Down-Regulation of MutS Homolog 2 (MSH2) Expression by Curcumin Enhances Cytotoxicity Induced by Gemcitabine in Human Lung Adenocarcinoma Cells
Received: 17-Nov-2017 / Accepted Date: 01-Dec-2017 / Published Date: 11-Dec-2017 DOI: 10.4172/2161-1165.1000332
Abstract
Gemcitabine (2′,2′-difluorodeoxycytidine) is a difluorinated analog of deoxycytidine. It is used clinically to treat patients with non-small-cell lung cancer (NSCLC). Curcumin is a yellow pigment derived from the rhizome of Curcuma longa, and has been proven to have antioxidant and antitumor properties. Human MutS homolog 2 (MSH2) is a key DNA mismatch repair protein that plays an important role in maintaining genomic stability. Depletion of MSH2 from cells can reverse resistance to certain DNA-damaging agents. In this study, exposure of human lung adenocarcinoma A549 and H1975 cells to gemcitabine increased protein phosphorylation of MKK3/6 and p38 MAPK in a time- and dose-dependent manner; this was accompanied by increased expression of MSH2 mRNA and protein. Gemcitabine-induced cytotoxicity was significantly enhanced by MSH2 siRNA transfection or inactivation of p38 MAPK by SB202190 or p38 MAPK siRNA transfection. However, overexpression of MSH2 cDNA reduced gemcitabine-induced cytotoxicity. Furthermore, curcumin enhanced gemcitabine-induced cytotoxicity via inactivation of MKK3/6-p38 MAPK and downregulation of MSH2. Enforced expression of constitutively active MKK6 rescued cell viability and restored MSH2 protein levels that were suppressed by curcumin and gemcitabine. Suppression of MSH2 expression and a combination with curcumin may be considered as potential therapeutic modalities for gemcitabine-resistant NSCLC cells.
Keywords: Gemcitabine; Curcumin; MSH2; p38 MAPK; Non-small cell lung cancer
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer death in the world [1], and the majority of patients with NSCLC present with advanced metastatic stages [2]. The cytotoxic chemotherapies can improve the median overall survival of NSCLC patients [3]. Gemcitabine (2′, 2′-difluorodeoxycytidine, dFdC) has been clinically utilized for NSCLC patients [4]. It′s cytotoxic effect requires intracellular transport and activation [5,6]. When gemcitabine introduced into the cells by membrane transporters, it undergoes intracellular phosphorylation and yields gemcitabine diphosphate (dFdCDP) and triphosphate (dFdCTP), which can be incorporated into DNA and RNA [5,7]. A previous study has shown that gemcitabine can be used in combination with platinum drugs to treat advancedstage NSCLC, and can act as a single agent for the treatment of pancreas adenocarcinoma [8]. The anti-proliferative activity of gemcitabine in pancreatic cancer results from p38 mitogen-activated protein kinase (MAPK) activation, which mediates cell apoptosis [9]. Moreover, down-regulation of gemcitabine-induced extracellular signal-regulated kinase 1/2 (ERK1/2) in hepatocellular and cholangiocellular carcinomas enhances cell death [10]. However, the molecular mechanisms leading to the lesser effectiveness of gemcitabine in lung adenocarcinoma cells than in squamous carcinoma cells in terms of growth inhibition and cytotoxicity are poorly understood and remain to be elucidated.
Human MutS homolog 2 (MSH2), a crucial element in the DNA mismatch repair (MMR) system, maintains genetic stability and avoids gene mutation in cells [11]. The mismatch recognition activity in MMR is carried out by either MutSα (MSH2-MSH6) or MutSβ (MSH2- MSH3). Deletion or mutation of MSH2 can cause genomic instability [12]. In addition, loss of MSH2 expression in tumors from patients with advanced NSCLC led to higher rates of response to oxaliplatinbased chemotherapy [13]. Moreover, both the p38 MAPK and c-Jun N-terminal kinase (JNK) pathways have been confirmed to mediate the ectopic expression of MSH2 under oxidative stress in renal carcinoma cells [14]. We previously found that down-regulation of the MKK3/6– p38 MAPK signal, with the subsequent reduction of MSH2 expression, enhanced the cytotoxic effect of pemetrexed in human lung squamous cells [15]. Whether gemcitabine can affect MSH2 expression in NSCLC is still unknown, and the role of p38 MAPK in MSH2 expression, regulating gemcitabine-induced cytotoxicity in NSCLC, has not been elucidated yet.
Curcumin (diferuloylmethane) is a phytopolylphenol pigment isolated from the plant Curcuma longa (turmeric) and has a variety of pharmacologic properties, including anti-inflammatory, antioxidant and anticancer properties [16,17]. A previous study showed that curcumin inhibited cisplatin-induced p38 MAPK activation and enhanced cisplatin-induced cytotoxicity in human lung cancer cells [18]; therefore, this study was designed to evaluate the role of MSH2 in cell survival in human lung adenocarcinoma cells when they were exposed to gemcitabine and curcumin. We hypothesized that the p38 MAPK signaling pathway was responsible for MSH2 expression and gemcitabine resistance, and that a combination of gemcitabine and curcumin may enhance gemcitabine-mediated cytotoxicity by decreasing the activation of p38 MAPK and the expression of MSH2 in human lung adenocarcinoma cell lines.
Materials and Methods
Cell lines and reagents
Human lung bronchioloalveolar carcinoma A549 cells (CCL-185) and lung adenocarcinoma H1975 cells (CRL-5908) were obtained from the American Type Culture Collection (Manassas, VA). Gemcitabine was obtained from Lilly (Fegersheim, France). Curcumin, cycloheximide and actinomycin D were purchased from Sigma-Aldrich (St Louis, MO, USA). SB202190, MG132, and lactacystin were purchased from Calbiochem-Novabiochem (San Diego, CA, USA).
Western blot analysis
After different treatments, equal amounts of proteins from each set of experiments were subjected to Western blot analysis. The relative protein blot intensities were determined using a computing densitometer equipped with the ImageQuant analysis program (Amersham Biosciences).
Quantitative real-time polymerase chain reaction (PCR)
Polymerase chain reactions (PCRs) were performed using an ABI Prism 7900HT, according to the manufacturer’s instructions. Amplification of specific PCR products was detected using the SYBR Green PCR Master Mix (Applied Biosystems). The designated primers were: for MSH2 forward primer, 5’- AAGCCCAGGATGCCATTG -3’, MSH2 reverse primer, 5’- CATTTGACACGTGAGCAAAGC -3’; GAPDH forward primer, 5’- CATGAGAAGTATGACAACAGCCT -3’; GAPDH reverse primer, 5’- AGTCCTTCCACGATACCAAAGT -3’. The PCR thermal cycling conditions were 10 min denaturation at 95°C; 40 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 20 s. For each sample, data was normalized to the housekeeping gene GAPDH.
Transfection of small interfering RNA and expression plasmids into NSCLC cells
Exponentially growing human lung cancer cells (106) were plated for 18 h, and then the MKK6E or MSH2 cDNA expression vectors were transfected into A549 or H1975 cells using Lipofectamine 2000 (Invitrogen). Cells were transfected with siRNA duplexes (100 nM) by using Lipofectamine 2000 (Invitrogen) for 24 h.
Cell viability analysis
Cells were cultured at 5000 per well in 96-well tissue culture plates. To assess cell viability, drugs were added after plating. At the end of the culture period, 20 μL of MTS solution (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI, USA) was added, the cells were incubated for a further 2 h, and the absorbance was measured at 490 nm using an ELISA plate reader (Biorad Technologies, Hercules, CA). Cell viability was calculated as follows: cell viability (%)=(OD of drug-treated sample/OD of untreated sample) × 100. The values of cell viability were calculated from the mean amount of 3 independent experiments.
Determination of cell death
After treatment, unattached and attached cells were collected and stained using trypan blue solution (Sigma-Aldrich, St Louis, MO, USA). The stain was excluded from living cells but could penetrate dead cells. The proportion of dead cells was determined by counting the cells stained with trypan blue using a hemocytometer.
Statistical analysis
For each protocol, 3 or 4 independent experiments were performed. Results were expressed as mean ± standard error of the mean (SEM). Statistical calculations were performed using SigmaPlot 2000 software (Systat Software, San Jose, CA). Differences in measured variables between the experimental and control groups were assessed using an unpaired t test. *P<0.05 was considered statistically significant.
Results
MSH2 mRNA and protein levels were increased after gemcitabine exposure
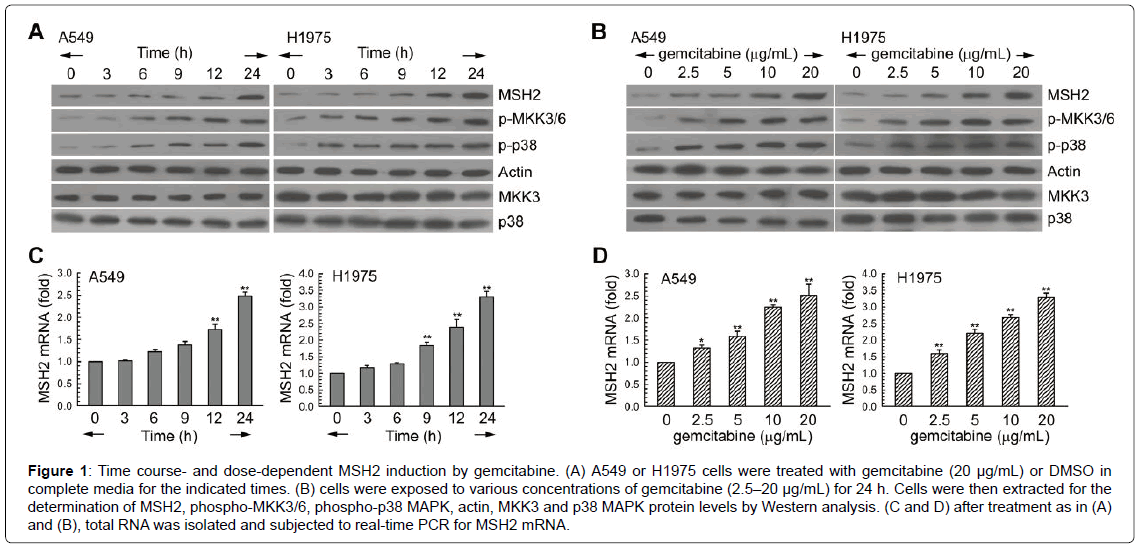
First, we examined the effects of gemcitabine on signal molecules in 2 human lung adenocarcinoma cell lines, A549 and H1975. As shown in (Figure 1A), gemcitabine treatment increased MSH2 protein levels, and this was accompanied by the activation of MKK3/6-p38 MAPK during different exposure times. In addition, these 2 NSCLC cell lines were treated with various concentrations of gemcitabine (2.5–20 μg/ mL) for 24 h, resulting in up-regulation of the protein levels of MSH2 in a dose-dependent manner (Figure 1B). To confirm the transcriptional regulation of MSH2 in the gemcitabine-treated A549 and H1975 cells, mRNA levels were examined using real-time PCR. In Figure 1C and 1D, gemcitabine increased MSH2 mRNA expression in a time- and dose-dependent manner.
Figure 1: Time course- and dose-dependent MSH2 induction by gemcitabine. (A) A549 or H1975 cells were treated with gemcitabine (20 μg/mL) or DMSO in complete media for the indicated times. (B) cells were exposed to various concentrations of gemcitabine (2.5–20 μg/mL) for 24 h. Cells were then extracted for the determination of MSH2, phospho-MKK3/6, phospho-p38 MAPK, actin, MKK3 and p38 MAPK protein levels by Western analysis. (C and D) after treatment as in (A) and (B), total RNA was isolated and subjected to real-time PCR for MSH2 mRNA.
Gemcitabine increased MSH2 mRNA and protein expression through up-regulation of p38 MAPK activity
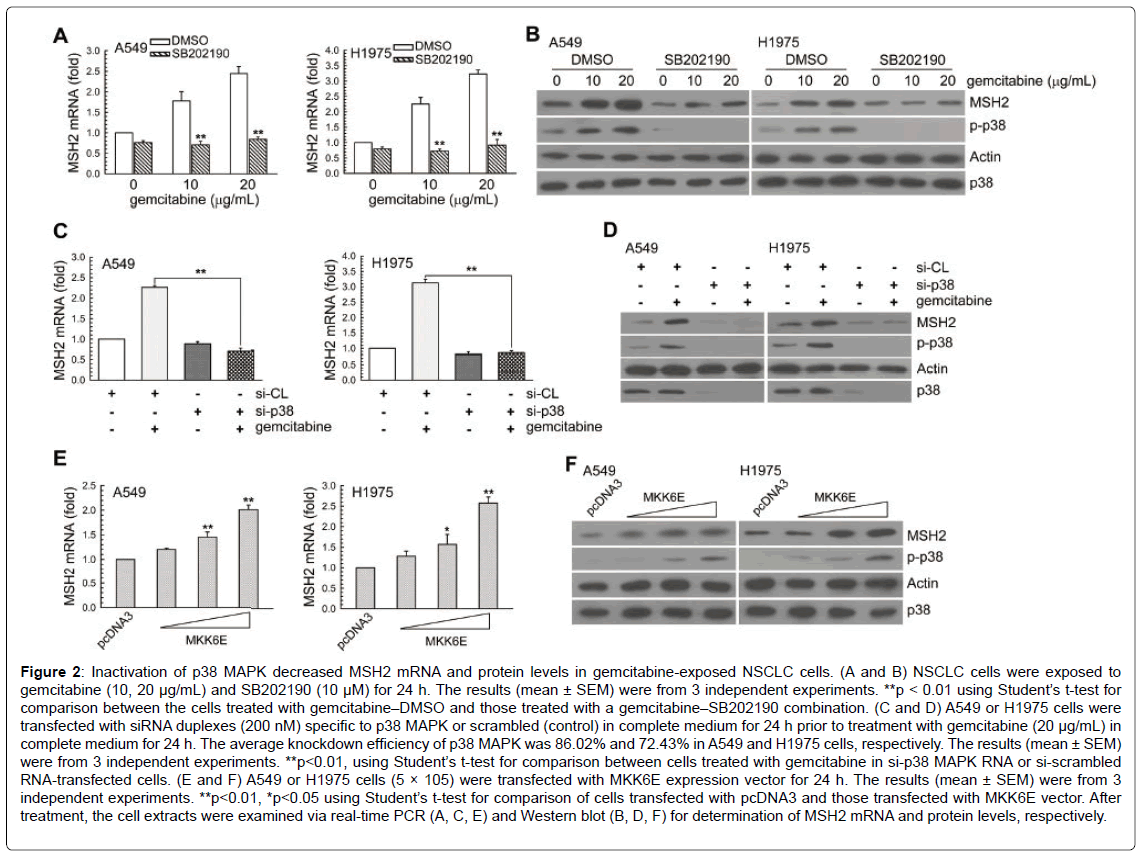
To examine whether the up-regulation of MSH2 expression by gemcitabine was a result of p38 MAPK signal activation, cells were pretreated with SB202190 (a p38 MAPK inhibitor) or transfected with specific p38 MAPK siRNA. In Figure 2A-2D, inactivation of p38 MAPK by the pharmacological inhibitor or the specific siRNA decreased the gemcitabine-induced MSH2 mRNA and protein levels. Furthermore, A549 or H1975 cells were transiently transfected with a plasmid carrying MKK6E, a constitutively active form of MKK6. Compared to transfection with the control vector-pcDNA3, transfection with MKK6E increased the p38 MAPK phosphorylation and MSH2 mRNA and protein levels in A549 or H1975 cells (Figure 2E and 2F).
Figure 2: Inactivation of p38 MAPK decreased MSH2 mRNA and protein levels in gemcitabine-exposed NSCLC cells. (A and B) NSCLC cells were exposed to gemcitabine (10, 20 μg/mL) and SB202190 (10 μM) for 24 h. The results (mean ± SEM) were from 3 independent experiments. **p < 0.01 using Student’s t-test for comparison between the cells treated with gemcitabine–DMSO and those treated with a gemcitabine–SB202190 combination. (C and D) A549 or H1975 cells were transfected with siRNA duplexes (200 nM) specific to p38 MAPK or scrambled (control) in complete medium for 24 h prior to treatment with gemcitabine (20 μg/mL) in complete medium for 24 h. The average knockdown efficiency of p38 MAPK was 86.02% and 72.43% in A549 and H1975 cells, respectively. The results (mean ± SEM) were from 3 independent experiments. **p<0.01, using Student’s t-test for comparison between cells treated with gemcitabine in si-p38 MAPK RNA or si-scrambled RNA-transfected cells. (E and F) A549 or H1975 cells (5 × 105) were transfected with MKK6E expression vector for 24 h. The results (mean ± SEM) were from 3 independent experiments. **p<0.01, *p<0.05 using Student’s t-test for comparison of cells transfected with pcDNA3 and those transfected with MKK6E vector. After treatment, the cell extracts were examined via real-time PCR (A, C, E) and Western blot (B, D, F) for determination of MSH2 mRNA and protein levels, respectively.
Blockage of p38 MAPK activation decreased the MSH2 mRNA and protein stability induced by gemcitabine
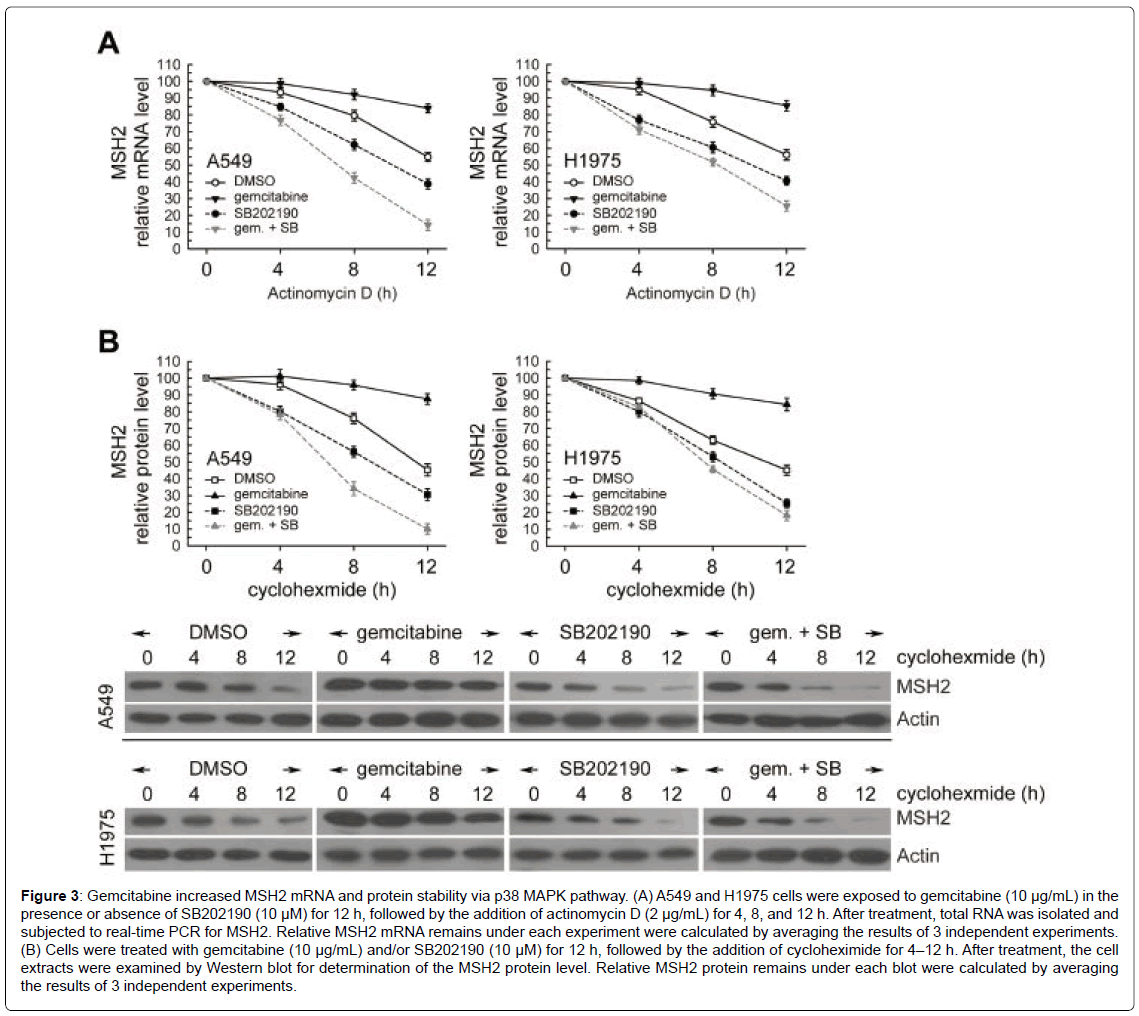
Next, we examined the possible mechanisms for post-transcriptional regulation of MSH2 transcripts under gemcitabine treatment. To evaluate the stability of MSH2 mRNA in gemcitabine-exposed A549 or H1975 cells, the cells were treated with gemcitabine for 12 h; actinomycin D was then added to block de novo RNA synthesis and the levels of existing MSH2 mRNA were measured using real-time PCR at 4, 8, and 12 h after treatment. After actinomycin D co-exposure, higher levels of MSH2 mRNA were observed with gemcitabine treatment than in the untreated cells (Figure 3A). Then, cycloheximide (an inhibitor of de novo protein synthesis) was added to gemcitabine treatment for 4, 8, and 12 h, and the remaining MSH2 protein was analyzed by Western blot. In Figure 3B, MSH2 protein levels were progressively reduced with time in the presence of cycloheximide. However, gemcitabine treatment significantly decreased MSH2 degradation after cycloheximide treatment, compared to the untreated cells. Moreover, more MSH2 protein remained after gemcitabine treatment, compared to the untreated cells (Figure 3B). Only ~45% of MSH2 remained in the H1975 cells treated with cycloheximide for 12 h; however, ~84% of MSH2 remained in cells co-treated with gemcitabine and cycloheximide, indicating that gemcitabine remarkably increased the stability of the MSH2 protein (Figure 3B). Moreover, SB202190 had a significantly decreased effect on gemcitabine-elicited MSH2 mRNA and protein stability in both A549 and H1975 cells (Figure 3A and 3B). These results indicated that gemcitabine increased MSH2 mRNA and protein levels by augmentation of mRNA and protein stability through p38 MAPK activation in gemcitabine-treated NSCLC cells.
Figure 3: Gemcitabine increased MSH2 mRNA and protein stability via p38 MAPK pathway. (A) A549 and H1975 cells were exposed to gemcitabine (10 μg/mL) in the presence or absence of SB202190 (10 μM) for 12 h, followed by the addition of actinomycin D (2 μg/mL) for 4, 8, and 12 h. After treatment, total RNA was isolated and subjected to real-time PCR for MSH2. Relative MSH2 mRNA remains under each experiment were calculated by averaging the results of 3 independent experiments. (B) Cells were treated with gemcitabine (10 μg/mL) and/or SB202190 (10 μM) for 12 h, followed by the addition of cycloheximide for 4–12 h. After treatment, the cell extracts were examined by Western blot for determination of the MSH2 protein level. Relative MSH2 protein remains under each blot were calculated by averaging the results of 3 independent experiments.
MSH2 expression was involved in gemcitabine-mediated cell growth inhibition and cytotoxicity
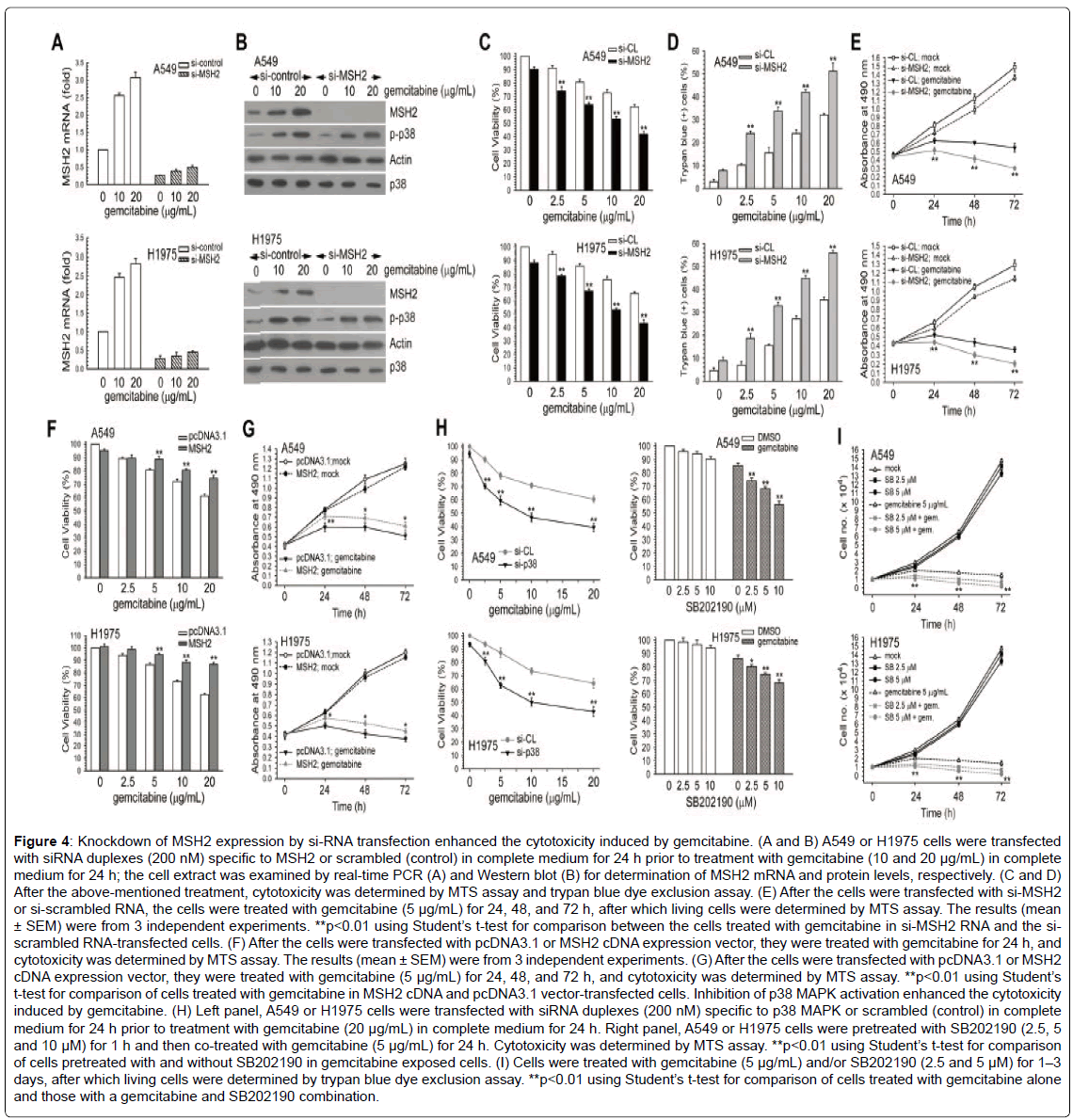
To determine whether MSH2 participated in the gemcitabineelicited inhibition of cell growth and cytotoxicity, we knocked down MSH2 using a specific siRNA duplex (si-MSH2 RNA). As shown in Figure 4A and 4B, si-MSH2 RNA effectively suppressed the MSH2 mRNA and protein expressions induced by various concentrations of gemcitabine. Of interest, the suppression of MSH2 protein expression by si-MSH2 RNA markedly increased the sensitivity of the cells to gemcitabine, compared to the si-control RNA-transfected cells (Figure 4C and 4D). In addition, the blockage of MSH2 expression significantly enhanced the antiproliferative effect brought about by gemcitabine (Figure 4E). Then, the cells were transfected with MSH2 cDNA vector and incubated with gemcitabine, and cell viability was analyzed using the MTS assay. The induction of cytotoxicity and growth inhibition by gemcitabine was significantly reduced following the overexpression of MSH2 in A549 and H1975 cells (Figure 4F and 4G). Therefore, MSH2 protected human lung adenocarcinoma cells from the cytotoxicity induced by gemcitabine.
Next, we investigated the roles of p38 MAPK activation induced by gemcitabine in the gemcitabine-elicited cytotoxicity in lung cancer cells. In the MTS assay, the inhibition of p38 MAPK by SB202190 or sip38 MAPK RNA, respectively, enhanced the cytotoxicity of gemcitabine (Figure 4H). We found that SB202190 also enhanced the gemcitabineinduced inhibition of cell growth in A549 and H1975 cells (Figure 4I). These results indicate that the mediation of MSH2 expression by p38 MAPK has protective effects against the induction of cytotoxicity by gemcitabine in NSCLC cell lines.
Figure 4: Knockdown of MSH2 expression by si-RNA transfection enhanced the cytotoxicity induced by gemcitabine. (A and B) A549 or H1975 cells were transfected with siRNA duplexes (200 nM) specific to MSH2 or scrambled (control) in complete medium for 24 h prior to treatment with gemcitabine (10 and 20 μg/mL) in complete medium for 24 h; the cell extract was examined by real-time PCR (A) and Western blot (B) for determination of MSH2 mRNA and protein levels, respectively. (C and D) After the above-mentioned treatment, cytotoxicity was determined by MTS assay and trypan blue dye exclusion assay. (E) After the cells were transfected with si-MSH2 or si-scrambled RNA, the cells were treated with gemcitabine (5 μg/mL) for 24, 48, and 72 h, after which living cells were determined by MTS assay. The results (mean ± SEM) were from 3 independent experiments. **p<0.01 using Student’s t-test for comparison between the cells treated with gemcitabine in si-MSH2 RNA and the siscrambled RNA-transfected cells. (F) After the cells were transfected with pcDNA3.1 or MSH2 cDNA expression vector, they were treated with gemcitabine for 24 h, and cytotoxicity was determined by MTS assay. The results (mean ± SEM) were from 3 independent experiments. (G) After the cells were transfected with pcDNA3.1 or MSH2 cDNA expression vector, they were treated with gemcitabine (5 μg/mL) for 24, 48, and 72 h, and cytotoxicity was determined by MTS assay. **p<0.01 using Student’s t-test for comparison of cells treated with gemcitabine in MSH2 cDNA and pcDNA3.1 vector-transfected cells. Inhibition of p38 MAPK activation enhanced the cytotoxicity induced by gemcitabine. (H) Left panel, A549 or H1975 cells were transfected with siRNA duplexes (200 nM) specific to p38 MAPK or scrambled (control) in complete medium for 24 h prior to treatment with gemcitabine (20 μg/mL) in complete medium for 24 h. Right panel, A549 or H1975 cells were pretreated with SB202190 (2.5, 5 and 10 μM) for 1 h and then co-treated with gemcitabine (5 μg/mL) for 24 h. Cytotoxicity was determined by MTS assay. **p<0.01 using Student’s t-test for comparison of cells pretreated with and without SB202190 in gemcitabine exposed cells. (I) Cells were treated with gemcitabine (5 μg/mL) and/or SB202190 (2.5 and 5 μM) for 1–3 days, after which living cells were determined by trypan blue dye exclusion assay. **p<0.01 using Student’s t-test for comparison of cells treated with gemcitabine alone and those with a gemcitabine and SB202190 combination.
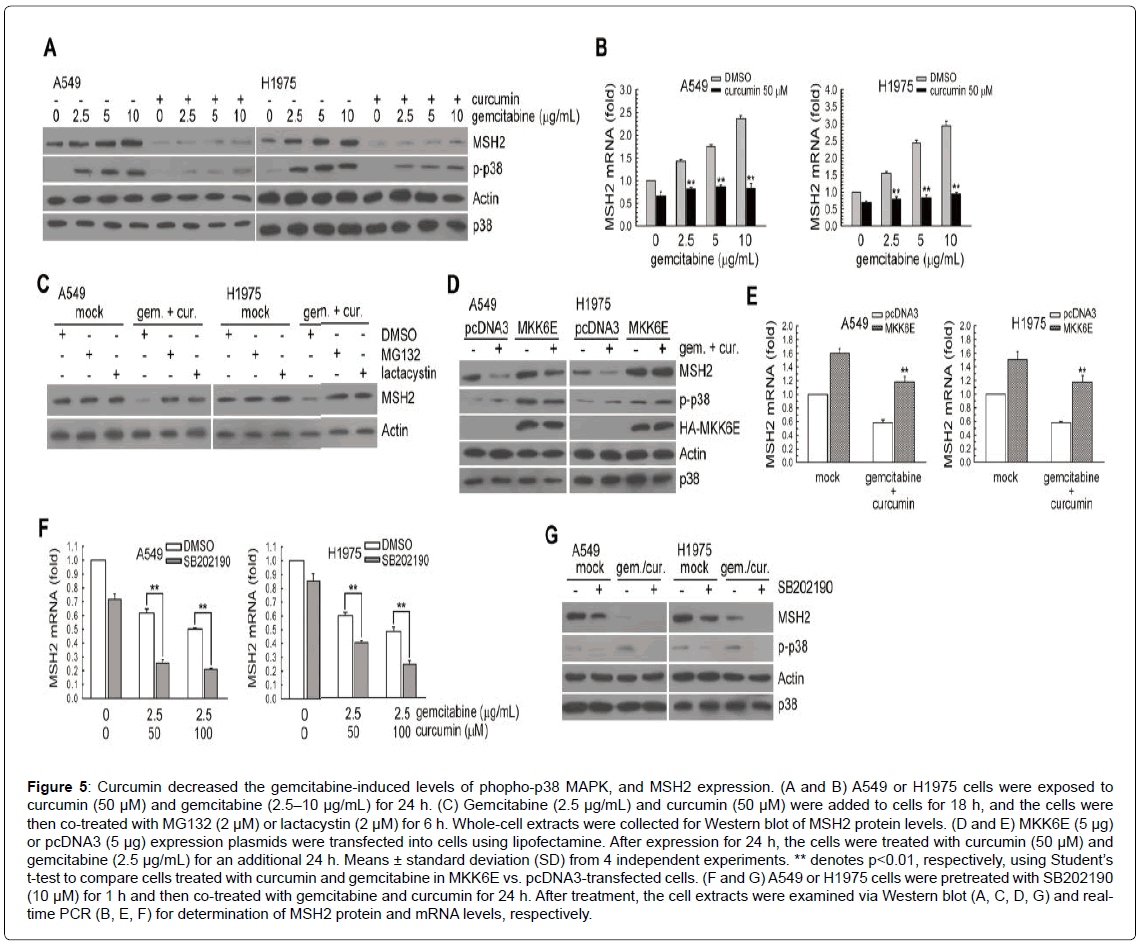
Induction of p38 MAPK phosphorylation and MSH2 expression by gemcitabine can be prevented by curcumin
A previous study showed that curcumin inhibited cisplatin-induced p38 MAPK activation and enhanced cisplatin-induced cytotoxicity in human lung cancer cells [18]; therefore, we tested whether curcumin could enhance the cytotoxicity of gemcitabine via modulating p38 MAPK activation and MSH2 expression. As shown in Figure 5A, under gemcitabine treatment, the protein levels of MSH2 and phospho-p38 MAPK were inhibited by curcumin. In addition, when compared to the untreated cells, treatment with curcumin or curcumin plus gemcitabine down-regulated the levels of MSH2 mRNA (Figure 5B). We also examined whether degradation of MSH2 by curcumin and gemcitabine co-treatment occurred through ubiquitin-26S proteasome-mediated proteolysis of MSH2. As shown in (Figure 5C), both MG132 and lactacystin (both common 26S proteasomal inhibitors) rescued the MSH2 protein levels decreased by curcumin and gemcitabine. Next, we determined whether the inactivation of p38 MAPK was involved in the downregulation of MSH2 protein expression after treatment with gemcitabine plus curcumin, and the constitutively active form of MKK6 (MKK6E) vectors was transfected into human lung cancer cells. MKK6E rescued the levels of phospho-p38 MAPK and MSH2 expression inhibited by treatment with curcumin and gemcitabine (Figure 5D and 5E). However, pretreatment with SB202190 further decreased MSH2 mRNA and protein levels in curcumin and gemcitabine co-treated A549 and H1975 cells (Figure 5F and 5G). Taken together, curcumin could down-regulate gemcitabine-induced p38 MAPK phosphorylation and MSH2 expression in A549 and H1975 cells.
Figure 5: Curcumin decreased the gemcitabine-induced levels of phopho-p38 MAPK, and MSH2 expression. (A and B) A549 or H1975 cells were exposed to curcumin (50 μM) and gemcitabine (2.5–10 μg/mL) for 24 . (C) Gemcitabine (2.5 μg/mL) and curcumin (50 μM) were added to cells for 18 h, and the cells were then co-treated with MG132 (2 μM) or lactacystin (2 μM) for 6 h. Whole-cell extracts were collected for Western blot of MSH2 protein levels. (D and E) MKK6E (5 μg) or pcDNA3 (5 μg) expression plasmids were transfected into cells using lipofectamine. After expression for 24 h, the cells were treated with curcumin (50 μM) and gemcitabine (2.5 μg/mL) for an additional 24 h. Means ± standard deviation (SD) from 4 independent experiments. ** denotes p<0.01, respectively, using Student’s t-test to compare cells treated with curcumin and gemcitabine in MKK6E vs. pcDNA3-transfected cells. (F and G) A549 or H1975 cells were pretreated with SB202190 (10 μM) for 1 h and then co-treated with gemcitabine and curcumin for 24 h. After treatment, the cell extracts were examined via Western blot (A, C, D, G) and realtime PCR (B, E, F) for determination of MSH2 protein and mRNA levels, respectively.
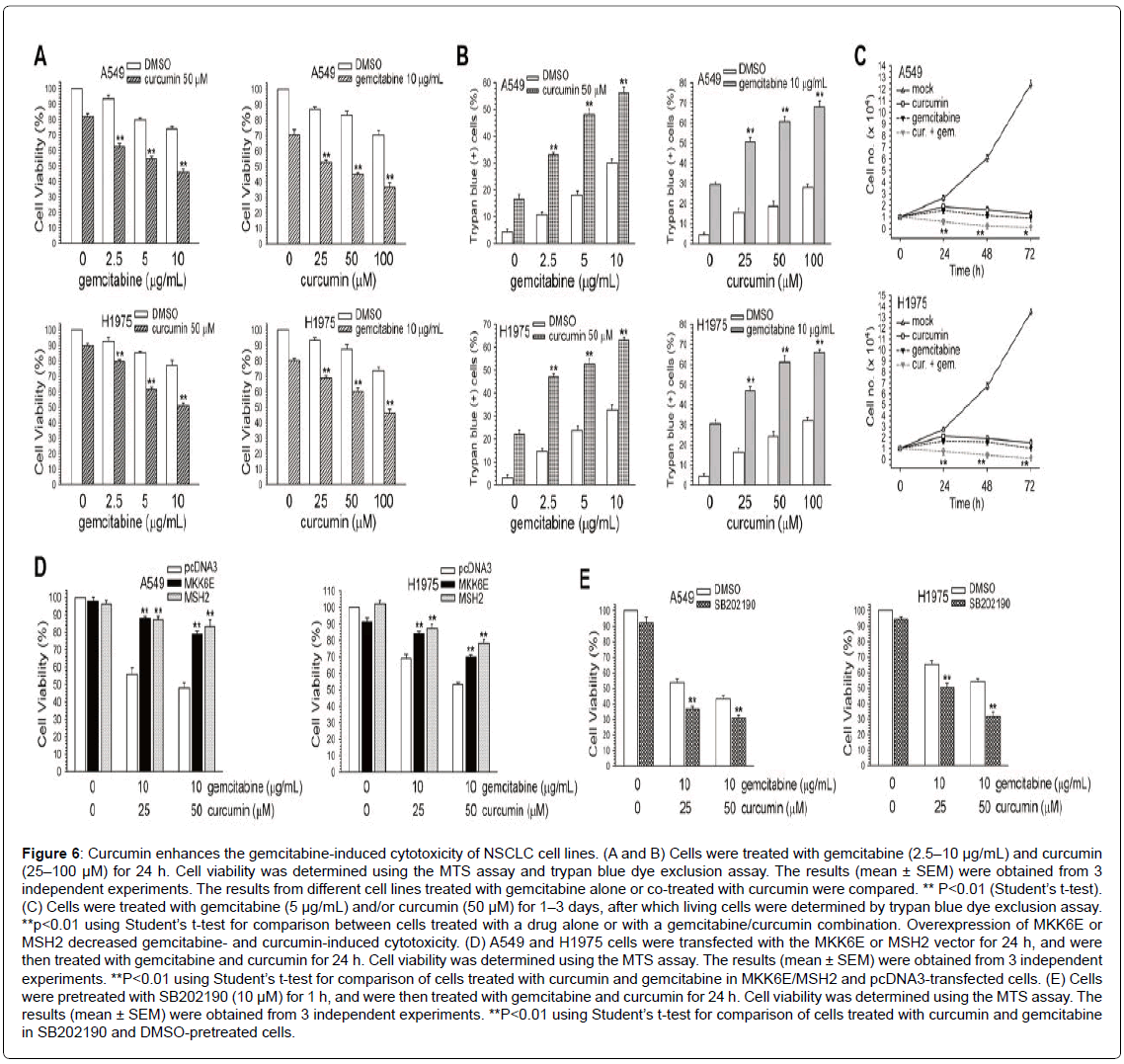
Combined treatment with curcumin enhances gemcitabine-induced cytotoxicity in NSCLC cells
We next examined whether curcumin could enhance the cytotoxicity of gemcitabine in A549 and H1975 cells. In the MTS assay, curcumin enhanced the cytotoxicity of cells treated with gemcitabine (Figure 6A). Consistent with these observations, cell death was higher in cells that were co-treated with the 2 drugs than in those that were treated with gemcitabine or curcumin alone, as determined using the trypan blue exclusion assay (Figure 6B). Moreover, curcumin significantly enhanced the antiproliferative effects induced by gemcitabine (Figure 6C).
Curcumin enhances gemcitabine-induced cytotoxicity via the inactivation of p38 MAPK and down-regulation of MSH2 expression
We determined whether activation of p38 MAPK and overexpression of MSH2 could protect the cells from cell death induced by gemcitabine and curcumin. A549 and H1975 cells were transfected with MKK6E or MSH2 cDNA vectors to evaluate the viability of cells treated with curcumin and/or gemcitabine. Transfection of MKK6E or MSH2 cDNA rescued the cells from the cell death induced by curcumin and gemcitabine in A549 and H1975 cells (Figure 6D). Inactivation of p38 MAPK activity by SB202190 could further enhance curcumin and gemcitabine-induced cytotoxicity (Figure 6E). Together, the activation of p38 MAPK and the expression of MSH2 protein and mRNA were suppressed by curcumin, leading to the enhancement of gemcitabineinduced cytotoxicity.
Figure 6: Curcumin enhances the gemcitabine-induced cytotoxicity of NSCLC cell lines. (A and B) Cells were treated with gemcitabine (2.5–10 μg/mL) and curcumin (25–100 μM) for 24 h. Cell viability was determined using the MTS assay and trypan blue dye exclusion assay. The results (mean ± SEM) were obtained from 3 independent experiments. The results from different cell lines treated with gemcitabine alone or co-treated with curcumin were compared. ** P<0.01 (Student’s t-test). (C) Cells were treated with gemcitabine (5 μg/mL) and/or curcumin (50 μM) for 1–3 days, after which living cells were determined by trypan blue dye exclusion assay. **p<0.01 using Student’s t-test for comparison between cells treated with a drug alone or with a gemcitabine/curcumin combination. Overexpression of MKK6E or MSH2 decreased gemcitabine- and curcumin-induced cytotoxicity. (D) A549 and H1975 cells were transfected with the MKK6E or MSH2 vector for 24 h, and were then treated with gemcitabine and curcumin for 24 h. Cell viability was determined using the MTS assay. The results (mean ± SEM) were obtained from 3 independent experiments. **P<0.01 using Student’s t-test for comparison of cells treated with curcumin and gemcitabine in MKK6E/MSH2 and pcDNA3-transfected cells. (E) Cells were pretreated with SB202190 (10 μM) for 1 h, and were then treated with gemcitabine and curcumin for 24 h. Cell viability was determined using the MTS assay. The results (mean ± SEM) were obtained from 3 independent experiments. **P<0.01 using Student’s t-test for comparison of cells treated with curcumin and gemcitabine in SB202190 and DMSO-pretreated cells.
Discussion
In this study, we first found that gemcitabine induced the expression of MSH2 via the activation of p38 MAPK. Knockdown of MSH2 expression enhanced gemcitabine-induced cytotoxicity in human lung adenocarcinoma cells. In a previous study, the inactivation of ERK1/2 enhanced cell death in gemcitabine-treated hepatocellular and cholangiocellular carcinoma cells [10]. In addition, nuclear factor κB (NF-κB) [19] and bcl-2 [20] were associated with gemcitabine resistance in pancreatic carcinoma cell lines. Low levels of BRCA1 were correlated with increased survival in NSCLC patients treated with gemcitabine plus cisplatin [21,22]. In our previous study, down-regulation of Rad51 expression enhanced the cytotoxicity of gemcitabine in NSCLC cell lines [23]. In this study, the overexpression of MSH2 enhanced the viability of lung cancer cells treated with gemcitabine, and down-regulation of MSH2 expression enhanced gemcitabine-induced cytotoxicity and cell growth arrest.
A previous study indicated that phosphorylation of p38 MAPK induced by vascular endothelial growth factor (VEGF) was significantly suppressed by curcumin pretreatment in human intestinal microvascular endothelial cells (HIMECs), and curcumin could attenuate VEGF-induced cyclo-oxygenase-2 (COX-2) expression through inhibition of MAPK pathway [24]. Previous results have indicated that curcumin induces cell apoptosis through p38 MAPKdependent up-regulation of FasL in human hepatocellular carcinoma cells [25]. Moreover, oridonin can potentiate the effects of gemcitabine in pancreatic cancer through the p38 MAPK signaling pathway, which is dependent on p53 activation [26]. In this study, curcumin blocked the activation of p38 MAPK in gemcitabine-treated NSCLC cell lines, and enhanced gemcitabine-induced cytotoxicity through suppressing the expression of MSH2.
A recent study found that when A549, NCI-H460, and NCI-H520 cells were treated with metuzumab and gemcitabine, there was a significant increase in deoxycytidine kinase (dCK) protein levels, compared to single-agent metuzumab or gemcitabine alone. Moreover, metuzumab could significantly enhance the chemosensitivity of human NSCLC cells to gemcitabine [27]. Our studies have shown that the downregulation of MSH2 in lung cancer cells could increase the chemosensitivity of cells to gemcitabine. Loss of MSH2 could result in cancer predisposition in both mice and humans due to the ensuing increase in genomic instability [28,29]. In another study, the p38 MAPK/MAPK-activated protein kinase 2 (MK2) signaling pathway was found to be a determinant mechanism of gemcitabine in killing pancreatic cancer cells [30]. Gemcitabine activates p38 MAPK to trigger apoptosis in response to cellular stress in pancreatic tumor cells [31]. MK2 was shown to be required for gemcitabine-induced cell death in vitro. Inhibition of MK2 led to the survival of osteosarcoma cells when treated with gemcitabine, through translesion polymerase activity [32]. Moreover, activation of p38 MAPK and MK2 by gemcitabine can induce phosphorylation of heat shock protein 27 (Hsp27), a chaperone related to the unfolded protein response, leading to growth suppression in vitro [33]. We are investigating whether MK2 is involved in p38 MAPKmediated MSH2 expression and cytotoxicity induced by gemcitabine in NSCLC cells. Suppression of MSH2 expression by blocking p38 MAPK pathways may provide a novel therapeutic modality to enhance cytotoxicity and overcome the drug resistance of gemcitabine in human lung adenocarcinoma cells.
Acknowledgements
This study was funded by the grant, MOST 105-2314-B-002-112 (J-C. Ko and Y-W. Lin), from the Ministry of Science and Technology, Taiwan, and by the National Taiwan University Hospital, Hsin-Chu Branch, Taiwan.
Conflict of Interest
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. ( 2007) Cancer statistics, 2007. CA:Â Cancer J Clin 57: 43-66.
- Silvestri GA, Spiro SG (2006) Carcinoma of the bronchus 60 years later. Thorax 61: 1023-1028.
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, et al. (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92-98.
- Martoni A, Marino A, Sperandi F, Giaquinta S, Di Fabio F, et al. (2005) Multicentre randomised phase III study comparing the same dose and schedule of cisplatin plus the same schedule of vinorelbine or gemcitabine in advanced non-small cell lung cancer. Eur J Cancer 41: 81-92.
- Mini E, Nobili S, Caciagli B, Landini I, Mazzei T (2006) Cellular pharmacology of gemcitabine. Annals oncol 17: 7-12.
- Ali S, El-Rayes BF, Aranha O, Sarkar FH, Philip PA (2005) Sequence dependent potentiation of gemcitabine by flavopiridol in human breast cancer cells. Breast Cancer Res Treat 90: 25-31.
- Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, et al. (2009) Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scan J Gastro 44:782-786.
- Heinemann V (2002) Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. SeminOncol29:9-16.
- Koizumi K, Tanno S, Nakano Y, Habiro A, Izawa T, et al. (2005) Activation of p38 mitogen-activated protein kinase is necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cells. Anticancer Res 25:3347-3353.
- Matsumoto K, Nagahara T, Okano J, Murawaki Y (2008)The growth inhibition of hepatocellular and cholangiocellular carcinoma cells by gemcitabine and the roles of extracellular signal-regulated and checkpoint kinases. Oncol Rep 20:863-872.
- Kunkel TA, Erie DA (2005) DNA mismatch repair. Annual Rev Biochem74:681-710.
- Kolodner RD (1995) Mismatch repair: mechanisms and relationship to cancer susceptibility. Trends Biochemical Sci20:397-401.
- Cooper WA, Kohonen-Corish MR, Chan C, Kwun SY, McCaughan B, et al. (2008) Prognostic significance of DNA repair proteins MLH1, MSH2 and MGMT expression in non-small-cell lung cancer and precursor lesions. Histopathology 52:613-622.
- Mo C, Dai Y, Kang N, Cui L, He W (2012) Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J BiolChem287:19242-19254.
- Tung CL, Chiu HC, Jian YJ, Jian YT, Chen CY, et al. (2014) Down-regulation of MSH2 expression by an Hsp90 inhibitor enhances pemetrexed-induced cytotoxicity in human non-small-cell lung cancer cells. Experimental Cell Res 322:345-354.
- Sharma RA, Gescher AJ, Steward WP (2005)Curcumin: the story so far. Eur J Cancer 41:1955-1968.
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV (2008)Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci65:1631-1652.
- Tung CL, Jian YJ, Chen JC, Wang TJ, Chen WC, et al. (2016) Curcumindownregulates p38 MAPK-dependent X-ray repair cross-complement group 1 (XRCC1) expression to enhance cisplatin-induced cytotoxicity in human lung cancer cells. NaunynSchmiedebergs Arch Pharmacol389:657-666.
- Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, et al. (2003) Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene 22:3243-3251.
- Okamoto K, Ocker M, Neureiter D, Dietze O, Zopf S, et al. (2007) Bcl-2-specific siRNAs restore gemcitabine sensitivity in human pancreatic cancer cells. J Cell Mol Med 11:349-361.
- Rosell R, Felip E, Taron M, Majo J, Mendez P, et al. (2004) Gene expression as a predictive marker of outcome in stage IIB-IIIA-IIIB non-small cell lung cancer after induction gemcitabine-based chemotherapy followed by resectional surgery. Clin Cancer Res 10:4215s-4219s.
- Taron M, Rosell R, Felip E, Mendez P, Souglakos J, et al. (2004) BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 13:2443-2449.
- Tsai MS,Kuo YH, Chiu YF, Su YC, Lin YW (2010) Down-regulation of Rad51 expression overcomes drug resistance to gemcitabine in human non-small-cell lung cancer cells. J PharmacolExpTher335:830-840.
- Binion DG, Otterson MF, Rafiee P (2008)Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57:1509-1517.
- Wang WZ, Li L, Liu MY, Jin XB, Mao JW, et al. (2013) Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci92:352-358.
- Bu HQ, Luo J, Chen H, Zhang JH, Li HH, et al. (2012) Oridonin enhances antitumor activity of gemcitabine in pancreatic cancer through MAPK-p38 signaling pathway. Int J Oncol41:949-958.
- Feng F, Wang B, Sun X, Zhu Y, Tang H, et al. (2017) Metuzumab enhanced chemosensitivity and apoptosis in non-small cell lung carcinoma. Cancer BiolTher18:51-62.
- Luo F, Brooks DG, Ye H, Hamoudi R, Poulogiannis G, et al. (2007) Conditional expression of mutated K-ras accelerates intestinal tumorigenesis in Msh2-deficient mice. Oncogene 26:4415-4427.
- Andrew SE, Reitmair AH, Fox J, Hsiao L, Francis A, et al. (1997) Base transitions dominate the mutational spectrum of a transgenic reporter gene in MSH2 deficient mice. Oncogene 15:123-129.
- Kopper F, Binkowski AM, Bierwirth C, Dobbelstein M (2014) The MAPK-activated protein kinase 2 mediates gemcitabine sensitivity in pancreatic cancer cells. Cell Cycle 13:884-889.
- Habiro A, Tanno S, Koizumi K, Izawa T, Nakano Y, et al. (2004) Involvement of p38 mitogen-activated protein kinase in gemcitabine-induced apoptosis in human pancreatic cancer cells. BiochemBiophy Res Commun316:71-77.
- Kopper F, Bierwirth C, Schon M, Kunze M, Elvers I, et al. (2013) Damage-induced DNA replication stalling relies on MAP K-activated protein kinase 2 activity. Proc NatAcadSci U.S.A 110:16856-16861.
- Nakashima M, Adachi S, Yasuda I, Yamauchi T, Kawaguchi J, et al. (2011) Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Let 313:218-225.
Citation: Ko JC, Peng YS, Wu CH, Chen JC, Zheng HY, et al. (2017) Down-Regulation of MutS Homolog 2 (MSH2) Expression by Curcumin Enhances Cytotoxicity Induced by Gemcitabine in Human Lung Adenocarcinoma Cells. Epidemiol 7:332. DOI: 10.4172/2161-1165.1000332
Copyright: © 2017 Jen-Chung Ko, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5007
- [From(publication date): 0-2017 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 4261
- PDF downloads: 746