the most important responses of plants against drought stress are associated with the accumulation of minerals [88] and enhanced synthesis of osmoprotectants, which are part of normal metabolism. Accumulation of these compounds helps the stressed cells in water retention [89] and in the maintenance of the structural integrity of the cell membranes. However, types of osmoprotectant metabolites and their relative contribution in lowering the osmotic potential differ greatly among plant species [90,91]. This suggests that different plant species may employ different drought tolerance strategies and the same case is hypothesized for cultivars/varieties. It has also been reported, however that many metabolites are conserved among species [91]. Metabolic adjustments in response to the adverse environmental conditions highlight pools of metabolites that play important roles in metabolism and physiology and may indicate which pathways perturbed by the stress [90]. Such metabolites include glycine betaine, trehalose, taurine and amino acids, principally Proline [42]. Proline is by far the most studied osmoprotectant. A large body of data suggests a positive correlation between Proline accumulation and plant stress [92]. In a study by Jungklang et al., Proline content was significantly high in Curcuma alismatifolia plants exposed to water stress at 30 days after imposing the stress [78]. Similar results were also obtained by Jungklang & Saengnil [78], Witt et al. and Bowne et al. [ 42]. Proline has been well documented as an osmotic regulator helping in reduction of osmotic damage [93,94]. It is further hypothesized that accumulation of Proline in leaves could possibly play a protection role aside from osmoregulation during water stress [ 78].
The wide use in nature of Proline as a stress adaptor molecule attests to its prime plausible role in stress response. Indeed, accumulation of proline and other osmoprotectant compounds in plant cells depict plants inherent tolerant mechanisms to harmful water deficit. Hyper release of proline under drought stress is suggested to accrue from the increased expression level of a critical gene in proline biosynthetic pathway, pyrroline-5-carboxylate synthetase [95,96] and the inhibition of proline dehydrogenase, a key enzyme in proline degradation. While acknowledging this knowledge, it is apparent however, that a majority of tomato cultivars and landraces remain unstudied in this regard. Moreover, studies have largely skewed towards proline, and less attention has been made to other potential osmoprotectants like glycine betaine, taurine and trehalose, which have demonstrated a critical role under other abiotic stresses like heat stress, hence a need for more studies on their plausible role in heat stress adaptation under water stress.
In conclusion, constitutive whole-plant traits contribute chiefly in plant water relations and plant dehydration avoidance under stress. Due to the critical water problems in many tropics, moisture is not sufficient to meet water demands for crops. Tomatoes demand high levels of moisture owing to their succulent nature. It is therefore desirable to breed tomato varieties that are able to withstand limited moisture levels. Selection efficiency for such breeding programs, should be holistic, integrated and comprehensive. It must consider manipulation of plant’s physiological and biochemical pathways and traits that explicitly and implicitly contribute to water stress tolerance ( Figure 4), than focusing on yield parameters alone as has been a common case.
Conflict of Interest
The author declares that there is no conflict of interest regarding the publication of this article.
Acknowledgement
The authors acknowledge support received from the Centre for Research, Agricultural Advancement, Teaching Excellence and Sustainability (CREATES) in Food and Nutrition Security at The Nelson Mandela African Institution of Science and Technology (NMAIST) during conception, preparation and publication of this article.
References
- Geoffrey SK, Hillary NK, Antony KM, Mariam M, Mary MC (2014) Challenges and strategies to improve tomato competitiveness along the tomato value chain in Kenya. International Journal of Business and Management 9: 205.
- Minja RR, Ndee A, Swai IS, Ojiewo CO (2011) Promising improved tomato varieties for eastern Tanzania. African Journal of Horticultural Science.
- Nuruddin MM, Madramootoo CA, Dodds GT (2003) Effects of water stress at different growth stages on greenhouse tomato yield and quality. HortScience 38: 1389-1393.
- Obreza TA, Pitts DJ, McGovern RJ, Spreen TH (1996) Deficit irrigation of micro-irrigated tomato affects yield, fruit quality, and disease severity. Journal of Production Agriculture 9: 270-275.
- Chadha ML, Oluoch MO, Saka AR, Mtukuso AP, Daudi AT (2003) Vegetable Research and Development in Malawi. Review and Planning Workshop Proceedings. Lilongwe, Malawi: Ministry of Agriculture, Irrigation and Food Security, Department of Agricultural Research Services, pp: 93-96.
- Sen-Hsuing L, Meki SS (2003) Vegetable production improvement at the Horticultural Development Training and Extension Center. Vegetable Research and Development in Malawi.
- Rembold F, Kerdiles H, Lemoine G, Perez-Hoyos A (2016) Impact of El Niño on agriculture in Southern Africa for the 2015/2016 main season. European Commission, JRC MARS Bulletin-Global Outlook Series.
- Soltys-Kalina D, Plich J, Strzelczyk-Żyta D, Śliwka J, Marczewski W (2016) The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding Science 66: 328-331.
- Deikman J, Petracek M, Heard JE (2012) Drought tolerance through biotechnology: improving translation from the laboratory to farmers’ fields. Current Opinion in Biotechnology 23: 243-250.
- Juenger TE (2013) Natural variation and genetic constraints on drought tolerance. Current Opinion in Plant Biology 16: 274-281.
- Cao KF (2000) Water relations and gas exchange of tropical saplings during a prolonged drought in a Bornean heath forest, with reference to root architecture. Journal of Tropical Ecology 16: 101-116.
- Engelbrecht BM, Comita LS, Condit R, Kursar TA, Tyree MT, et al. (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80.
- Kursar TA, Engelbrecht BM, Burke A, Tyree MT, EI Omari B, et al. (2009) Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Functional Ecology 23: 93-102.
- Acevedo E (1991) Improvement of winter cereal crops in Mediterranean environments: use of yield, morphological and physiological traits. Breeding for Drought Resistance in Wheat.
- Clarke JM, Townley-Smith TF (1984) Screening and selection techniques for improving drought resistance. Crop Breeding: A Contemporary Basis, pp: 137-162.
- Maréchaux I, Bartlett MK, Sack L, Baraloto C, Engel J, et al. (2015) Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Functional Ecology 29: 1268-1277.
- Obidiegwu JE, Bryan GJ, Jones HG, Prashar A (2015) Coping with drought: stress and adaptive responses in potato and perspectives for improvement. Frontiers in Plant Science 6: 542.
- Morgan JM (1984) Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology 35: 299-319.
- Turner NC, Jones MM (1980) Turgor maintenance by osmotic adjustment: a review and evaluation. pp: 87-103.
- DaCosta M, Huang B (2006) Osmotic adjustment associated with variation in bentgrass tolerance to drought stress. Journal of the American Society for Horticultural Science 131: 338-344.
- Wilson JR, Ludlow MM, Fisher MJ, Schulze E (1980) Adaptation to water stress of the leaf water relations of four tropical forage species. Functional Plant Biology 7: 207-220.
- Yuan XK, Yang ZQ, Li YX, Liu Q, Han W (2016) Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 54: 28-39.
- Ni Z, Liu Z, Huo H, Li ZL, Nerry F, et al. (2015) Early water stress detection using leaf-level measurements of chlorophyll fluorescence and temperature data. Remote Sensing 7: 3232-3249.
- Schapendonk AHCM, Spitters CJT, Groot PJ (1989) Effects of water stress on photosynthesis and chlorophyll fluorescence of five potato cultivars. Potato Research 32: 17-32.
- Isoda A (2010) Effects of water stress on leaf temperature and chlorophyll fluorescence parameters in cotton and peanut. Plant Production Science 13: 269-278.
- Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. Journal of Experimental Botany 50: 1199-1206.
- Méthy M, Olioso A, Trabaud L (1994) Chlorophyll fluorescence as a tool for management of plant resources. Remote Sensing of Environment 47: 2-9.
- Schreiber UBWN, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis, Springer, Berlin, Heidelberg, pp: 49-70.
- Razavi F, Pollet B, Steppe K, Van Labeke MC (2008) Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 46: 631-633.
- Porcar-Castell A, Tyystjärvi E, Atherton J, Van der Tol C, Flexas J, et al. (2014) Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: mechanisms and challenges. Journal of Experimental Botany 65: 4065-4095.
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. Journal of Experimental Botany 51: 659-668.
- Lichtenthaler HK, Miehé JA (1997) Fluorescence imaging as a diagnostic tool for plant stress. Trends in Plant Science 2: 316-320.
- Zhu XG, Baker NR, Ort DR, Long SP (2005) Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II. Planta 223: 114-133.
- Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). Journal of Experimental Botany 50: 1533-1540.
- Zlatev Z (2009) Drought-induced changes in chlorophyll fluorescence of young wheat plants. Biotechnology & Biotechnological Equipment 23: 438-441.
- Baquedano FJ, Castillo FJ (2006) Comparative ecophysiological effects of drought on seedlings of the Mediterranean water-saver Pinus halepensis and water-spenders Quercus coccifera and Quercus ilex. Trees 20: 689.
- Boundless (2017) Movement of Water and Minerals in the Xylem Boundless Biology Boundless.
- Pietragalla J, Alistair P (2012) Canopy temperature, stomatal conductance and water relation traits-Chapter 2: Stomatal conductance.
- Taiz L, Zeiger E (1991) Plant physiology. Redwood City, Plant Physiology: Redwood City.
- Cornic G, Fresneau C (2002) Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany 89: 887-894.
- Bowne JB, Erwin TA, Juttner J, Schnurbusch T, Langridge P, et al. (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Molecular Plant 5: 418-429.
- Easlon HM, Richards JH (2009) Drought response in self-compatible species of tomato (Solanaceae). American Journal of Botany 96: 605-611.
- Chaves MM (1991) Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42: 1-16.
- Tembe KO, George N, Chemining W, Jane A, Willis O, et al. (2017) Effect of water stress on yield and physiological traits among selected African tomato (Solanum lycopersicum) land races. International Journal of Agronomy and Agricultural Research (IJAAR) 10: 78-85.
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, et al. (2002) How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89: 907-916.
- Pirzad A, Shakiba MR, Zehtab-Salmasi S, Mohammadi SA, Darvishzadeh R, et al. (2011) Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. Journal of Medicinal Plants Research 5: 2483-2488.
- Van Rensburg L, Krüger GHJ (1994) Evaluation of components of oxidative stress metabolism for use in selection of drought tolerant cultivars of Nicotiana tabacum L. Journal of Plant Physiology 143: 730-737.
- Jagtap V, Bhargava S, Streb P, Feierabend J (1998) Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. Journal of Experimental Botany 49: 1715-1721.
- Beeflink (1985) Ecology of Coastal Vegetation. 2nd Edition, W Junk Publication, USA, p: 640.
- Bradford KJ, Hsiao TC (1982) Physiological responses to moderate water stress. In Physiological Plant Ecology II, Springer, Berlin, Heidelberg, pp: 263-324.
- Chartzoulakis K, Noitsakis B, Therios I (1993) Photosynthesis, plant growth and dry matter distribution in kiwifruit as influenced by water deficits. Irrigation Science 14: 1-5.
- Chen CT, Li CC, Kao CH (1991) Senescence of rice leaves XXXI. Changes of chlorophyll, protein, and polyamine contents and ethylene production during senescence of a chlorophyll-deficient mutant. Journal of Plant Growth Regulation 10: 201.
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany 32: 93-101.
- Montagu KD, Woo KC (1999) Recovery of tree photosynthetic capacity from seasonal drought in the wet–dry tropics: the role of phyllode and canopy processes in Acacia auriculiformis. Functional Plant Biology 26: 135-145.
- Elvira S, Alonso R, Castillo FJ, Gimeno BS (1998) On the response of pigments and antioxidants of Pinus halepensis seedlings to Mediterranean climatic factors and long-term ozone exposure. The New Phytologist 138: 419-432.
- Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annual Review of Plant Physiology 35: 15-44.
- Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany.
- Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signaling & Behavior 3: 156-165.
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909-930.
- Miller GAD, Suzuki N, Ciftciâ€Yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment 33: 453-467.
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH (1996) Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences 93: 5116-5121.
- Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. International Journal of Molecular Sciences 14: 7370-7390.
- Marschner H (2011)Â Marschner's mineral nutrition of higher plants. Academic Press.
- Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant and Soil 335: 155-180.
- Kant S, Kafkafi U (2002) Potassium and Abiotic Stresses in Plants. In Potassium for Sustainable Crop Production. Pasricha NS, Bansal SK, (eds.). Potash Institute of India: Gurgaon, India, pp: 233-251.
- Jones MM, Osmond CB, Turner NC (1980) Accumulation of solutes in leaves of sorghum and sunflower in response to water deficits. Functional Plant Biology 7: 193-205.
- Ford CW, Wilson JR (1981) Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Functional Plant Biology 8: 77-91.
- Akıncı Ş, Lösel DM (2012) Plant water-stress response mechanisms. In Water stress. InTech.
- Lindhauer MG (1985) Influence of K nutrition and drought on water relations and growth of sunflower (Helianthus annuus L.). Journal of Plant Nutrition and Soil Science 148: 654-669.
- Kozlowski TT, Kramer PJ, Pallard SG (1991) The physiological ecology of woody plants. San Diego: Academic Press.
- Jin SH, Huang JQ, Li XQ, Zheng BS, Wu JS, et al. (2011) Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiology 31: 1142-1151.
- Tomemori H, Hamamura K, Tanabe K (2002) Interactive effects of sodium and potassium on the growth and photosynthesis of spinach and komatsuna. Plant Production Science 5: 281-285.
- Pervez H, Ashraf M, Makhdum MI (2004) Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.). Photosynthetica 42: 251-255.
- Benlloch-González M, Arquero O, Fournier JM, Barranco D, Benlloch M (2008) K+ starvation inhibits water-stress-induced stomatal closure. Journal of Plant Physiology 165: 623-630.
- Ritchie GA, Landis TD (2006) Seedling quality tests: Root electrolyte leakage. Forest Nursery Notes USDA For Serv PNW Region Winter.
- Bolat I, Dikilitas M, Ercisli S, Ikinci A, Tonkaz T (2014) The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. The Scientific World Journal.
- Jungklang J, Saengnil K, Uthaibutra J (2015) Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi Journal of Biological Sciences.
- Al Busaidi KTS, Farag KM (2015) The use of electrolyte leakage procedure in assessing heat and salt tolerance of Ruzaiz date palm (Phoenix dactylifera L.) cultivar regenerated by tissue culture and offshoots and treatments to alleviate the stressful injury. Journal of Horticulture and Forestry 7: 104-111.
- Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany 78: 389-398.
- Mao L, Pang H, Wang G, Zhu C (2007) Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biology and Technology 44: 42-47.
- Hassanzadeh M, Ebadi A, Panahyan-e-Kivi M, Eshghi AG, Jamaati-e-Somarin S, et al. (2009) Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamum indicum L.) genotypes at early flowering stage. Research Journal of Environmental Sciences 3: 345-350.
- Tanentzap FM, Stempel A, Ryser P (2015) Reliability of leaf relative water content (RWC) measurements after storage: consequences for in situ measurements. Botany 93: 535-541.
- Lugojan C, Ciulca S (2011) Evaluation of relative water content in winter wheat. Journal of Horticulture, Forestry and Biotechnology 15: 173-177.
- Munné-Bosch S, Peñuelas J (2004) Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Science 166: 1105-1110.
- Ozkur O, Ozdemir F, Bor M, Turkan I (2009) Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environmental and Experimental Botany 66: 487-492.
- Patanè C, Scordia D, Testa G, Cosentino SL (2016) Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Science 249: 25-34.
- Samarah N, Mullen R, Cianzio S (2004) Size distribution and mineral nutrients of soybean seeds in response to drought stress. Journal of Plant Nutrition 27: 815-835.
- Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant, Cell & Environment 21: 535-553.
- Silvente S, Sobolev AP, Lara M (2012) Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS One 7: e38554.
- Sanchez DH, Schwabe F, Erban A, Udvardi MK, Kopka J (2012) Comparative metabolomics of drought acclimation in model and forage legumes. Plant, Cell & Environment 35: 136-149.
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, et al. (2012) Role of proline under changing environments: a review. Plant Signaling & Behavior 7: 1456-1466.
- Slama I, Ghnaya T, Savouré A, Abdelly C (2008) Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. Comptes Rendus Biologies 331: 442-451.
- Reddy PS, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, et al. (2015) Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiology and Biochemistry 94: 104-113.
- Ueda A, Shi W, Sanmiya K, Shono M, Takabe T (2001) Functional analysis of salt-inducible proline transporter of barley roots. Plant and Cell Physiology 42: 1282-1289.
- Ishitani M, Nakamura T, Han SY, Takabe T (1995) Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Molecular Biology 27: 307-315.
Citation: Kamanga RM, Mbega E, Ndakidemi P (2018) Drought Tolerance Mechanisms in Plants: Physiological Responses Associated with Water Deficit Stress in Solanum lycopersicum. Adv Crop Sci Tech 6: 362. DOI: 10.4172/2329-8863.1000362
Copyright: © 2018 Kamanga RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13339
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 11740
- PDF downloads: 1599