Dramatic Response to Hormone Therapy Plus CDK4/6 Inhibitor of a Retinal Detachment Secondary to Luminal Advanced Breast Cancer
Received: 23-Apr-2021 / Accepted Date: 07-May-2021 / Published Date: 14-May-2021
Abstract
Luminal breast cancer is hormone-receptor positive (estrogen-receptor and/or progesterone-receptor positive) and human epidermal growth factor receptor 2-negative (HER2-). Currently, the standard treatment of luminal metastatic breast cancer is the combination of hormone therapy with CDK4/6 (Cyclin-Dependent Kinases 4/6) inhibitors. The overall response rates for the combination are between 40% to 50%, increasing Progression Free Survival (PFS) and Overall Survival (OS) compared with hormone therapy alone. Herein, we report a clinical case showing the management of a patient with luminal breast cancer in different stages of the disease, highlighting the dramatic response of cyclin inhibitor plus fulvestrant to a bilateral retinal detachment due to ocular metastases.
Keywords: Luminal breast cancer, Hormone Therapy, CDK4/6 inhibitor, Carcinoma, Meningioma
Introduction
Breast Cancer (BC) may be a common women-related malignant neoplasm disease. Estrogen Receptor–positive (ER-positive) breast cancer represents approximately 70% of all BC. ER-positive carcinoma are often further stratified into pathological subtypes, like ductal or mixed ductal and lobular, mucinous, and tubular carcinomas, which are mentioned as luminal carcinoma. Luminal breast tumors are highly heterogeneous in terms of histology and response to treatment. Luminal A and B are two main ER-positive carcinoma subtypes, supported different organic phenomenon profiles, prognosis, and clinical therapy responses.
The difference between luminal A and B is especially associated with the expression of hormone receptors. Luminal B tumors have lower levels of ER expression, lower or no levels of Progesterone Receptor (PR) expression, but higher tumor grade and better Ki-67– positive staining than luminal A tumors. Endocrine therapy, like ER down regulators, selective ER modulators, and aromatase inhibitors, is taken into account to be the first treatment for luminal A and luminal B. However, within the clinic, the most therapy for luminal B is chemotherapy, thanks to the lower sensitivity of those patients to endocrine treatment or drug resistance. In fact, endocrine resistance is an unavoidable problem in clinical therapy of luminal tumors. Development of latest therapy methods to avert endocrine resistance is an urgent challenge in clinical medicine. It is documented that the cell cycle is driven by Cyclin-Dependent Kinases (CDKs), like CDK4 and CDK6, which also are closely related to tumor initiation and progression. The activity of cyclin D and CDK4/6 complexes is taken into account to play the main role in tumor cell proliferation driven by estrogen, especially in carcinoma. In recent years, it’s been established that targeting the cell cycle for anticancer treatment may be a rational option that would be combined with endocrine therapy.
CDK inhibitors, which target overactive CDK activities in tumor cells, are widely utilized in preclinical or clinical trials. In the clinic, three CDK4/6 inhibitors, namely, palbociclib, ribociclib, and abemaciclib have been successfully used in combination with other endocrine therapy drugs for ER-positive and Human Epidermal growth factor Receptor-2 (HER2)–negative advanced breast cancer treatment; in addition, significant Overall Survival (OS) benefits have been confirmed at ESMO2019 conference.
Despite the very fact that the new guidelines for the therapy of advanced carcinoma includes a CDK4/6 inhibitor combined with endocrine treatment because the first- or second-line drug in most countries, most patients eventually develop acquired drug resistance to CDK4/6 inhibitors. Several factors affect the effectiveness of CDK4/6 inhibitors, like continuous expression of G1-S-phase cyclins and gene mutations in key cell signaling pathways. Research on the molecular mechanisms or clinical strategies to beat CDK4/6 inhibitor resistance is ongoing. Therefore, the main emerging consideration in treatment of advanced luminal carcinoma is now CDK4/6 inhibitor resistance.
Case Presentation
53-year-old woman with a history of arterial hypertension treated with enalapril and bariatric surgery for morbid obesity. Postmenopausal status since 51 years-old. No toxics consume.
The patient consulted in June 2017 due to a nodule in the infero- outer quadrant of the right breast of 8 months of evolution that progressively increased in size. The diagnostic was an early luminal B breast cancer.
In July 2017, a quadrantectomy of the right breast with and a sentinel lymph node biopsy (SLNB) was performed, with a postsurgical result of infiltrating ductal carcinoma grade 2 pT2 (27 mm) without lymphatic, vascular or perineural invasion, N0 (negative SLNB), oestrogen receptor positive (RE+++), progesterone receptor positive (RP+++), HER2 negative and percentage of Ki67 expression was 20%, anatomical stage II-A. To aid in the decision to administer adjuvant chemotherapy, a genomic platform was requested, with a result of low risk of recurrence, so adjuvant chemotherapy was obviated. The patient received adjuvant radiation therapy (42 Gy) completed in September 2017, and began hormonal therapy based on anastrozole 1 mg daily associated with oral calcium supplementation. Since then, she kept normal analytical controls without evidence of disease in the mammography and ultrasound scans.
In May 2019, the patient consulted in emergency room for 2-week history of headache and blurred vision of both eyes.
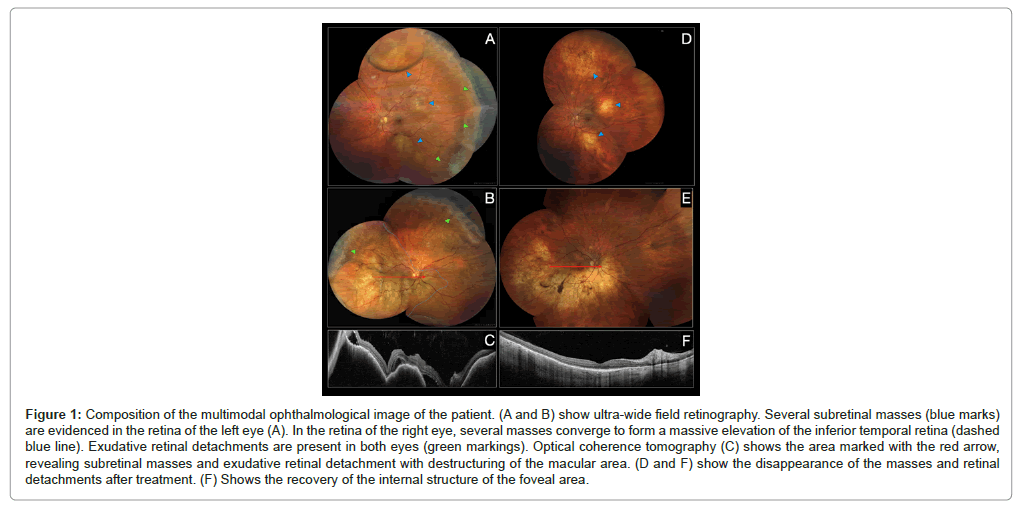
The cardiopulmonary and abdominal examination was normal. The neurological examination revealed a left palpebral ptosis without other findings. In the ophthalmological examination, the fundus revealed an extensive elevation of the entire inferior hemiretin, related to a subretinal mass in the right eye with various foci of exudative retinal detachment. The left eye showed a mass under the lower temporal arcade with exudative retinal detachment (Figures 1A and 1B).Optical coherence tomography (OCT) confirmed the presence of subretinal masses with exudative retinal detachment (Figure 1C). She was admitted to the hospital for treatment and complementary tests 9 (Figures 1D-1F).
Figure 1: Composition of the multimodal ophthalmological image of the patient. (A and B) show ultra-wide field retinography. Several subretinal masses (blue marks) are evidenced in the retina of the left eye (A). In the retina of the right eye, several masses converge to form a massive elevation of the inferior temporal retina (dashed blue line). Exudative retinal detachments are present in both eyes (green markings). Optical coherence tomography (C) shows the area marked with the red arrow, revealing subretinal masses and exudative retinal detachment with destructuring of the macular area. (D and F) show the disappearance of the masses and retinal detachments after treatment. (F) Shows the recovery of the internal structure of the foveal area.
Results
Laboratory tests revealed mild anemia with hemoglobin of 10.5 g/dL, a moderate increase in alkaline phosphatase and gamma glutamyltransferase levels, as well as a carcinoembryonic antigen (CEA) of 202 ng/ml and a cancer antigen 15-3 (CA15.3) of 73 ng/ml.
The brain magnetic resonance imaging (MRI) revealed two focal left parasagittal extra-axial frontal lesions compatible with meningioma; and in the orbits MRI, laminar thickenings in both eyeballs, one in the posteroinferior aspect of the right eyeball and another similar but less important in the cranial aspect of the left eyeball, compatible with retinal detachment secondary to metastasis in Figure 2.
Figure 2: Initial orbits MRI (left side) reveals laminar thickenings in both eyeballs, one in the postero-inferior aspect of the right eyeball and another similar but less important in the cranial aspect of the left eyeball, compatible with retinal detachment secondary to metastasis. Post-treatment orbits MRI (right side) shows a radiological complete response without pathologic findings.
In the extension computed tomography (CT) scan, multiple lung, liver, adrenal, skin metastases, metastatic bone infiltration and bilateral mammary nodules were observed. The bone scan revealed blastic bone metastases in the shoulders, humerus, sternum, costal arches, spine, pelvis, femurs, and tibia.
On breast ultrasound, numerous solid nodules were found in both breasts. A biopsy of a nodule in the upper-outer quadrant of the right breast was performed with a diagnosis of luminal B like infiltrating ductal carcinoma of the breast grade 2 (RE+++, RP++, HER2 negative, Ki67 20%, TILs 5%, PIK3CA negative).
Due to relapse of multilevel disease in the course of treatment with aromatase inhibitor, it was decided to start first-line treatment with fulvestrant and palbociclib (CDK4/6 inhibitor) associated with zoledronic acid.
In September 2019, a partial radiological, clinical and biochemical response was observed. The CT scan showed a reduction in metastatic lesions, decreasing CA15.3 levels.
In the last control, in March 2021 and after 24 cycles of palbociclib, a radiological, and biochemical response was maintained without pathologic findings in the orbits MRI in Figure 2. In the ophthalmic examination, a complete disappearance of the retinal masses was evidenced. In the areas where the masses were found, a pigmentary alteration with a mottled appearance persisted in Figures 1D and 1E. The examination also revealed that the exudative retinal detachments had been reabsorbed. OCT confirmed the anatomical recovery of the macular area Figure 1F. Surprisingly, visual acuity had recovered to 20/20 and the patient reported no visual symptoms.
Discussion
Breast cancer is the most common neoplastic disorder diagnosed in women worldwide. Despite recent advances in early diagnosis and effective treatment, it is estimated that around a third of patients having been diagnosed with breast cancer will develop metastatic disease [1].
Ocular metastases from breast cancer, although rare, can occur. Metastatic lesions in other locations such as the lungs, the central nervous system, or the bones are usually detected before the diagnosis of ocular metastases [2]. In some rarer cases, ocular metastasis may represent the initial manifestation of breast cancer [3]. Survival of patients with metastatic ophthalmic disease depends on the level of organ dysfunction and tumor burden caused by neoplastic spread. The incidence of ocular metastases in advanced breast cancer ranges from 5% to 30%. These types of eye injuries often go unnoticed because they are generally asymptomatic, in contrast to metastatic disease in other organs [2]. It has been observed that bilateral ocular involvement could occur in around 30%-40% of all women with metastatic breast cancer in the eyes [4].
The eyeball itself represents the anatomical structure most frequently affected in ocular metastases. The vast majority of ocular metastatic sites occur due to hematogenous spread [5]; for this reason, the uveal tissue (iris, ciliary body, and choroid), especially the choroid, is the main ocular site of breast cancer metastases. Uveal involvement occurs in up to 10% of metastatic breast cancer cases [2,6].
Microenvironmental factors and large choroidal vascularization have been proposed as possible explanations for increased metastatic spread in the choroid [7]. The uveal tract, composed by the iris, the ciliary body, and the choroidal layer, with a large vascular network, is the site of the eyeball where most metastatic lesions occur (aorund 80%).
On rare occasions, metastases may also be seen in other ophthalmic structures, such as the optic disc, conjunctiva, lacrimal gland, and extrabulbar structures. Retinal metastases are very unusual and only a few cases have been described in the literature.
Most patients with metastatic choroidal disease are asymptomatic. Some of the most common symptoms are blurred vision, ocular pain, visual field defects, metamorphopsia, floaters, and photopsia. Patients with advanced breast cancer with affectation of the soft tissues of the orbit could present symptoms, such as proptosis, ptosis, diplopia, and even pain. Enophthalmos is occasionally seen as a result of soft-tissue retraction, usually with a scirrhous form of breast cancer [2].
Computed tomography scans and magnetic resonance imaging are often ordered, but may fail to identify intraocular lesions because they measure only a few millimeters in height. Nevertheless, retinal detachment, if present, is usually observed using these imaging techniques [8].
Although systemic therapy can affect the growth of ocular metastases, the diagnosis of ocular involvement is usually made in symptomatic patients. There is currently no consensus on the ideal treatment strategy. Most patients have a limited life expectancy, and complex or surgical treatments are generally not recommended. Recent advances in systemic therapy have significantly improved response rates for some patients who may benefit from less aggressive treatment, while still preserving vision. Although external beam radiation therapy is the most widely used local treatment, more advanced forms of radiation therapy that are associated with fewer side effects may be proposed in selected cases [9,10]. In patients with a shorter life expectancy, systemic therapies can induce a regression of choroidal metastases and could be sufficient to temporarily reduce visual symptoms [11]. However, on certain occasions, resistance to systemic treatment is acquired and ocular relapse usually requires radiotherapy for a longer local control. Less invasive local treatments, such as photodynamic therapy and intravitreal injection of anti-VEGF, can also help preserve vision and improving the quality of life [8,12].
Nowadays, the treatment of hormone-receptor positive (HR+) HER2 negative advanced breast cancer consists in the combination of hormone therapy and CDK4/6 inhibitors [1]. CDK4/6 inhibitors are a class of drugs that target enzymes called CDK4 and CDK6. These enzymes are important in cell division. CDK4/6 inhibitors are designed to stop tumor cell growth [13]. The CDK4/6 inhibitors currently used to treat metastatic breast cancer are palbociclib, ribociclib and abemaciclib. CDK4/6 inhibitors are all approved in combination with endocrine therapy such as aromatase inhibitors or fulvestrant; abemaciclib is also approved as a single agent [14].
In the first-line setting, all the agents-together with an aromatase inhibitor substantially prolonged PFS with a consistent hazard ratio of around 0.5 in all phase III trials [15-17]. The data for second-line settings and beyond is also very consistent, with a substantial prolongation of PFS demonstrated for fulvestrant together with abemaciclib, ribociclib, or palbociclib [18-20]. Treatment with palbociclib plus fulvestrant increased median PFS compared with fulvestrant alone from 4.6 months to 11.2 months. Palbociclib plus fulvestrant resulted in a median 6.9 months gain in OS, although this was not statistically significant (34.9 months for palbociclib plus fulvestrant compared with 28 months for placebo plus fulvestrant) [20]. Therapy with CDK4/6 inhibitors is well tolerated and side effects are manageable. Hematological toxicities are most frequent with palbociclib and ribociclib. Abemaciclib has a lower incidence of neutropenia but much greater incidence of all grades of diarrhea compared with other CDK4/6 inhibitors [21].
This case highlights the importance of a complete ophthalmological examination in these kind of patients. Advances in ophthalmic multimodal imaging such as ultra-wide field retinography and spectral domain OCT can provide important information about ocular metastases. Ultra-wide-field retinography can detect masses and exudative retinal detachments even though they are in the retinal periphery. Spectral domain OCT can help assess the response to treatment of these lesions. Both are non-invasive, painless tests and are available in most ophthalmology services. Furthermore, this case report shows the relevance of starting a systemic treatment in breast cancer patients with ocular metastases, avoiding local therapies such as surgery or radiotherapy.
Conclusion
To the best of our knowledge, there has been no report of a complete remission of a bilateral retinal detachment due to ocular lobular breast cancer metastases treated with fulvestrant plus CDK4/6 inhibitor. Systemic therapy in luminal breast cancer may be sufficient to obtain a good response to ocular metastases, thus avoiding other treatments that may have more local side effects, such as radiotherapy. Optical coherence tomography and ultra-wide-field retinography allow accurate diagnosis and quickly non-invasively response assessment.
References
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 12: 1623-1649.
- Georgalas I, Paraskevopoulos T, Koutsandrea C, Kardara E, Malamos P (2015) Ophthalmic metastasis of breast cancer and ocular side effects from breast cancer treatment and management: Mini review. BioMed Res Int 9: 1-8.
- Olfa G, Riadh H, Sameh T, Fafani BH, Slim BA (2009) Breast cancer discovered from choroidal metastasis: Apropos of a case and review of the literature. Can J Ophthalmol 44: 23-26.
- Dagoglu N, Mahadevan A (2019) Breast cancer metastases to the eye. Breast Dis.
- Ferry AP, Font RL (1974) Carcinoma metastatic to the eye and orbit: A clinicopathologic study of 227 cases. Arch Ophthalmol 92: 276-286.
- Kreusel KM, Wiegel T, Stange M, Bornfeld N, Foerster MH (2000) Intramokulare metastasen bei metastasiertem mammakarzinom der Frau inzidenz, risikofaktoren and therapie. Ophthalmologe 97: 342-346.
- McCartney A (1993) Intraocular metastasis. Br J Ophthalmol 77: 133.
- Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM (2019) New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res 68: 144-176.
- Rudoler SB, Shields CL, Corn BW, De Potter P, Hyslop T (1997) Functional vision is improved in the majority of patients treated with external-beam radiotherapy for choroid metastases: a multivariate analysis of 188 patients. J Clin Oncol 15: 1244-1251.
- Rosset A, Zografos L, Coucke P, Monney M, Mirimanoff R (1998) Radiotherapy of choroidal metastases. Radiot and Onc 46: 263-268.
- Manquez ME, Shields CL, Karatza EC, Shields JA (2005) Regression of choroidal metastases from breast carcinoma using aromatase inhibitors. Br J Ophthalmol 89: 776-777.
- Shahar J, Avery RL, Heilweil GA, Barak A, Zemel E (2006) Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina Phila 26: 262-269.
- Goel S, DeCristo MJ, McAllister SS, Zhao JJ (2018) CDK4/6 inhibition in cancer: Beyond cell cycle arrest. Trends Cell Biol 28: 911-925.
- Murphy CG (2019) The role of CDK4/6 inhibitors in breast cancer. Curr Treat Options Oncol 20: 1-3.
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 16: 25-35.
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. NÂ Engl J Med 375: 1738-1748.
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S (2017) MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35: 3638-3646.
- Sledge Jr GW, Toi M, Neven P, Sohn J, Inoue K (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35: 2875-2884.
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Monaleesa-3. J Clin Oncol 36: 2465-2472.
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17: 425-439.
- Eggersmann TK, Degenhardt T, Gluz O, Wuerstlein R, Harbeck N (2019) CDK4/6 inhibitors expand the therapeutic options in breast cancer: palbociclib, ribociclib and abemaciclib. BioDrugs 33: 125-135.
Citation: Abad MN, Salvador MO, Fariñas SC, Hernández JM, Navarro VC et al. (2021) Dramatic Response to Hormone Therapy Plus CDK4/6 Inhibitor of a Retinal Detachment Secondary to Luminal Advanced Breast Cancer. J Clin Exp Pathol 11: 399.
Copyright: © 2021 Abad MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2091
- [From(publication date): 0-2021 - Apr 16, 2025]
- Breakdown by view type
- HTML page views: 1503
- PDF downloads: 588